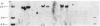Abstract
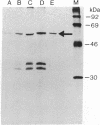
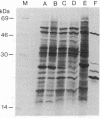
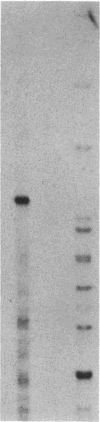
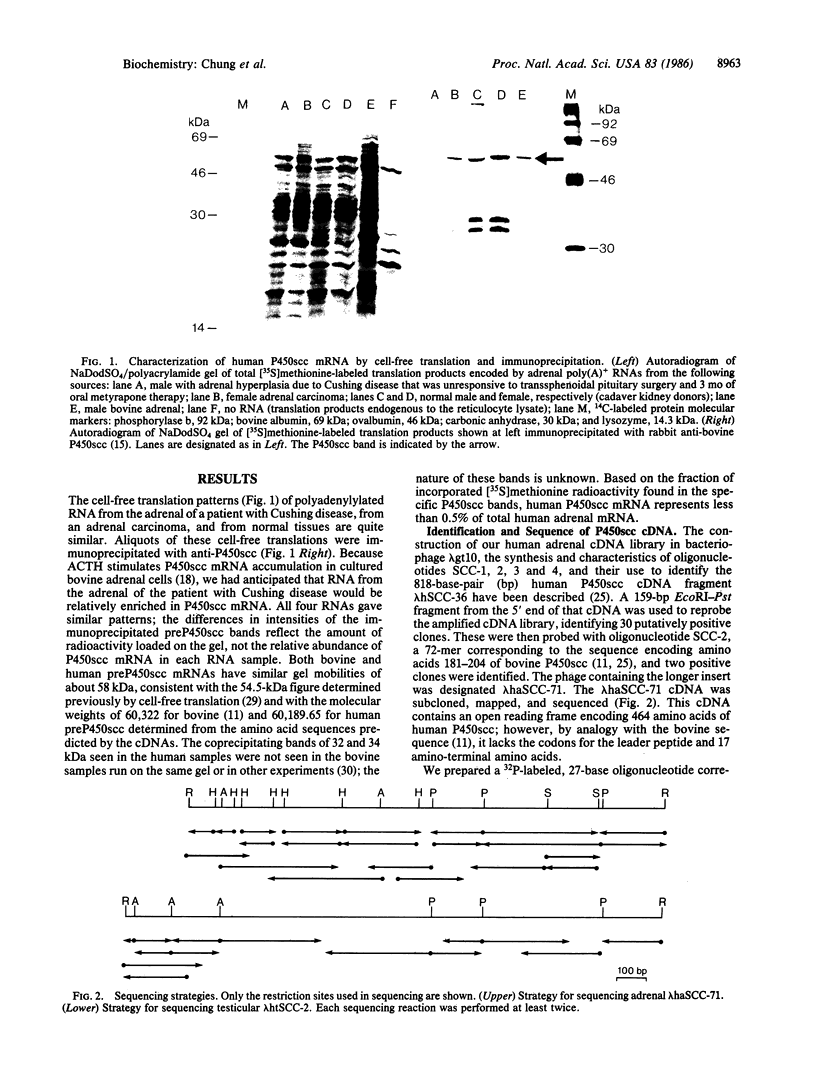
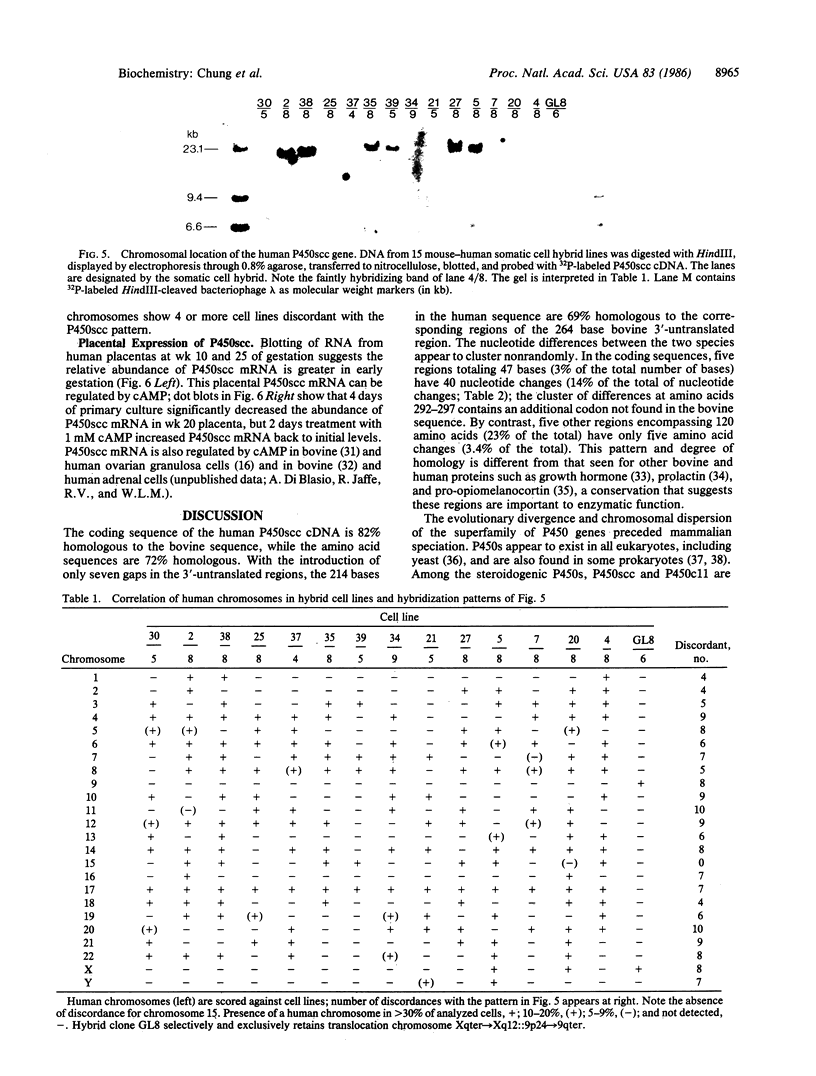
Conversion of cholesterol to pregnenolone is mediated by P450scc [cholesterol, reduced-adrenal-ferrodoxin: oxygen oxidoreductase (side-chain-cleaving), EC 1.14.15.67]. RNA from several human adrenal samples was translated in vitro and immunoprecipitated with anti-bovine P450scc, indicating that P450scc mRNA represents about 0.5% of human adrenal mRNA in normal, hypertrophied, and malignant adrenals. A 1626-base-pair human adrenal P450scc cDNA was cloned in bacteriophage lambda gt10. Primer extension data indicated P450scc mRNA is about 1850 bases long and that all adrenal P450scc mRNA has the same 5' end. A full-length clone containing 1821 bases was obtained from a human testis cDNA library to yield the complete sequence. The encoded human preP450scc contains 521 amino acids with a molecular weight of 60189.65. The testis and adrenal sequences were identical; the human cDNA and amino acid sequences are 82% and 72% homologous, respectively, with the bovine sequences. P450scc cDNA was used to probe DNA from a panel of mouse-human somatic cell hybrids, showing that the single human P450scc gene lies on chromosome 15. The human P450scc gene is expressed in the placenta in early and midgestation; primary cultures of placental tissue indicate P450scc mRNA accumulates in response to cyclic AMP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Porter R. R. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci U S A. 1985 Jan;82(2):521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Cloning and characterization of the bovine gene for steroid 21-hydroxylase (P-450c21). DNA. 1985 Jun;4(3):211–219. doi: 10.1089/dna.1985.4.211. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Structure of a bovine gene for P-450c21 (steroid 21-hydroxylase) defines a novel cytochrome P-450 gene family. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4243–4247. doi: 10.1073/pnas.83.12.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois R. N., Simpson E. R., Kramer R. E., Waterman M. R. Induction of synthesis of cholesterol side chain cleavage cytochrome P-450 by adrenocorticotropin in cultured bovine adrenocortical cells. J Biol Chem. 1981 Jul 10;256(13):7000–7005. [PubMed] [Google Scholar]
- Funkenstein B., Waterman M. R., Simpson E. R. Induction of synthesis of cholesterol side chain cleavage cytochrome P-450 and adrenodoxin by follicle-stimulating hormone, 8-bromo-cyclic AMP, and low density lipoprotein in cultured bovine granulosa cells. J Biol Chem. 1984 Jul 10;259(13):8572–8577. [PubMed] [Google Scholar]
- Haniu M., Armes L. G., Tanaka M., Yasunobu K. T., Shastry B. S., Wagner G. C., Gunsalus I. C. The primary structure of the monoxygenase cytochrome P450CAM. Biochem Biophys Res Commun. 1982 Apr 14;105(3):889–894. doi: 10.1016/0006-291x(82)91053-1. [DOI] [PubMed] [Google Scholar]
- Hauffa B. P., Miller W. L., Grumbach M. M., Conte F. A., Kaplan S. L. Congenital adrenal hyperplasia due to deficient cholesterol side-chain cleavage activity (20, 22-desmolase) in a patient treated for 18 years. Clin Endocrinol (Oxf) 1985 Nov;23(5):481–493. doi: 10.1111/j.1365-2265.1985.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Yoshioka H., Yamane M., Gotoh O., Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C. E., Gonzalez F. J., McBride O. W., Nebert D. W. Assignment of the human 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible cytochrome P1-450 gene to chromosome 15. Nucleic Acids Res. 1985 Mar 25;13(6):2009–2016. doi: 10.1093/nar/13.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoate C. R., Simpson E. R., Boyd G. S. Spectral properties of rat adrenal-mitochondrial cytochrome P-450. Eur J Biochem. 1974 Mar 1;42(2):539–551. doi: 10.1111/j.1432-1033.1974.tb03369.x. [DOI] [PubMed] [Google Scholar]
- John M. E., John M. C., Ashley P., MacDonald R. J., Simpson E. R., Waterman M. R. Identification and characterization of cDNA clones specific for cholesterol side-chain cleavage cytochrome P-450. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5628–5632. doi: 10.1073/pnas.81.18.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri M., Takemori S., Itagaki E., Suhara K., Gomi T. Characterization of purified cytochrome P-450scc and P-450(11)beta from bovine adrenocortical mitochondria. Adv Exp Med Biol. 1976;74:281–289. [PubMed] [Google Scholar]
- Koritz S. B., Kumar A. M. On the mechanism of action of the adrenocorticotrophic hormone. The stimulation of the activity of enzymes involved in pregnenolone synthesis. J Biol Chem. 1970 Jan 10;245(1):152–159. [PubMed] [Google Scholar]
- Kramer R. E., Rainey W. E., Funkenstein B., Dee A., Simpson E. R., Waterman M. R. Induction of synthesis of mitochondrial steroidogenic enzymes of bovine adrenocortical cells by analogs of cyclic AMP. J Biol Chem. 1984 Jan 25;259(2):707–713. [PubMed] [Google Scholar]
- Matteson K. J., Chung B. C., Miller W. L. Molecular cloning of DNA complementary to bovine adrenal P450scc mRNA. Biochem Biophys Res Commun. 1984 Apr 16;120(1):264–270. doi: 10.1016/0006-291x(84)91443-8. [DOI] [PubMed] [Google Scholar]
- Matteson K. J., Chung B. C., Urdea M. S., Miller W. L. Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology. 1986 Apr;118(4):1296–1305. doi: 10.1210/endo-118-4-1296. [DOI] [PubMed] [Google Scholar]
- Matteson K. J., Picado-Leonard J., Chung B. C., Mohandas T. K., Miller W. L. Assignment of the gene for adrenal P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase) to human chromosome 10. J Clin Endocrinol Metab. 1986 Sep;63(3):789–791. doi: 10.1210/jcem-63-3-789. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Coit D., Baxter J. D., Martial J. A. Cloning of bovine prolactin cDNA and evolutionary implications of its sequence. DNA. 1981;1(1):37–50. doi: 10.1089/dna.1.1981.1.37. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Leisti S., Johnson L. K. Synthesis of growth hormone, prolactin, and proopiomelanocortin by intact adult ovine pituitary tissue in vitro. Endocrinology. 1982 Oct;111(4):1358–1367. doi: 10.1210/endo-111-4-1358. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Martial J. A., Baxter J. D. Molecular cloning of DNA complementary to bovine growth hormone mRNA. J Biol Chem. 1980 Aug 25;255(16):7521–7524. [PubMed] [Google Scholar]
- Mohandas T., Heinzmann C., Sparkes R. S., Wasmuth J., Edwards P., Lusis A. J. Assignment of human 3-hydroxy-3-methylglutaryl coenzyme A reductase gene to q13----q23 region of chromosome 5. Somat Cell Mol Genet. 1986 Jan;12(1):89–94. doi: 10.1007/BF01560731. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Fujii-Kuriyama Y., Okada Y., Sogawa K., Hirose T., Inayama S., Omura T. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4647–4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- Ohashi M., Simpson E. R., Mason J. I., Waterman M. R. Biosynthesis of cholesterol side-chain cleavage cytochrome P-450 in human fetal adrenal cells in culture. Endocrinology. 1983 Jun;112(6):2039–2045. doi: 10.1210/endo-112-6-2039. [DOI] [PubMed] [Google Scholar]
- Omura T., Sato R., Cooper D. Y., Rosenthal O., Estabrook R. W. Function of cytochrome P-450 of microsomes. Fed Proc. 1965 Sep-Oct;24(5):1181–1189. [PubMed] [Google Scholar]
- Phillips I. R., Shephard E. A., Povey S., Davis M. B., Kelsey G., Monteiro M., West L. F., Cowell J. A cytochrome P-450 gene family mapped to human chromosome 19. Ann Hum Genet. 1985 Oct;49(Pt 4):267–274. doi: 10.1111/j.1469-1809.1985.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Rabe T., Weidenhammer K., Runnebaum B. Characterization of human cholesterol side chain cleavage enzyme (EC 1.14.15x) of human term placental mitochondria. J Steroid Biochem. 1983 Mar;18(3):333–340. doi: 10.1016/0022-4731(83)90112-7. [DOI] [PubMed] [Google Scholar]
- Simpson E. R. Cholesterol side-chain cleavage, cytochrome P450, and the control of steroidogenesis. Mol Cell Endocrinol. 1979 Mar;13(3):213–227. doi: 10.1016/0303-7207(79)90082-0. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Kahri A. I., Lähteenmäki P. The effect of dehydroepiandrosterone and its sulphate on human midterm placental secretion of HCG, progesterone, estrone and estradiol-17 beta in tissue culture. J Steroid Biochem. 1981 Nov;14(11):1153–1155. doi: 10.1016/0022-4731(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Miller W. L. Developmental expression of genes for the stereoidogenic enzymes P450scc (20,22-desmolase), P450c17 (17 alpha-hydroxylase/17,20-lyase), and P450c21 (21-hydroxylase) in the human fetus. J Clin Endocrinol Metab. 1986 Nov;63(5):1145–1150. doi: 10.1210/jcem-63-5-1145. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Tapanainen J., Chung B. C., Matteson K. J., Miller W. L. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986 Jul;63(1):202–207. doi: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- Warner B. D., Warner M. E., Karns G. A., Ku L., Brown-Shimer S., Urdea M. S. Construction and evaluation of an instrument for the automated synthesis of oligodeoxyribonucleotides. DNA. 1984 Oct;3(5):401–411. doi: 10.1089/dna.1984.3.401. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel C. A., Snyder J. M., MacDonald P. C., Simpson E. R. Regulation of cholesterol and progesterone synthesis in human placental cells in culture by serum lipoproteins. Endocrinology. 1980 Apr;106(4):1054–1060. doi: 10.1210/endo-106-4-1054. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Marsh B., Mohandas T. K., Shapiro L. J. Isolation of genomic clones homologous to transcribed sequences from human X chromosome. Somat Cell Mol Genet. 1984 Nov;10(6):561–571. doi: 10.1007/BF01535221. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Aoyama Y., Kumaoka H., Kubota S. A highly purified preparation of cytochrome P-450 from microsomes of anaerobically grown yeast. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1005–1010. doi: 10.1016/0006-291x(77)90521-6. [DOI] [PubMed] [Google Scholar]
- Zuber M. X., John M. E., Okamura T., Simpson E. R., Waterman M. R. Bovine adrenocortical cytochrome P-450(17 alpha). Regulation of gene expression by ACTH and elucidation of primary sequence. J Biol Chem. 1986 Feb 15;261(5):2475–2482. [PubMed] [Google Scholar]