Abstract
Intraductal papillary neoplasm of the bile duct (IPNB) is a variant of bile duct carcinoma that is characterized by intraductal growth and better outcomes compared with common cholangiocarcinoma. IPNBs are mainly found in patients from Far Eastern areas, where hepatolithiasis and clonorchiasis are endemic. According to the immunohistochemical profiles of the mucin core proteins, IPNBs are classified into four types: pancreaticobiliary, intestinal, gastric, and oncocytic. Approximately 40%-80% of IPNBs contain a component of invasive carcinoma or tubular or mucinous adenocarcinoma, suggesting that IPNB is a disease with high potential for malignancy. It is difficult to make an accurate preoperative diagnosis because of IPNB’s low incidence and the lack of specificity in its clinical manifestation. The most common abnormal preoperative imaging findings of IPNB are intraductal masses and the involvement of bile duct dilation. Simultaneous proximal and distal bile duct dilation can be detected in some cases, which has diagnostic significance. Cholangiography and cholangioscopy are needed to confirm the pathology and demonstrate the extent of the lesions. However, pathologic diagnosis by biopsy cannot reflect the actual stage in many cases because different foci may be of different stages and because mixed pathologic findings may exist in the same lesion. Surgical resection is the major treatment. Systematic cholangioscopy with staged biopsies and frozen sections is recommended during resection to ensure that no minor tumors are left and that curative resection is achieved. Staging, histologic subtype, curative resection and lymph node metastasis are factors affecting long-term survival.
Keywords: Intraductal neoplasm, Papillary cholangiocarcinoma, Biliary papillomatosis, Mucinous, Prognosis
Core tip: In this review, we have provided a more comprehensive understanding of “intraductal papillary neoplasm of the bile duct” than in other research articles. We found that preoperative pathologic diagnosis by biopsy could not reflect the actual stage in many cases because different foci might be of different stages and because mixed pathologic findings might exist in the same lesion. Staging, histologic subtype, curative resection and lymph node metastasis were factors affecting long-term survival.
INTRODUCTION
In the biliary system there is a class of tumor that is characterized by predominant intraductal papillary growth, which can occur anywhere along the biliary tree. Growths are usually multifocal, with or without macroscopically visible mucin secretion, and can be of any type of pathological transformation, from low-grade dysplasia to invasive carcinoma. According to these features, these growths used to be identified by various names such as biliary papillomatosis, mucin-producing cholangiocarcinoma, mucin-hypersecreting bile duct tumor, and biliary intraductal papillary mucinous neoplasm. They share common phenotypic changes in tumorigenesis or tumor-progression and show more favorable prognoses compared with non-papillary cholangiocarcinoma. The clinical entity intraductal papillary neoplasm of the bile duct (IPNB) was added to the 2010 World Health Organization (WHO) classification, and it includes intraductal papillary cholangiocarcinoma and its precursor lesions[1]. Previous studies have usually focused on one or several aspects of this disease and have added to our knowledge about their clinicopathological features. This review aims to summarize this knowledge and provide a more comprehensive understanding of IPNB.
EPIDEMIOLOGY
IPNB is mainly found in patients in Far Eastern areas, such as Taiwan, Japan, and Korea, where hepatolithiasis and clonorchiasis are endemic. It is a rare disease, and papillary cholangiocarcinoma accounts for approximately 4%-38% of all bile duct adenocarcinomas. Current reports are of sporadic cases without a tendency to familial aggregation. IPNB most commonly develops in patients between 50 and 70 years of age and shows different sex preponderances in different regions such that the male-to-female ratio is nearly 1:2 in Taiwan, 1:1 in Japan, and 2:1 in Korea and Western countries[2-5].
ETIOLOGY AND PATHOGENESIS
Although the specific etiology and pathogenesis are unclear, IPNB is known to have two major risk factors: hepatolithiasis and clonorchiasis. Yeh et al[6] reported that nearly 87% of patients with IPNB had hepatolithiasis in Taiwan. Another study[7] from Korea suggested that 31% of IPNB patients had hepatolithiasis and 18% had clonorchiasis, but there was no such association in Western countries[2]. This phenomenon suggests that racial and environmental factors may play a role in the development of IPNB in addition to the two major risk factors.
The time lag between the development of hepatolithiasis and IPNB is 6-8 years, and intraductal carcinoma in situ can take 1-2 years to evolve into an invasive lesion. Considering the findings of mixed pathologic types in each case, as well as papillary adenocarcinoma in the background of papillary adenoma, a process of inflammatory cell-repair dysplasia and malignant transformation is likely[6,8,9] (Figure 1). Approximately 40%-80% of IPNBs contain a component of invasive carcinoma or tubular or mucinous adenocarcinoma, suggesting that IPNB is a disease with a high potential for malignancy[2,3,6,8,9]. Immunohistochemical study of surgically removed specimens shows that almost all IPNBs express CK7, CK20, and mucin (MUC)5AC, which are markers of biliary, intestinal, and gastric epithelium, respectively. This finding indicates that IPNB tumor cells retain a biliary immunophenotype and obtain intestinal and gastric immunophenotypes during carcinogenesis. MUC1 expression is frequently associated with the development of invasive lesions[2,4,6,10-14], especially tubular adenocarcinoma, while mucinous carcinoma is usually associated with negativity for MUC1 but positivity for MUC2. Sasaki et al[15] found that overexpression of enhancer of zeste homolog 2 might be associated with malignant behavior in IPNB, in parallel with up-regulated MUC1 expression and down-regulated MUC6 expression. Recently Nakanuma et al[16] provided evidence that peribiliary glands (PBGs) contain cells implicated in the origin of IPNB. Cardinale et al[17] suggested that IPNB might arise from biliary tree stem/progenitor cells (BTSCs) located in PBGs. In response to risk factors such as inflammation, BTSCs might undergo a series of genetic changes and progress from dysplasia to invasive carcinoma.
Figure 1.

A representative case of intraductal papillary neoplasm of the bile duct with macroscopically visible mucin secretion. Within a single tumor (A), the coexistence of adenoma (B), borderline lesion (C), and adenocarcinoma (D) was found (hematoxylin and eosin stain, × 200 ).
PATHOLOGY
IPNB usually appears as singular or multiple grayish-tan to yellow, friable, papillary masses anywhere along the biliary tree, and small lesions may at times be remote from the main tumor. Histologically, IPNB is defined by tumors that show papillary proliferation of neoplastic biliary epithelial cells with delicate fibrovascular stalks within the bile duct, the macroscopic or microscopic existence of mucin, and dilation of the proximal or remote bile duct. Hematoxylin and eosin staining and immunohistochemical profiling of the mucin core proteins are used to classify IPNB into four types[7](Table 1). The pancreaticobiliary type consists of columnar cells with eosinophilic cytoplasm and round nuclei. This type is often positive for MUC1 but negative for MUC2. The intestinal type resembles an intestinal villous neoplasm, and the neoplastic cells consistently express MUC2 and MUC5AC but not MUC1. The gastric type consists of columnar cells resembling gastric foveae that express MUC5AC but are negative for MUC1 and MUC2. The oncocytic type consists of cells with abundant, intensely eosinophilic cytoplasm that consistently express MUC5AC with focal expression of MUC1 and/or MUC2. The pancreaticobiliary type is the most common and is usually associated with invasive lesions, while the oncocytic and gastric types are rare. According to the degree of dysplasia and depth of invasion, IPNB is classified into four stages: IPNB with low-to-intermediate grade dysplasia; IPNB with high-grade dysplasia; intraductal growth-type cholangiocarcinoma, AJCC stage T1; and intraductal growth-type cholangiocarcinoma, AJCC stage T2 or higher.
Table 1.
Histologic subtypes classified by mucin core protein and cytokeratins
| Histologic subtype |
Profile of MUCs |
Cytokeratin |
|||
| MUC1 | MUC2 | MUC5AC | CK7 | CK20 | |
| Pancreaticobiliary | + | - | + | + | + |
| Intestinal | - | + | + | + | + |
| Gastric | - | - | + | + | + |
| Oncocytic | Focal+ | Focal+ | + | + | + |
MUC: Mucin core proteins; CK: Cytokeratin.
CLINICAL MANIFESTATION
The most common clinical manifestations of patients with IPNB are right hypochondralgia (35%-88.5%), repeated episodes of acute cholangitis (5%-59%), and obstructive jaundice (20%-36%). Anemia and loss of body weight are relatively less common. Some patients are asymptomatic[2,3,5-9]. Acute cholangitis, which is not a common presentation of conventional cholangiocarcinoma, is the second most common manifestation of IPNB. First, friable tumor emboli can easily detach from their origins, leading to acute obstruction of the bile duct. Second, more patients are diagnosed with biliary stones in IPNB than in typical cholangiocarcinoma. Third, macroscopic mucin hypersecretion can be observed in nearly one third of IPNB patients. Theoretically, abundant mucin discharge into the bile duct may intermittently impede bile flow, leading to obstructive jaundice and cholangitis, which can also cause volatile jaundice. The majority of IPNBs are found in the hilum and left liver; however, despite these variable locations, the primary site does not affect the course of the disease or prognosis[2].
LABORATORY TESTS
Laboratory data show common manifestations of obstruction of the bile duct such as elevation of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, direct bilirubin, gamma-glutamyl transpeptidas, alkaline phosphatase, etc. Yeh et al[18] found that an increased ALT level (> 36 U/L, P = 0.022) in IPNB was the only independent factor that could differentiate it from conventional cholangiocarcinoma. However, the specific relationship was not clearly elaborated, and this finding has not been supported by any other reports. Lee et al[8] observed elevation of CA19-9 in 20 of 50 IPNB patients, and the mean level was higher in patients with mucin hypersecretion. Yeh et al[6] found that an elevated serum CA19-9 level was detected in 35% of benign lesions, while 61% of malignant lesions had elevated levels; however, there was no significant difference. Thus, the elevation of CA19-9 may be due to common cholestasis or cholangitis associated with mucin overproduction[19-22]. Additionally, an elevated CEA level was detected in nearly 25% of malignant IPNBs, so CEA may be of some value in differentiating intraductal papillary cholangiocarcinoma from its precursor lesions.
IMAGING FEATURES
The most common abnormal preoperative imaging findings for IPNB are intraductal masses and the involvement of bile duct dilation. Simultaneous proximal and distal bile duct dilation can be detected in some cases, which has diagnostic significance. Imaging patterns can be specifically classified into five subtypes[7,23] (Figure 2). Type 1 shows diffuse duct dilation with a grossly visible intraductal mass (45.4%). Type 2 shows diffuse and marked duct ectasia as in type 1 but without a grossly visible mass (23.7%). Type 3 shows an intraductal papillary mass with localized duct dilation (19.6%). Type 4 shows mild ductal dilation filled with intraductal cast-like lesions (4.1%). Type 5 shows a focal stricture-like lesion with mild proximal duct dilation (7.2%).
Figure 2.
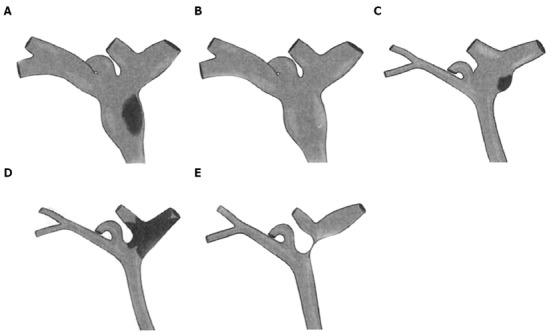
Schematic drawings of intraductal papillary neoplasms of the bile duct showing the five imaging patterns. A: Type 1: Diffuse duct ectasia with a grossly visible papillary mass; B: Type 2: Diffuse duct ectasia without a visible mass; C: Type 3: An intraductal polypoid mass within localized duct dilation; D: Type 4: Intraductal cast-like lesions; E: Type 5: A focal stricture-like lesion with mild proximal ductal dilation.
Ultrasound and ultrasonography
Ultrasound is sensitive for the detection of bile duct dilation, but it is only able to detect a low-echoic mass in nearly 41.2% of cases[8]. Although it helps to differentiate a stone from a tumor in most cases, the accuracy of ultrasound depends on the skill of the investigator. In addition, the presence of mucin cannot be detected on ultrasound because it is equally anechoic as bile. Endoscopic ultrasonography (EUS) and intraductal ultrasonography (IDUS) are useful for assessing the depth of invasion and involvement of the lymph nodes even in the presence of thick mucin, which is important to judge the resectability and predict prognosis. Therefore, it is difficult to distinguish between inflammatory wall thickness and the superficial spread of a tumor using EUS or IDUS[24,25].
Computed tomography and magnetic resonance image
Computed tomography (CT) can detect tumors larger than 1 cm and dilated bile ducts, and its sensitivity is 50%. The enhancement pattern of a tumor is related to the tumoral blood volume and blood flow as well as the prevalence of stromal space. IPNB is usually confined to the mucosa of the bile duct and suspended on small fibrovascular stalks, so it more often shows washout in enhancement scanning rather than the gradually persistent or progressive enhancement observed for conventional cholangiocarcinoma. IPNB appears as a slightly lower signal than hepatic parenchyma in T1WI and as a slightly higher signal in T2WI on magnetic resonance image (MRI) axial scanning. The enhancement pattern on MRI is similar to CT scan[26-28]. Neither CT nor MRI can detect the presence of mucin.
Cholangiography
IPNB often involves the biliary epithelium either diffusely or multifocally, and the actual extent of the lesions usually exceeds CT, MRI and other conventional imaging findings. Cholangiography, including indirect (magnetic resonance cholangiography, MRC) and direct [endoscopic retrograde cholangiography (ERC), percutaneous transhepatic cholangiography (PTC)] cholangiography, is useful for showing the entire bile duct to define the extent of the IPNB[5,25]. MRC is noninvasive and can demonstrate the extent of narrowing or dilation of the bile duct and multifocal intraductal tumors, but it cannot detect the presence of overproduced mucin. ERC and PTC can show multiple small irregular filling defects in the bile duct wall. In patients with mucin overproduction, hypersecreted mucin draining through the ampulla and a patulous ampulla are the characteristic findings. On cholangiography, diffuse bile duct dilation and amorphous filling defects in the bile duct are characteristic[5] (Figure 3). However, a large amount of mucin secretion and obstruction by the tumor may prevent the complete opacification of the entire biliary system to locate the tumors. Cholangiography cannot detect multiple small tumors or lesions confined to the mucosa, and it cannot differentiate tumors from stones or benign strictures of the bile duct.
Figure 3.
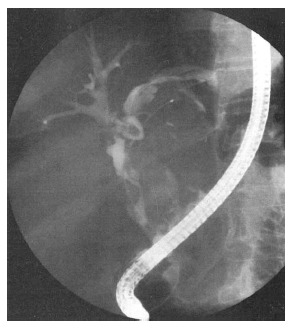
Endoscopic retrograde cholangiography showing an amorphous filling defect, suggesting the presence of mucobilia.
Cholangioscopy
Peroral cholangioscopy (POCS) and percutaneous transhepatic cholangioscopy (PTCS) can visualize the bile duct directly and confirm the histology and extent of the lesions to ensure that appropriate treatment is provided. In a study by Lee et al [8], PTCS revealed additional lesions in nearly one third of IPNB patients after radiologic imaging examinations including ERC and MRC. Therefore, preoperative cholangioscopy has been suggested to be essential. If the papillary orifice is dilated with or without mucin secretion, POCS can be performed immediately after ERC, resulting in an accurate early diagnosis of IPNB. This approach avoids the complications caused by PTCS, such as catheter dislodgement, hemobilia, and tumor seeding of the sinus tract[25,29].
Position-emission tomography/computed tomography
Malignant IPNB with a large mural nodule will present an increased fludeoxyglucose uptake. FDG PET has advantages in the detection of unsuspected distant metastases and in patients with renal dysfunction[24,30,31].
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
It is difficult to make an accurate diagnosis preoperatively because of IPNB’s low incidence and lack of a specific clinical manifestation. The combined application of different imaging techniques is very helpful (Table 2). Noninvasive imaging modalities such as CT and MRI usually fail to detect minor tumors and mucin; thus, cholangiography and cholangioscopy are needed to confirm pathology and demonstrate the extent of the lesions. Kang et al[32] reported that the accuracy of predicting macroscopic multiplicity based on preoperative radiologic imaging findings was 53.5%, with a false positive rate of 25.8% and a false-negative rate of 37.7%. In multifocal IPNB, different foci may be of different stages, and mixed pathologic findings may exist within the same lesion. This phenomenon suggests that pathologic diagnosis by biopsy cannot reflect the actual stage in many cases[8].
Table 2.
Major utility of different imaging techniques
| Techniques | Utility |
| Ultrasound | Detection of bile duct dilation |
| Differentiation from a stone | |
| EUS/IDUS | Assessing the depth of invasion and lymph node involvement |
| CT/MRI | Detect tumors larger than 1 cm and bile duct dilation |
| Differentiation from conventional cholangiocarcinoma | |
| Cholangiography | Define the extent of tumors |
| Detection and drainage of mucin in ERC and PTC | |
| Cholangioscopy | Confirm the histology and extent of lesions |
| Adjuvant treatments | |
| PET/CT | Detection of unsuspected distant metastases |
EUS: Endoscopic ultrasonography; IDUS: Intraductal ultrasonography; ERC: Endoscopic retrograde cholangiography; PTC: Percutaneous transhepatic cholangiography; CT: Computed tomography; MRI: Magnetic resonance image; PET: Positron emission tomography.
The differential diagnoses of IPNB includes recurrent pyogenic cholangitis with bile duct stones[31], mass-forming cholangiocarcinomas[18,27], and biliary mucinous cystic neoplasms (MCNs) (cystadenoma/cystadenocarcinoma)[33-36]. Both IPNB and recurrent pyogenic cholangitis with bile duct stones involve intermittent and incomplete biliary obstruction and intraluminal masses or filling defects on imaging. Mucin plugs or sloughed masses may be confused with stones. Invasive methods such as ERC or cholangioscopy may be necessary to differentiate these diseases. Mass-forming cholangiocarcinoma often appears as a single intrahepatic mass with upstream bile duct dilation and gradually persistent or progressive enhancement on CT and MRI imaging. However, IPNB usually appears as multifocal papillary lesions with both upstream and downstream bile duct dilation with or without visible mucin overproduction that shows washout on enhancement scanning. The vast majority of MCN patients are female; 90% of cases are histologically benign but have the potential to recur and undergo malignant transformation. Multilocular cysts with separation or a cyst-in-cyst appearance are distinctive. Mucin produced by MCNs is confined and does not enter the biliary duct. Ovarian-like stroma is the characteristic microscopic finding. On the contrary, there is no such sex preponderance in IPNB, and 40%-80% of IPNBs are malignant. IPNBs communicate with the bile duct, and there is no ovarian-like stroma pathologically.
TREATMENTS
Surgical resection
Patients without distant metastasis are considered for surgical resection[37-39]. Preoperative IDUS or EUS is used for extrahepatic bile duct assessment and to look for the presence of lymph nodes. Cholangioscopy should be performed to determine the extent of the lesions and to draw up the optimal surgical strategy. During resection, systematic cholangioscopy is performed with staged biopsies and frozen sections. Patients with IPNBs localized to the intrahepatic bile duct are treated with hepatectomy. Patients with IPNBs involving one of the two intrahepatic bile ducts are treated with resection of the affected hemiliver and the common bile duct. For IPNBs localized to the extrapancreatic portion of the bile duct, complete resection of the bile duct from the biliary confluence to the intrapancreatic portion with extended lymphadenectomy is recommended. In cases of positive distal frozen sections, resection of the bile duct is performed with or without pancreatic resection (transduodenal resection). A partial liver resection can be performed when a proximal frozen section is positive in a single intrahepatic duct. Hilar lymphadenectomy has been suggested to be essential for tumors localized to the hilum or common bile duct, but a policy of selective application of caudate lobectomy and portal vein resection can be applied when it is necessary to achieve tumor clearance[40].
Liver transplantation
Surgical resection may remain incomplete due to the high risk of recurrence given positive margins in cases with superficial mucosal spread or recurrence on the remnant bile duct because of the high incidence of multifocal involvement. Resection of the entire biliary tree by liver transplantation and duodenopancreatectomy can be theoretically regarded as the only curative treatment. So far, case reports[41-44] on this approach indicate that patients with positive lymph nodes or major tumor invasion or associated severe comorbidities have not been eligible for liver transplantation. However, the preoperative assessment is usually insufficient for the majority of IPNBs. Thus, a strategy of initial resection to select patients without positive lymph nodes or advanced tumor invasion by definitive analysis of the specimen who would actually benefit from a subsequent liver transplantation seems to be reasonable[37].
Palliative treatment
In case major surgery is not indicated, palliative treatments are recommended[34]. Palliative intrahepatic tubing or percutaneous transhepatic biliary drainage can alleviate jaundice and cholangitis to prolong survival. Recently, new approaches such as percutaneous cholangioscopic laser ablation, cholangioscopic electrocoagulation, iridium-192 intraluminal therapy, and argon plasma coagulation are also useful for improved survival[45].
PROGNOSIS AND FOLLOW-UP
The prognosis of patients with IPNB has been consistently better than of those with conventional bile duct cholangiocarcinoma[40,46-49], and this finding may be related to the inherent biology of IPNB or its primarily intraductal growth pattern. Likewise, there is significant heterogeneity among these tumors. We summarize several factors affecting IPNB patient survival below.
Staging
There was no uniform staging system for IPNB before the 2010 WHO classification. However, it seems to be clear that the overall survival and recurrence-free survival of patients with IPNB is worse as one progresses from low-grade dysplasia to invasive carcinoma on the pathological scale[5,6]. Rocha et al[2] found that both depth of invasion and percentage of invasive carcinoma components correlated with survival (Figure 4). The depth of invasion, graded as ≥ 5 mm, < 5 mm, or none, was associated with survivals of 39, 128, and 144 mo, respectively (P < 0.007). In addition, the percentage of invasive carcinoma components, graded as ≥ 10%, < 10%, or none, was associated with survivals of 42 mo, 128 mo and 144 mo, respectively (P < 0.03).
Figure 4.
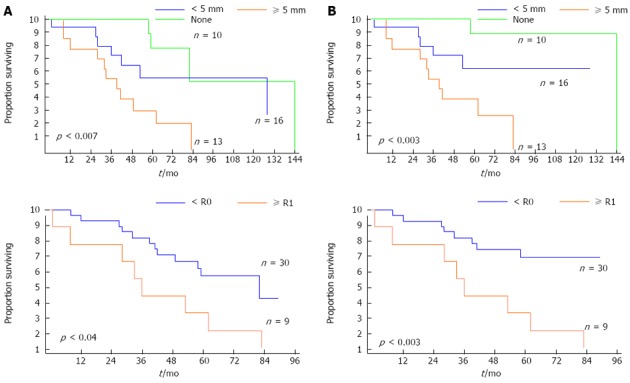
Kaplan-Meier survival estimates of overall survival (A) and disease-specific survival (B) according to the depth of extraductal invasion (none, < 5 mm, and ≥ 5 mm) and resection type (R0 vs R1). P < 0.05 was considered significant.
Histologic subtypes
Kim et al[7] studied a group of 97 patients with IPNB and found that the histologic subtypes of IPNB were associated with different clinicopathologic features and prognoses. Specifically, the pancreaticobiliary type was distinct from the gastric and intestinal types with respect to higher histologic grades, more lymph node metastases, more postoperative recurrences, and worse clinical outcomes. The MUC1-high expressing group showed a shorter disease-specific and recurrence-free survival than the MUC1-low expressing group. In addition, patients with mucinous carcinoma showed a better prognosis compared with patients with tubular adenocarcinoma[3,13].
Curative resection
Lee et al[8] reported that the disease-free survival rate for patients who underwent curative resection was 81% at 5 years in a group of 58 cases of IPNB, and the mean survival period was 60.87 ± 5.86 mo (95%CI: 49.38-72.36), while it was 36.72 ± 6.61 mo (95%CI: 23.77-49.67) in patients who underwent palliative treatments such as percutaneous transhepatic biliary drainage (PTBD). Rocha et al[2] found that R0 resection was associated with an improved median survival time of 82 mo compared with 36 mo in the R1 resection group (Figure 4). Positive resection margins were strongly associated with shorter overall and recurrence-free survival rates, even for low-to-intermediate grade dysplasia[3].
Lymph node metastasis
Lymph node metastasis is rare in early-stage IPNB. Even in patients with invasive carcinoma, it is relatively less common than conventional cholangiocarcinoma. Yeh et al[5] found that the mean survival times with malignant IPNB with and without lymph node metastasis were 12.1 ± 5.1 mo (95%CI: 2.0-22.0) and 39.0 ± 6.7 mo (95%CI: 25.9-52.1), respectively.
A high rate of recurrence after surgical resection has been noticed. Incomplete preoperative assessment of the extent of IPNB might be an important contributing factor. Because small papillary tumors may not be detected by conventional radiologic methods, these undetected lesions, which are usually remote from the main tumor, may be the foci of recurrence[50,51]. In addition, positive margins related to the superficial spreading pattern of IPNB may be the reason for recurrence in many cases[8]. The recurrence rate at 5 years in benign IPNBs has been reported at nearly 20%, which rises to 60% in malignant cases, and most recurrences are locoregional[2,7]. Therefore, to improve the prognosis, full preoperative evaluation of the extent of disease is essential; and to detect recurrences, follow-up appointments scheduled every 3 mo for the first year and every 6 mo beginning in the second year are recommended. MRC is the optimal imaging method, while cholangioscopy can also be performed percutaneously or through the jejunal loop[34].
DISCUSSION
Recent studies[2,4,12,13,16,52] have revealed striking similarities between IPNB and pancreatic intraductal papillary mucinous neoplasm (IPMN). In both organs, these neoplasms arise within the duct system and show a predominantly intraductal growth pattern macroscopically and papillary proliferation with delicate fibrovascular cores and four types of tumor cells microscopically, occasional association with multiple lesions, possible progression to tubular adenocarcinoma and mucinous carcinoma, and more favorable biological behaviors and clinical outcomes. Based on these similarities, IPNB is recognized as a biliary counterpart of IPMN and can be differentiated from conventional cholangiocarcinoma. However, there are several differences between IPNB and IPMN. The most frequent phenotype is intestinal in IPMN but pancreaticobiliary in IPNB, which is more often associated with invasive carcinoma. The other important difference between IPNB and IPMN is with respect to mucin hypersecretion. Mucin is macroscopically identifiable in most cases of IPMN but only in one third of IPNB cases. Considering the existence of goblet cells (one of the mucin-producing cells) and the expression of secretory-type mucin core proteins such as MUC2 and MUC5AC, this difference might be caused by the amount of mucin production.
Ohtsuka et al[9] separated IPNB with or without hypersecretion of mucin into two groups, and found that they were similar in terms of clinical features but somewhat different in pathological findings. IPNB without mucin hypersecretion showed a tubulopapillary growth pattern and uniform degree of cytoarchitectural atypia throughout the neoplasm, which was different from the mixed pathologic transformations in IPNB with mucin hypersecretion. Therefore, whether IPNB with and without mucin hypersecretion are different subtypes or they are distinct clinical entities needs further study.
In conclusion, intraductal papillary neoplasm of the bile duct is a rare biliary tumor with a better prognosis than conventional cholangiocarcinoma. Its specific mechanism of pathogenesis and progression has not yet been well defined[14,15,53-55], and its clinicopathologic features are similar to IPMN. Curative resection is the major treatment and an important favorable factor for long-term survival, especially in patients with early-stage IPNB.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30970623 and No.31071137; International Science and Technology Cooperation Projects, No. 2010DFA31840 and No. 2010DFB33720; Program for New Century Excellent Talents in University, No. NCET-11-0288; and Beijing Natural Science Foundation, No. 5112030
P- Reviewers: Maraveyas A, Tsuyuguchi T S- Editor: Zhai HH L- Editor: Cant MR E- Editor: Liu XM
References
- 1.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC; 2010. p. 417. [Google Scholar]
- 2.Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352–1360. doi: 10.1002/hep.25786. [DOI] [PubMed] [Google Scholar]
- 3.Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;57:787–793. doi: 10.1016/j.jhep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–1343. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- 5.Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, Chen MF. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248–253. doi: 10.1097/01.sla.0000217636.40050.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh TS, Tseng JH, Chen TC, Liu NJ, Chiu CT, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma of the intraductal growth-type and its precursor lesions. Hepatology. 2005;42:657–664. doi: 10.1002/hep.20837. [DOI] [PubMed] [Google Scholar]
- 7.Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, Jang KT, Heo JS, Choi SH, Choi DW, et al. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012;107:118–125. doi: 10.1038/ajg.2011.316. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783–793. doi: 10.1002/cncr.20031. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512–521. doi: 10.1097/PAS.0b013e3182103f36. [DOI] [PubMed] [Google Scholar]
- 10.Sclabas GM, Barton JG, Smyrk TC, Barrett DA, Khan S, Kendrick ML, Reid-Lombardo KM, Donohue JH, Nagorney DM, Que FG. Frequency of subtypes of biliary intraductal papillary mucinous neoplasm and their MUC1, MUC2, and DPC4 expression patterns differ from pancreatic intraductal papillary mucinous neoplasm. J Am Coll Surg. 2012;214:27–32. doi: 10.1016/j.jamcollsurg.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350–358. doi: 10.1016/j.jhep.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Klöppel G, Kosmahl M. Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J Hepatol. 2006;44:249–250. doi: 10.1016/j.jhep.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327–338. doi: 10.1097/00000478-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Amaya S, Sasaki M, Watanabe Y, Tsui WM, Tsuneyama K, Harada K, Nakanuma Y. Expression of MUC1 and MUC2 and carbohydrate antigen Tn change during malignant transformation of biliary papillomatosis. Histopathology. 2001;38:550–560. doi: 10.1046/j.1365-2559.2001.01103.x. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki M, Matsubara T, Yoneda N, Nomoto K, Tsuneyama K, Sato Y, Nakanuma Y. Overexpression of enhancer of zeste homolog 2 and MUC1 may be related to malignant behaviour in intraductal papillary neoplasm of the bile duct. Histopathology. 2013;62:446–457. doi: 10.1111/his.12016. [DOI] [PubMed] [Google Scholar]
- 16.Nakanuma Y, Sato Y. Cystic and papillary neoplasm involving peribiliary glands: a biliary counterpart of branch-type intraductal papillary mucinous [corrected] neoplasm? Hepatology. 2012;55:2040–2041. doi: 10.1002/hep.25590. [DOI] [PubMed] [Google Scholar]
- 17.Cardinale V, Wang Y, Carpino G, Reid LM, Gaudio E, Alvaro D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology. 2012;55:2041–2042. doi: 10.1002/hep.25587. [DOI] [PubMed] [Google Scholar]
- 18.Yeh CN, Jan YY, Yeh TS, Hwang TL, Chen MF. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann Surg Oncol. 2004;11:606–611. doi: 10.1245/ASO.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474–479. doi: 10.1053/ejso.1999.0925. [DOI] [PubMed] [Google Scholar]
- 20.Kim HR, Lee CH, Kim YW, Han SK, Shim YS, Yim JJ. Increased CA 19-9 level in patients without malignant disease. Clin Chem Lab Med. 2009;47:750–754. doi: 10.1515/CCLM.2009.152. [DOI] [PubMed] [Google Scholar]
- 21.Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, Pinto E, Roviello F. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–339. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Ong SL, Sachdeva A, Garcea G, Gravante G, Metcalfe MS, Lloyd DM, Berry DP, Dennison AR. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci. 2008;53:3213–3217. doi: 10.1007/s10620-008-0289-8. [DOI] [PubMed] [Google Scholar]
- 23.Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, Cho HJ, Kim KA, Choi SY. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29:683–700. doi: 10.1148/rg.293085729. [DOI] [PubMed] [Google Scholar]
- 24.Takanami K, Yamada T, Tsuda M, Takase K, Ishida K, Nakamura Y, Kanno A, Shimosegawa T, Unno M, Takahashi S. Intraductal papillary mucininous neoplasm of the bile ducts: multimodality assessment with pathologic correlation. Abdom Imaging. 2011;36:447–456. doi: 10.1007/s00261-010-9649-x. [DOI] [PubMed] [Google Scholar]
- 25.Tsuyuguchi T, Sakai Y, Sugiyama H, Miyakawa K, Ishihara T, Ohtsuka M, Miyazaki M, Yokosuka O. Endoscopic diagnosis of intraductal papillary mucinous neoplasm of the bile duct. J Hepatobiliary Pancreat Sci. 2010;17:230–235. doi: 10.1007/s00534-009-0153-z. [DOI] [PubMed] [Google Scholar]
- 26.Han JK, Lee JM. Intrahepatic intraductal cholangiocarcinoma. Abdom Imaging. 2004;29:558–564. doi: 10.1007/s00261-004-0189-0. [DOI] [PubMed] [Google Scholar]
- 27.Kim JE, Lee JM, Kim SH, Baek JH, Moon SK, Yu IS, Kim SH, Lee JY, Han JK, Choi BI. Differentiation of intraductal growing-type cholangiocarcinomas from nodular-type cholangiocarcinomas at biliary MR imaging with MR cholangiography. Radiology. 2010;257:364–372. doi: 10.1148/radiol.10092105. [DOI] [PubMed] [Google Scholar]
- 28.Yoon HJ, Kim YK, Jang KT, Lee KT, Lee JK, Choi DW, Lim JH. Intraductal papillary neoplasm of the bile ducts: description of MRI and added value of diffusion-weighted MRI. Abdom Imaging. 2013;38:1082–1090. doi: 10.1007/s00261-013-9989-4. [DOI] [PubMed] [Google Scholar]
- 29.Lim JH, Jang KT. Mucin-producing bile duct tumors: radiological-pathological correlation and diagnostic strategy. J Hepatobiliary Pancreat Sci. 2010;17:223–229. doi: 10.1007/s00534-009-0154-y. [DOI] [PubMed] [Google Scholar]
- 30.Takanami K, Hiraide T, Kaneta T, Hayashi H, Unno M, Fujishima F, Fukuda H, Yamada S, Takahashi S. FDG PET/CT findings in malignant intraductal papillary mucinous neoplasm of the bile ducts. Clin Nucl Med. 2010;35:83–85. doi: 10.1097/RLU.0b013e3181c7bff0. [DOI] [PubMed] [Google Scholar]
- 31.Inoue H, Isaji S, Takei Y. Intraductal papillary neoplasm of bile duct detected on fluorodeoxyglucose-positron emission tomography performed for cancer scanning. Clin Gastroenterol Hepatol. 2009;7:e71. doi: 10.1016/j.cgh.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Kang MJ, Jang JY, Lee KB, Han IW, Kim SW. Impact of macroscopic morphology, multifocality, and mucin secretion on survival outcome of intraductal papillary neoplasm of the bile duct. J Gastrointest Surg. 2013;17:931–938. doi: 10.1007/s11605-013-2151-3. [DOI] [PubMed] [Google Scholar]
- 33.Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, Quaglia A, Nakanuma Y, Heaton N, Portmann B. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24:1079–1089. doi: 10.1038/modpathol.2011.71. [DOI] [PubMed] [Google Scholar]
- 34.Li T, Ji Y, Zhi XT, Wang L, Yang XR, Shi GM, Zhang W, Tang ZY. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. Clin Gastroenterol Hepatol. 2009;7:586–593. doi: 10.1016/j.cgh.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Miao R, Liu H, Du X, Liu L, Lu X, Zhao H. Intrahepatic biliary cystadenoma and cystadenocarcinoma: an experience of 30 cases. Dig Liver Dis. 2012;44:426–431. doi: 10.1016/j.dld.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, Yamamoto M, Isayama H, Tabata M, Kinoshita H, et al. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci. 2013:Epub ahead of print. doi: 10.1002/jhbp.23. [DOI] [PubMed] [Google Scholar]
- 37.Vibert E, Dokmak S, Belghiti J. Surgical strategy of biliary papillomatosis in Western countries. J Hepatobiliary Pancreat Sci. 2010;17:241–245. doi: 10.1007/s00534-009-0151-1. [DOI] [PubMed] [Google Scholar]
- 38.Hwang S, Lee SG, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH. Extended extrahepatic bile duct resection to avoid performing pancreatoduodenectomy in patients with mid bile duct cancer. Dig Surg. 2008;25:74–79. doi: 10.1159/000118025. [DOI] [PubMed] [Google Scholar]
- 39.Kim JK, Hwang HK, Park JS, Cho SI, Yoon DS, Chi HS. Left hemihepatectomy and caudate lobectomy and complete extrahepatic bile duct resection using transduodenal approach for hilar cholangiocarcinoma arsing from biliary papillomatosis. J Surg Oncol. 2008;98:139–142. doi: 10.1002/jso.21089. [DOI] [PubMed] [Google Scholar]
- 40.Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Roggin K, Cymes K, Fong Y, DeMatteo RP, D’Angelica M, Koea J, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–712; discussion 712-714. doi: 10.1097/01.sla.0000160817.94472.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imvrios G, Papanikolaou V, Lalountas M, Patsiaoura K, Giakoustidis D, Fouzas I, Anagnostara E, Antoniadis N, Takoudas D. Papillomatosis of intra- and extrahepatic biliary tree: Successful treatment with liver transplantation. Liver Transpl. 2007;13:1045–1048. doi: 10.1002/lt.21207. [DOI] [PubMed] [Google Scholar]
- 42.Charre L, Boillot O, Goffette P, Geubel A, Gigot JF, Sempoux C, Lerut J. Long-term survival after isolated liver transplantation for intrahepatic biliary papillomatosis. Transpl Int. 2006;19:249–252. doi: 10.1111/j.1432-2277.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 43.Beavers KL, Fried MW, Johnson MW, Zacks SL, Gerber DA, Weeks SM, Fair JH, Odell P, Shrestha R. Orthotopic liver transplantation for biliary papillomatosis. Liver Transpl. 2001;7:264–266. doi: 10.1053/jlts.2001.22322. [DOI] [PubMed] [Google Scholar]
- 44.Dumortier J, Scoazec JY, Valette PJ, Ponchon T, Boillot O. Successful liver transplantation for diffuse biliary papillomatosis. J Hepatol. 2001;35:542–543. doi: 10.1016/s0168-8278(01)00126-x. [DOI] [PubMed] [Google Scholar]
- 45.Brauer BC, Fukami N, Chen YK. Direct cholangioscopy with narrow-band imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct (with videos) Gastrointest Endosc. 2008;67:574–576. doi: 10.1016/j.gie.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 46.Albores-Saavedra J, Murakata L, Krueger JE, Henson DE. Noninvasive and minimally invasive papillary carcinomas of the extrahepatic bile ducts. Cancer. 2000;89:508–515. doi: 10.1002/1097-0142(20000801)89:3<508::aid-cncr5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 47.Hoang MP, Murakata LA, Katabi N, Henson DE, Albores-Saavedra J. Invasive papillary carcinomas of the extrahepatic bile ducts: a clinicopathologic and immunohistochemical study of 13 cases. Mod Pathol. 2002;15:1251–1258. doi: 10.1097/01.MP.0000036450.61830.8E. [DOI] [PubMed] [Google Scholar]
- 48.Tajima Y, Kuroki T, Fukuda K, Tsuneoka N, Furui J, Kanematsu T. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg. 2004;91:99–104. doi: 10.1002/bjs.4366. [DOI] [PubMed] [Google Scholar]
- 49.Fu Y, Yang W, Wu W, Yan K, Xing BC, Chen MH. Radiofrequency ablation for postoperative recurrences of intrahepatic cholangiocarcinoma. Chin J Cancer Res. 2011;23:295–300. doi: 10.1007/s11670-011-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SS, Kim MH, Lee SK, Kim TK, Seo DW, Park JS, Hwang CY, Chang HS, Min YI. MR cholangiography versus cholangioscopy for evaluation of longitudinal extension of hilar cholangiocarcinoma. Gastrointest Endosc. 2002;56:25–32. doi: 10.1067/mge.2002.125363. [DOI] [PubMed] [Google Scholar]
- 51.Abraham SC, Lee JH, Boitnott JK, Argani P, Furth EE, Wu TT. Microsatellite instability in intraductal papillary neoplasms of the biliary tract. Mod Pathol. 2002;15:1309–1317. doi: 10.1097/01.MP.0000038461.80167.34. [DOI] [PubMed] [Google Scholar]
- 52.Minagawa N, Sato N, Mori Y, Tamura T, Higure A, Yamaguchi K. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol. 2013;39:554–558. doi: 10.1016/j.ejso.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Yao C, Zhang M, Zou J, Li H, Wang D, Zhu J, Guo Z. Functional modules with disease discrimination abilities for various cancers. Sci China Life Sci. 2011;54:189–193. doi: 10.1007/s11427-010-4129-7. [DOI] [PubMed] [Google Scholar]
- 54.Li G, Miao R, Zhao H. Progression and prospects of translational medicine in China. Sci China Life Sci. 2012;55:1022–1025. doi: 10.1007/s11427-012-4397-5. [DOI] [PubMed] [Google Scholar]
- 55.Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, Jeliazkova P, Sipos B, Siveke JT, Terris B, et al. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod Pathol. 2013:Epub ahead of print. doi: 10.1038/modpathol.2013.112. [DOI] [PubMed] [Google Scholar]


