Abstract
AIM: To assess the association between smoking and alcohol consumption and extrahepatic cholangiocarcinoma (ECC) through a meta-analysis of clinical observational studies.
METHODS: A literature search was conducted using Embase and MEDLINE databases from inception to 31 May 2013 without language limitations, and by manually searching the references of retrieved articles. Case-control and cohort studies that investigated the association between smoking or alcohol consumption and ECC were included. The quality of these studies was assessed using the Newcastle-Ottawa quality assessment scale. Summary relative risks and corresponding 95%CI were calculated using a random-effects model. Publication bias was assessed by Begg’s funnel plot and Egger’s test.
RESULTS: A total of 12 eligible articles (11 case-control studies and one cohort study) were included in this meta-analysis. Eleven studies reported the association between smoking and ECC. Pooled analysis indicated that smokers had an increased risk of ECC development as compared with non-smokers (summary RR = 1.23; 95%CI: 1.01-1.50). This correlation was present in population-based studies (n = 5; summary RR = 1.47; 95%CI: 1.06-2.05) but not in hospital-based studies (n = 6; summary RR = 1.10; 95%CI: 0.88-1.37) and in non-Asian regions (n = 7; summary RR = 1.39; 95%CI: 1.03-1.87) but not in Asia (n = 4; summary RR = 1.08; 95%CI: 0.85-1.38). Seven studies reported an association between consuming alcohol and ECC. Pooled analysis indicated that alcohol drinkers had a similar risk of ECC development as did individuals who did not drink alcohol (summary RR = 1.09; 95%CI: 0.87-1.37). There was moderate heterogeneity among the studies and no evidence of publication bias.
CONCLUSION: Smoking is associated with an increased risk of ECC, but alcohol consumption is not. Further population-based studies, particularly cohort studies, are warranted to enable definitive conclusions.
Keywords: Extrahepatic cholangiocarcinoma, Smoking, Alcohol consumption, Meta-analysis, Relative risk
Core tip: Little is known about the etiology of extrahepatic cholangiocarcinoma (ECC) because of its rarity and high fatality. Smoking and alcohol consumption are potential risk factors for ECC development. However, reported relations between these two risk factors and ECC are conflicting. Our meta-analysis identified a positive association between smoking and the risk of ECC. The association between alcohol consumption and the risk of ECC was positive but not significant. Further investigations are required.
INTRODUCTION
An extrahepatic cholangiocarcinoma (ECC) is a malignant tumor that arises from cholangiocytes and involves the biliary tree within the hepatoduodenal ligament[1]. It is a relatively rare but often lethal neoplasm that accounts for about 80% of cholangiocarcinomas in the Western world[2]. The prognosis of ECC is poor, and the 5-year survival rate for patients with ECC after resection is as low as 20%-40%[3]. Hilar cholangiocarcinoma is typically classified as extrahepatic[4]. Although the incidence of ECC seems to be constant (annual percent changes = 1%)[1,5,6], it varies across regions, with the highest incidence in Southeast Asia and the lowest in Australia[7,8]. Such geographic variation may be associated with different genetic and environmental factors, including dietary patterns and lifestyle effects.
Although little is known about the etiology of ECC, several risk factors have been proposed to be involved in the development of this disease[9-11]. Epidemiological studies have found that a history of cholecystectomy, cholecystitis, parasitic infection, or primary sclerosing cholangitis is a risk factor for ECC[11-13]. There are other potential factors, such as hepatitis virus infection, obesity, diabetes, and host genetic polymorphisms, but these are less well established[4]. The low incidence of ECC precludes carrying out well-designed, single-center, case-control or prospective cohort studies with sufficient size and statistical power to determine the potential risk factors.
Smoking is associated with the risk of nonpulmonary cancer at many sites, including the liver and pancreas[14,15], and consuming alcohol is related to cancer of the upper digestive tract[16]. The metabolites of smoking and alcohol have carcinogenic properties[17]. However, the reported correlations between these two risk factors and ECC are inconsistent[10,11,18-27]. The lack of consistency across studies may be due to the small number of cases, differences in the study populations, differences in methodological designs or exposure definitions, or a shortage of data concerning confounding factors.
To provide a quantitative assessment of the correlations between these two factors and the risk of ECC, we performed a meta-analysis of published studies following the meta-analysis of observational studies in epidemiology guidelines[28] .
MATERIALS AND METHODS
Data sources and searches
Two investigators (Ye XH and Huai JP) independently performed a computerized search of MEDLINE (from 1 January 1966 to 31 May 2013) and Embase (from 1 January 1974 to 31 May 2013) databases to identify potentially relevant articles. Searches were performed using the following text words and/or Medical Subject Headings: “tobacco”, “smoking”, “alcohol”, “beverages”, “ethanol”, “cholangiocarcinoma”, “extrahepatic”, “bile duct cancer”, and “epidemiologic studies”; the search results were restricted to studies performed after 1990 to avoid any possible inconsistencies in the diagnostic criteria used. The bibliographies of all relevant articles were reviewed manually to identify additional relevant articles. No language restrictions were imposed.
Study selection
Studies were included if they fulfilled the following criteria: (1) case-control or cohort design and published in manuscript form; (2) smoking or alcohol consumption included as an exposure of interest; (3) ECC included as an outcome of interest; and (4) RR in cohort studies or OR in case-control studies and their 95%CI (or sufficient data to calculate them) reported. If data on the same population were reported in multiple papers, the most informative report was selected. Studies were excluded if the data were not specified for ECC, or if they reported data for another type of cancer. Articles or reports that were not peer reviewed were not included.
Data extraction
Two investigators (Ye XH and Huai JP) independently extracted the following data from all included studies: first author’s last name, publication year, geographic location of the study population, study design, methods used to determine risk factors and ECC, sample size (cases and controls or cohort size), variables adjusted for in the analysis, and RR estimates with corresponding 95%CI. From each study, the risk estimates that indicated the greatest degree of control for potential confounders were extracted, and discrepancies were resolved by consensus.
Assessment of study quality
The quality of the included studies was assessed using the Newcastle-Ottawa scale[29]. The scale consists of nine items that cover three dimensions: (1) patient selection (four items); (2) comparability of the two study arms (two items); and (3) assessment of outcome (three items). A point is awarded for each item that is satisfied by the study. The total score therefore ranges from zero to nine, with higher scores indicating higher quality. Studies that scored seven or more points were considered to be of high quality. The Newcastle-Ottawa scale score was assessed independently by both of the reviewers. Discrepancies in the score were resolved through discussion between the reviewers.
Statistical analysis
Different measures of RR were included in this meta-analysis: case-control studies (odds ratio) and cohort studies (rate ratio, hazard ratio). In practice, these measures of effect yielded similar estimates of RR because of the low absolute risk of ECC.
Summary RR estimates with their corresponding 95%CI were calculated with a random-effects model using the methods of DerSimonian and Laird, which consider both within- and between-study variations[30]. Most of the included studies reported the RR of ECC for smokers vs nonsmokers and alcohol drinkers vs non-alcohol drinkers. If studies reported separate RRs for males and females or for different levels of alcohol consumption, we calculated the pooled RR and its corresponding 95%CI. We conducted further analyses stratified by study design, geographic region, and adjustment for cholelithiasis.
Heterogeneity was evaluated using the Q-statistic and quantified by I2[31]. For the Q test, a P value of > 0.10 was considered to indicate no statistically significant heterogeneity. I2 is the proportion of total variation contributed by between-study variation. In addition, a sensitivity analysis was carried out to estimate the effects of each included study on the overall pooled RR. Publication bias was assessed using Begg’s funnel plot and Egger’s test[32,33]. All statistical analyses were carried out using STATA software (vs 12.0; STATA, College Station, Texas, United States). A two-tailed P value of < 0.05 was considered to be significant.
RESULTS
Search results and study characteristics
Twelve articles (11 case-control studies and 1 cohort study) were included in this meta-analysis (Figure 1). Eleven studies reported the association between smoking and ECC, and seven studies reported the association between alcohol consumption and ECC. Briefly, our initial search identified 361 articles, and 318 were excluded by examining the titles and abstracts. Reasons for the exclusion included duplicate citations, reviews, experimental studies, meta-analyses and other irrelevant articles. Forty-three full-text articles were considered for detailed evaluation. One additional relevant study was identified by manually reviewing the references of all 43 articles. Thirty-one of these 43 articles were subsequently excluded from the meta-analysis: 4 did not specify the cancer type, 25 were duplicate reports based on the same population, and 2 did not evaluate the association between each risk factor and ECC. The remaining 12 studies were published between 1993 and 2013 and included a total of 1834 incident cases (Table 1). The studies were carried out in Asia (n = 4), North America (n = 6), and Europe (n = 2; Table 1). Ten of the 12 studies were of high quality (Newcastle-Ottawa scale score ≥ 7; Table 2).
Figure 1.
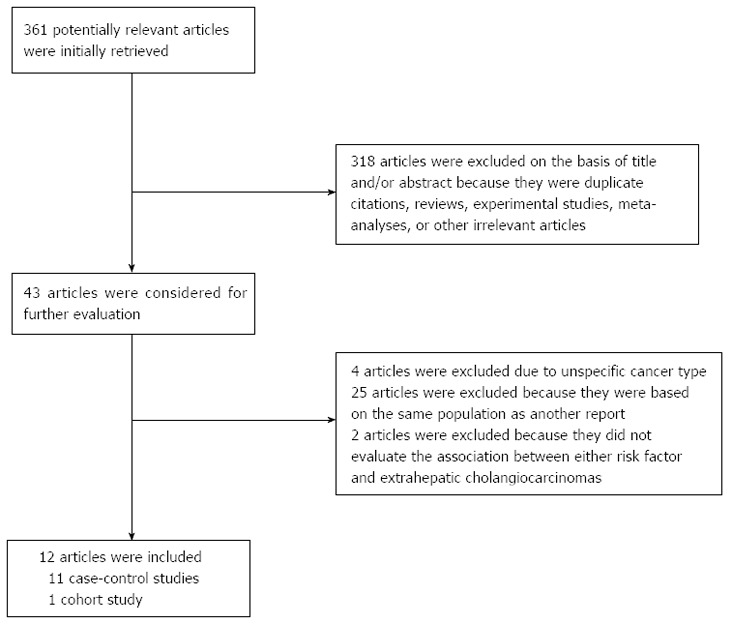
Flow chart of the study selection.
Table 1.
Characteristics of the 12 studies that reported an association between smoking or alcohol consumption and the risk of extrahepatic cholangiocarcinoma
| Author and year | Country | Design | Source | Number of cases | Number of controls | Risk factor assessment | ECC ascertainment | Smoking RR (95% CI) | Alcohol RR (95% CI) | Adjustments |
| Ghadirian et al[18] 1993 | Canada | Case-control | Population | 24 | 239 | Questionnaire | NA | 2.820 (1.010-7.860) | - | Age, sex, other smoking habits, alcohol consumption, schooling, respondent status |
| Chow et al[19] 1994 | United States | Case-control | Population | 64 | 255 | Questionnaire | Pathological | 1.630 (0.900-2.970)1 | 0.600 (0.290-1.220)1 | Age, ethnic origin, smoking status (adjusted for alcohol consumption) |
| Khan et al[20] 1999 | United States | Case-control | Hospital | 31 | 138 | Medical records | Pathological | 0.630 (0.210-1.880) | - | Age, female gender, ethnicity, cholelithiasis, socioeconomic status |
| Zhang et al[21] 2004 | China | Case-control | Population | 99 | 373 | Questionnaire | Cancer registry | 1.490 (0.870-2.560)2 | 1.290 (0.780-2.170)2 | Age, total energy, cholelithiasis, hypertension, history of salty food intake, smoking status (adjusted for alcohol consumption) |
| Welzel et al[11] 2007 | United States | Case-control | Population | 549 | 102782 | Medical records | Cancer registry | 1.700 (1.000-3.000) | - | Age, sex, race, geographic location, state buy-in status |
| Shaib et al[10] 2007 | United States | Case-control | Hospital | 163 | 236 | Medical records | Pathological + imaging | 1.300 (0.800-1.900)3 | 1.290 (0.190-8.910)1 | Race, age, gender, HBV, HCV markers (adjusted for alcohol consumption) |
| El-Serag et al[22] 2009 | United States | Cohort | - | - | - | Registry | Cancer registry | - | 1.060 (0.600-1.870) | Age, sex, baseline visit date, type of visit, a preceding visit |
| Tao et al[23] 2010 | China | Case-control | Hospital | 129 | 380 | Medical records | Pathological + imaging | 0.900 (0.500-1.300)3 | 1.200 (0.800-1.900)3 | - |
| Cai et al[24] 20114 | China | Case-control | Hospital | 313 | 608 | Medical records | Pathological | 0.900 (0.640-1.248)3 | - | - |
| Onal et al[25] 2012 | Turkey | Case-control | Hospital | 89 | 48 | Questionnaire | Pathological + imaging | 1.900 (0.900-4.200)3 | 4.010 (0.480-33.62)3 | - |
| Brandi et al[26] 2013 | Italy | Case-control | Population | 59 | 212 | Questionnaire | Pathological | 0.780 (0.400-1.500) | - | Age, sex, region of residence |
| Zhou et al[27] 2013 | China | Case-control | Hospital | 239 | 478 | Medical records | Pathological + imaging | 1.301 (0.863-1.962) | 1.053 (0.670-1.655) | Age, sex, cirrhosis, cholelithiasis, cholecystectomy, DM, family history of other cancer |
The summary RRs and 95%CI were derived by pooling the relative risks for each sex;
Only males were included in the summary relative risks because of the rarity of smoking in females;
Univariate OR was calculated because adjusted ORs were not available;
Only hilar cholangiocarcinomas were included; ECC: Extrahepatic cholangiocarcinoma; HBV: Hepatitis B virus; HCV: Hepatitis C virus; DM: Diabetes mellitus; NA: Not available.
Table 2.
Quality of the studies used in this analysis
| Author and year |
Quality indicators of Newcastle-Ottawa quality assessment scale |
Score | ||||||||
|
Selection |
Comparability |
Exposure/outcome |
||||||||
| Ia | Ib | Ic | Id | IIa | IIb | IIIa | IIIb | IIIc | ||
| Ghadirian et al[18] 1993 | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 6 |
| Chow et al[19] 1994 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 7 |
| Khan et al[20] 1999 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Zhang et al[21] 2004 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 7 |
| Welzel et al[11] 2007 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Shaib et al[10] 2007 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| El-Serag et al[22] 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 8 |
| Tao et al[23] 2010 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Cai et al[24] 2011 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Onal et al[25] 2012 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | 7 |
| Brandi et al[26] 2013 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | 6 |
| Zhou et al[27] 2013 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | 7 |
For case-control studies; Ia: Indicates cases with independent validation; Ib: Indicates consecutive or representative cases; Ic: Indicates community controls; Id: Indicates controls with no history of ECC; IIa: Indicates that study controls were comparable for age and sex; IIb: Indicates that study controls were comparable on all additional factor(s) reported; IIIa: Indicates that the same method of ascertainment was used for cases and controls; IIIb: Indicates that assessment of exposure was from a secure record; IIIc: Indicates that the non-response rate was similar in both groups. For cohort studies; Ia: indicates that the exposed cohort was representative of the population; Ib: Indicates that the non-exposed cohort was drawn from the same population; Ic: Indicates that the exposure ascertainment was from secure records or a structured interview; Id: Indicates that ECC was not present at start of study; IIa: Indicates that the cohorts were comparable for age and sex; IIb: Indicates that the cohorts were comparable on all additional factor(s) reported; IIIa: Indicates that ECC was assessed from a secure record; IIIb: Indicates that follow-up was long enough for ECC to occur; IIIc: Indicates that follow-up was complete. ECC: Extrahepatic cholangiocarcinoma.
Control subjects in the 11 case-control studies were recruited from a population-based[11,18,19,21,26] or hospital-based setting[10,20,23-25,27] (Table 1). Most studies used a questionnaire or hospital records to evaluate smoking or alcohol consumption status (Table 1). ECC was diagnosed on the basis of histological and imaging methods in 10 studies and according to diagnostic codes in 1 study[11]; the method of diagnosis was not reported in 1 study[18] (Table 1). Adjustments were made for potential confounders of one or more factors in nine of 12 studies (Table 1).
One study[18] reported an increased risk of ECC in smokers as compared with non-smokers, whereas 10 studies reported a similar risk of ECC in smokers and non-smokers (Table 1). All seven studies reported a similar risk of ECC in alcohol drinkers and non-alcohol drinkers (Table 1). One study[10] stratified alcohol consumption as moderate/heavy and found an increased risk of ECC in heavy drinkers as compared with non-drinkers (RR = 3.6; 95%CI: 1.5-9.4).
Only one study had a prospective cohort design and evaluated the association between alcohol consumption and the risk of ECC[22]. A total of 75 incidence cases of ECC were reported. An increased risk of ECC was observed in alcohol drinkers as compared with non-alcohol drinkers, but this was not significant.
Smoking and risk of ECC
Eleven case-control studies were identified that reported the association between smoking and the risk of ECC[10,11,18-21,23-27]. The summary RR for ECC was 1.23 (95%CI: 1.01-1.50) in a random-effects model for smokers vs non-smokers (Figure 2A). There was moderate heterogeneity among studies (Q = 14.69, P = 0.144 for heterogeneity, I2 = 31.9%).
Figure 2.
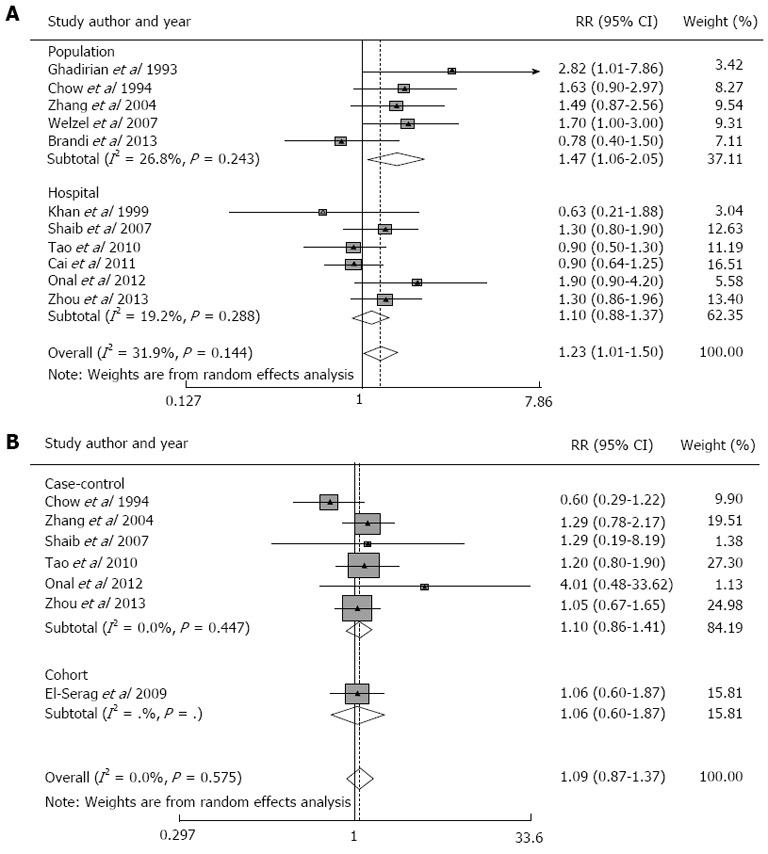
Relative risk of extrahepatic cholangiocarcinoma. A: Smokers as compared with non-smokers in population- and hospital-based case-control studies; B: Alcohol drinkers as compared with non-alcohol drinkers in case-control and cohort studies.
Subgroup meta-analyses were conducted according to geographical region, study design and confounders. The summary RR for ECC was significant for studies conducted outside of Asia[10,11,18-20,25,26] (n = 7; summary RR = 1.39; 95%CI: 1.03-1.87; P = 0.218 for heterogeneity, I2 = 27.5%) but not for studies conducted in Asia (n = 4; summary RR = 1.08; 95% CI, 0.85-1.38; P = 0.278 for heterogeneity, I2 = 22.1%; Table 3). The summary RR for ECC was significant for population-based case-control studies[11,18,19,21,26] (n = 5; summary RR = 1.47; 95%CI: 1.06-2.05; P = 0.243 for heterogeneity, I2 = 26.8%) but not for hospital-based case-control studies (n = 6; summary RR = 1.10; 95%CI: 0.88-1.37; P = 0.288 for heterogeneity, I2 = 19.2%; Table 3). The summary RR was not significant for studies that controlled for cholelithiasis[2,21,27] (summary RR = 1.28; 95%CI: 0.94-1.76; P = 0.383 for heterogeneity, I2 = 0%; Table 3).
Table 3.
Subgroup analyses of the association between smoking and extrahepatic cholangiocarcinoma and alcohol consumption and extrahepatic cholangiocarcinoma
| No. of studies | RR (95%CI) |
Tests for heterogeneity |
|||
| Q | P | I2 | |||
| Smoking | |||||
| Geographical region | |||||
| Non-Asia | 7 | 1.39 (1.03-1.87) | 8.280 | 0.218 | 27.5% |
| Asia | 4 | 1.08 (0.85-1.38) | 3.850 | 0.278 | 22.1% |
| Study design | |||||
| Population-based | 5 | 1.47 (1.06-2.05) | 5.470 | 0.243 | 26.8% |
| Hospital-based | 6 | 1.10 (0.88-1.37) | 6.190 | 0.288 | 19.2% |
| Adjustment for cholelithiasis | 3 | 1.28 (0.94-1.76) | 1.917 | 0.383 | 0.0% |
| Alcohol drinking | |||||
| Geographical region | |||||
| Non-Asia | 4 | 0.94 (0.56-1.56) | 3.560 | 0.313 | 15.7% |
| Asia | 3 | 1.17 (0.90-1.53) | 0.360 | 0.835 | 0.0% |
| Study design | |||||
| Population-based | 2 | 0.92 (0.44-1.94) | 2.890 | 0.089 | 65.4% |
| Hospital-based | 4 | 1.16 (0.86-1.58) | 1.520 | 0.678 | 0.0% |
| Case-control | 6 | 1.10 (0.86-1.41) | 4.750 | 0.447 | 0.0% |
| Cohort | 1 | 1.06 (0.60-1.87) | - | - | - |
Alcohol consumption and risk of ECC
Six case-control studies and one prospective cohort study were identified that reported an association between alcohol consumption and the risk of ECC. The summary RR for ECC was 1.09 (95%CI: 0.87-1.37) in a random-effects model for alcoholic drinkers vs non-alcoholic drinkers (Figure 2B). There was no heterogeneity among studies (Q = 4.76, P = 0.575 for heterogeneity, I2 = 0%).
Subgroup meta-analyses were conducted according to geographical region and study design. The summary RR was not significant for studies conducted outside of Asia (n = 4; summary RR = 0.94; 95%CI: 0.56-1.56; P = 0.313 for heterogeneity, I2 = 15.7%) or in Asia (n = 3; summary RR = 1.17; 95%CI 0.90-1.53; P = 0.835 for heterogeneity, I2 = 0%; Table 3). The summary RR was not significant for case-control studies (n = 6; summary RR = 1.10; 95%CI: 0.86-1.41; P = 0.447 for heterogeneity, I2 = 0%) or for the cohort study (RR = 1.06; 95%CI 0.60-1.87; Table 3). The summary RRs for the population-based[19,21] (n = 2; summary RR = 0.92; 95%CI 0.44-1.94; P = 0.089 for heterogeneity, I2 = 65.4%) and hospital-based[10,23,25,27] (n = 4; summary RR = 1.16; 95%CI: 0.86-1.58; P = 0.678 for heterogeneity, I2 = 0%) case-control studies were not significant (Table 3).
Publication bias and sensitivity analysis
A funnel plot showed no evidence of publication bias (Figure 3). P values for Begg’s adjusted rank correlation test and Egger’s regression asymmetry test were 0.161 and 0.296, respectively, which indicate that publication bias probably had little effect on summary estimates.
Figure 3.
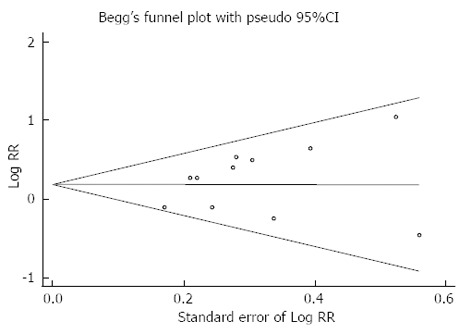
A Begg’s funnel plot with pseudo 95% confidence limits showing the symmetrical distribution of included studies. This indicates that there was no publication bias.
Sensitivity analysis was performed to assess the influence of individual studies on the overall risk of ECC by excluding each individual study and recalculating the pooled RR. Similar RR and 95%CI were generated with the exclusion of each study, indicating the high degree of stability of the results (Figure 4).
Figure 4.
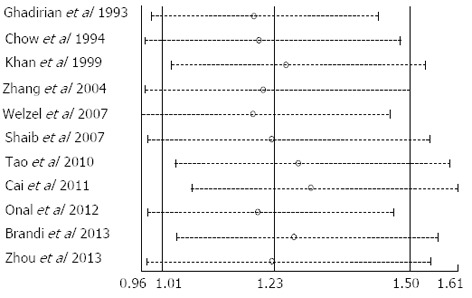
Influence of each individual study on the relative risks of extrahepatic cholangiocarcinoma in smokers as compared with non-smokers. Data show the RR (open circle) and 95%CI (dashed horizontal line) when the study named on the left was omitted. Random-effects estimates (exponential form) were used. RR: Relative risk.
DISCUSSION
In this meta-analysis, we assessed the association between smoking and the risk of ECC and between alcohol consumption and the risk of ECC. A previous meta-analysis evaluated the association between alcohol consumption and the risk of extrahepatic bile system cancer[34], and a more recent meta-analysis investigated the association between smoking and the risk of gallbladder cancer[35]. Although both of these previous studies investigated the risk of extrahepatic bile system cancer, the risk of ECC was not specified. To the best of our knowledge, this is the first study to provide comprehensive evidence of the association between smoking and alcohol consumption and the risk of ECC. In this meta-analysis we found that smokers had a 23% increased risk of ECC as compared with non-smokers. The association between alcohol consumption and the risk of developing ECC was positive but not significant.
Although the incidence of ECC remained low among smokers and alcohol drinkers, our results carry substantial clinical and public health implications. The incidence of ECC has been on the rise worldwide in recent years, although this type of malignancy is uncommon[4]. The number of habitual smokers is rising in spite of current anti-smoking campaigns[36], and a rapid increase in the consumption of alcohol has been documented in many regions[37]. It is estimated that there are currently more than 500 million alcohol drinkers in China[16,36] and approximately 37% of Chinese adults are heavy drinkers[37] .
In the subgroup analysis, we found that smoking was associated with an increased risk of ECC in non-Asian regions but not in Asia. This difference may be associated with ethnicity or with differences in the types of tobacco use between the two areas. For example, cigars contain more nicotine than regular cigarettes[38], and the pH of cigar smoke is higher than that of cigarettes, allowing more complete delivery of nicotine into the bloodstream[38,39]. Only 0.3% of the Chinese population use cigars[40], compared with 6.7% of the American population[40]. However, most of the studies included in our meta-analysis did not report the type of tobacco use, and thus we could not conduct further analysis. Although the risk of ECC was similar in smokers and non-smokers in Asia, attention should still be paid to the potential association between smoking and ECC in this population. The number of smokers in China increased from 320 to 350 million from 2005 to 2007[16,36] and 72% of Chinese citizens aged 15 years or older have been exposed to tobacco[41,42].
The potential mechanism by which smoking and alcohol consumption are associated with ECC remains unknown. Direct carcinogenic properties of smoking might be mediated by various metabolites generated in cigarettes including formaldehyde, benzene, and chromium. As early as the 1970s, it was suggested that tobacco compounds exert carcinogenic effects on the epithelial cells of the bile ducts as a result of exposure via blood flow[43] (Figure 5), and this may underlie the relation between smoking and ECC. For alcohol consumption, it is more likely that there is an indirect and bidirectional effect of carcinogenesis on the development of ECC. Moderate alcohol intake protects against gallstone formation, and gallstones are a risk factor for biliary tract cancer[17]. Metabolites of alcohol are produced in the liver and excreted into the bile duct and may interact with cholesterol metabolism. Alcohol also enhances the activation of different precarcinogenic elements[17]. Therefore, alcohol may be associated with ECC via co-effects of different mechanisms.
Figure 5.
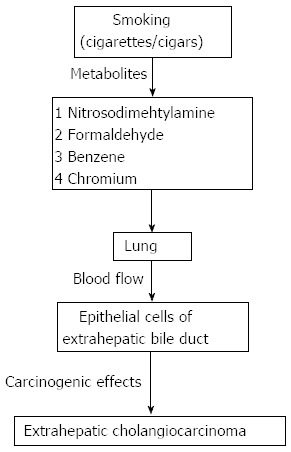
Proposed mechanisms by which smoking may be associated with the formation of extrahepatic cholangiocarcinoma.
As with all meta-analyses of observational studies, our results have several potential limitations. First, definitions of both smoking and alcohol consumption were not consistent across the included studies. In addition, a dose-response relationship between alcohol consumption and ECC was observed in one study[10], in which the risk of ECC development was higher in heavy drinkers who consumed at least 80 g of ethanol per day. However, we could not further evaluate this dose-response relationship because of a paucity of data. The majority of studies included in this meta-analysis were case-control studies, which are more susceptible to selection and recall bias than are cohort studies. Associations between smoking or alcohol consumption and the risk of ECC in case-control studies may be confounded by changes in lifestyle after the diagnosis of ECC. In addition, 6 of the 11 case-control studies were hospital based, and these cases may not represent the general population of patients with ECC. This may have introduced selection bias into our results. Furthermore, moderate heterogeneity was observed across studies, and this may also bias the results. This heterogeneity results from diversity of the study designs, analysis of populations from different geographic locations, and the selection of participants for the different studies. These biases may distort the true associations, and data provided by this meta-analysis should thus be interpreted with caution.
Confounding effects may also have influenced the results of this meta-analysis. As noted above, moderate alcohol consumption is inversely related to gallstone disease[17,44]. When we limited the meta-analysis to studies that were adjusted for cholelithiasis, the association between smoking and ECC was no longer significant, suggesting that a confounding effect may exist. The possibility of residual confounding such as gallstone formation cannot be excluded because of a paucity of data.
Although it is possible that small studies with null results were less likely to be published than large studies with significant results, we found no evidence from funnel plot analysis and formal statistical tests for such bias.
In conclusion, the results from this meta-analysis suggest that smoking, but not alcohol consumption, is associated with a higher risk of ECC. However, the possibility that the association may be influenced by bias or confounding variables cannot be fully excluded. Further well-designed prospective studies are warranted to clarify the association between smoking and alcohol consumption and the risks of ECC.
COMMENTS
Background
Data based on epidemiological studies related to the associations between smoking and alcohol consumption and extrahepatic cholangiocarcinoma (ECC) remain conflicting. The aim of this meta-analysis was to assess the association of each risk factor with ECC.
Research frontiers
Until now, several studies have assessed the association between smoking and alcohol consumption and the risk of ECC in various regions and ethnicities; however, the results have been mixed and inconsistent. No quantitative summary of the evidence has ever been provided.
Innovations and breakthroughs
This meta-analysis identified that smoking was associated with an increased risk of ECC, especially in population-based studies and studies conducted in non-Asian regions. A positive but non-significant increased risk of ECC was observed in alcohol drinkers as compared with non-alcohol drinkers.
Applications
These results suggest that smoking is associated with an increased risk of ECC, especially in population-based studies and in studies conducted in non-Asian regions. Lifestyle changes may contribute to reducing the incidence of ECC.
Peer review
This was a well-performed meta-analysis of currently available studies on the association between smoking and alcohol consumption and ECC. The authors concluded that smoking rather than alcohol consumption may be associated with increased risk of ECC, with an emphasis on population-based studies and in non-Asian regions. This study was well designed and performed, and the results are well discussed.
Footnotes
P- Reviewers: Morales-Gonzalez JA, Rota M, Zhu X S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Welzel TM, McGlynn KA, Hsing AW, O’Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood R, Brewster DH, Fraser LA, Brown H, Hayes PC, Garden OJ. Do increases in mortality from intrahepatic cholangiocarcinoma reflect a genuine increase in risk? Insights from cancer registry data in Scotland. Eur J Cancer. 2003;39:2087–2092. doi: 10.1016/s0959-8049(03)00544-6. [DOI] [PubMed] [Google Scholar]
- 6.Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490–495. doi: 10.1016/j.dld.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 9.Wadsworth C, Lim A, Taylor-Robinson S, Khan S. The risk factors and diagnosis of cholangiocarcinoma. Hepatol Int. 2013;7:377–393. doi: 10.1007/s12072-012-9407-y. [DOI] [PubMed] [Google Scholar]
- 10.Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, Todoroki T, Jedpiyawongse A, Kittiwatanachot P, Sripa B, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 13.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–526. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–1511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 15.La Torre G, de Waure C, Specchia ML, Nicolotti N, Capizzi S, Bilotta A, Clemente G, Ricciardi W. Does quality of observational studies affect the results of a meta-analysis?: the case of cigarette smoking and pancreatic cancer. Pancreas. 2009;38:241–247. doi: 10.1097/MPA.0b013e318190d795. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Yang H, Cao J. Association between alcohol consumption and cancers in the Chinese population--a systematic review and meta-analysis. PLoS One. 2011;6:e18776. doi: 10.1371/journal.pone.0018776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moerman CJ, Bueno de Mesquita HB, Runia S. Smoking, alcohol consumption and the risk of cancer of the biliary tract; a population-based case-control study in The Netherlands. Eur J Cancer Prev. 1994;3:427–436. doi: 10.1097/00008469-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ghadirian P, Simard A, Baillargeon J. A population-based case-control study of cancer of the bile ducts and gallbladder in Quebec, Canada. Rev Epidemiol Sante Publique. 1993;41:107–112. [PubMed] [Google Scholar]
- 19.Chow WH, McLaughlin JK, Menck HR, Mack TM. Risk factors for extrahepatic bile duct cancers: Los Angeles County, California (USA) Cancer Causes Control. 1994;5:267–272. doi: 10.1007/BF01830247. [DOI] [PubMed] [Google Scholar]
- 20.Khan ZR, Neugut AI, Ahsan H, Chabot JA. Risk factors for biliary tract cancers. Am J Gastroenterol. 1999;94:149–152. doi: 10.1111/j.1572-0241.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Gao Y, Asif R, Deng J, Liu E, Wu K, Cheng J, Gloria G, Hsing A. Cigarette Smoking,Alcohol Consumption and Risk of Biliary Tract Cancers: A Population-based Case-control Study in Shanghai, China. Zhongguo Aizheng Fangzhi Zaizhi. 2004;31:597–600. [Google Scholar]
- 22.El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116–123. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, Zhang SM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30:215–221. doi: 10.1111/j.1478-3231.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 24.Cai WK, Sima H, Chen BD, Yang GS. Risk factors for hilar cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2011;17:249–253. doi: 10.3748/wjg.v17.i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onal IK, Parlak E, Kekilli M, Kurt M, Alioglu H, Disibeyaz S, Sayilir A, Beyazit Y, Sasmaz N. Hepatitis B and C Virus Infection and Cholangiocarcinoma: A Case-Control Study in Turkey. Int J Hematol Oncol. 2012;22:187–191. [Google Scholar]
- 26.Brandi G, Di Girolamo S, Farioli A, de Rosa F, Curti S, Pinna AD, Ercolani G, Violante FS, Biasco G, Mattioli S. Asbestos: a hidden player behind the cholangiocarcinoma increase? Findings from a case-control analysis. Cancer Causes Control. 2013;24:911–918. doi: 10.1007/s10552-013-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Zhou Q, Lin Q, Chen R, Gong Y, Liu Y, Yu M, Zeng B, Li K, Chen R, et al. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer. 2013;133:1867–1875. doi: 10.1002/ijc.28196. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute. Available from: http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kan HP, Huang YQ, Tan YF, Zhou J. Meta-analysis of alcohol consumption and risk of extrahepatic bile system cancer. Hepatol Res. 2011;41:746–753. doi: 10.1111/j.1872-034X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 35.Wenbin D, Zhuo C, Zhibing M, Chen Z, Ruifan Y, Jie J, Cheng Q, Zhenming G. The effect of smoking on the risk of gallbladder cancer: a meta-analysis of observational studies. Eur J Gastroenterol Hepatol. 2013;25:373–379. doi: 10.1097/MEG.0b013e32835a870b. [DOI] [PubMed] [Google Scholar]
- 36.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Organization WH. Global Status Report on Alcohol. Document# WHO. In: HSC/SAB/99-11 , editor. Geneva: World Health Organization; 1999. [Google Scholar]
- 38.Brunnemann KD, Hoffmann D. The pH of tobacco smoke. Food Cosmet Toxicol. 1974;12:115–124. doi: 10.1016/0015-6264(74)90327-7. [DOI] [PubMed] [Google Scholar]
- 39.Yang T, Li F, Yang X, Wu Z, Feng X, Wang Y, Wang X, Abdullah AS. Smoking patterns and sociodemographic factors associated with tobacco use among Chinese rural male residents: a descriptive analysis. BMC Public Health. 2008;8:248. doi: 10.1186/1471-2458-8-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyland A, Cummings KM, Shopland DR, Lynn WR. Prevalence of cigar use in 22 North American communities: 1989 and 1993. Am J Public Health. 1998;88:1086–1089. doi: 10.2105/ajph.88.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Cai B. The impact of tobacco on lung health in China. Respirology. 2003;8:17–21. doi: 10.1046/j.1440-1843.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Ou JX, Bai CX. Tobacco smoking in China: prevalence, disease burden, challenges and future strategies. Respirology. 2011;16:1165–1172. doi: 10.1111/j.1440-1843.2011.02062.x. [DOI] [PubMed] [Google Scholar]
- 43.Wynder EL, Mabuchi K, Maruchi N, Fortner JG. Epidemiology of cancer of the pancreas. J Natl Cancer Inst. 1973;50:645–667. doi: 10.1093/jnci/50.3.645. [DOI] [PubMed] [Google Scholar]
- 44.De Santis A, Attili AF, Ginanni Corradini S, Scafato E, Cantagalli A, De Luca C, Pinto G, Lisi D, Capocaccia L. Gallstones and diabetes: a case-control study in a free-living population sample. Hepatology. 1997;25:787–790. doi: 10.1002/hep.510250401. [DOI] [PubMed] [Google Scholar]


