Abstract
Chronic hepatitis B virus (HBV) infection is the key driving force of liver disease progression, resulting in the development of hepatic dysfunction, cirrhosis and hepatocellular carcinoma (HCC). The primary aim of therapy is to suppress or eliminate HBV replication to reduce the activity of hepatitis, thus reducing the risk of, or slowing the progression of, liver disease. Nucleos(t)ide analogues (Nucs) may result in rapid suppression of HBV replication with normalization of serum transaminases and restore liver function, thus increasing survival in patients with hepatic decompensation. Long-term Nuc therapy may result in histological improvement or reversal of advanced fibrosis and reduction in disease progression, including the development of HCC. The long-term benefits of a finite course of interferon (IFN)-α therapy also include a sustained and cumulative response, as well as hepatitis B surface antigen seroclearance and reduction in the development of cirrhosis and/or HCC. Pegylated IFN and newer Nucs may achieve better long-term outcomes because of improved efficacy and a low risk of drug resistance. However, treatment outcomes are still far from satisfactory. Understanding the effects of anti-HBV treatment against HCC incidence and recurrence after hepatectomy or liver transplantation is required for further improvement of outcome.
Keywords: Hepatocelluar carcinoma, Antiviral therapy, Carcinogenesis, Recurrence, Nucleos(t)ide analogues, Interferon, Retrospective study, Clinical trial
Core tip: Chronic hepatitis B virus (HBV) infection is the key driving force of hepatocellular carcinoma (HCC). In this review, we discussed the mechanism of HBV induction of HCC and described the current trends in anti-HBV therapy. The associations of anti-HBV therapy with prevention of HCC incidence and recurrence after curative operations were also summarized. Moreover, based on our center’s experiences, a standardized antiviral strategy was suggested which greatly benefited those patients who underwent hepatectomy and liver transplantation with regard to better clinical results.
INTRODUCTION
Hepatitis B virus (HBV) is a member of the Hepadnaviridae family, which includes small enveloped DNA viruses. HBV infection affects > 2 billion people worldwide and is a significant cause of liver cirrhosis and hepatocellular carcinoma (HCC), which increases morbidity and mortality in these patients[1]. HBV targets and replicates in hepatocytes, and the risk of developing HCC among HBV carriers is 10 to 100-fold greater compared with that in uninfected people[2]. Treatment with antiviral drugs such as nucleos(t)ide analogues (Nucs) or interferon (IFN)-α may result in rapid suppression of HBV replication and thus reduce the progression of fibrosis and the development of HCC[3]. Although localized or systemic radiation and chemotherapy have been used to eliminate the tumor mass, surgical resection or liver transplantation are still the most effective treatments, but relapse is common[4]. It is widely accepted that comprehensive treatment is required for prevention of HBV-associated HCC development and recurrence. Thus, this review highlights the mechanism of HBV induction of HCC and discusses the current trends in anti-HBV therapy for prevention of HCC and its recurrence.
HBV INFECTION INDUCES CHRONIC INFLAMMATION AND CANCER TRANSFORMATION
HBV persistently replicates in immortalized hepatocytes in vitro without overt cellular damage or death, implying that the viruses are not directly cytopathic, and the pathogenesis of hepatitis is immune mediated[5,6]. Liver injury in response to inflammatory hepatitis elicits an inflammatory response in non-parenchymal cells (NPCs), such as myeloid Kupffer cells and hepatic stellate cells. Toll-like receptor-nuclear factor (NF)-κB signaling activation may trigger an innate immune response and inhibit virus replication in HBV-transgenic mice[7]. NPCs secrete NF-κB-regulated hepato-mitogens [e.g., tumor necrosis factor (TNF)-α, interleukin (IL)-6, and hepatocyte growth factor], which promote compensatory proliferation of quiescent hepatocytes carrying HBV-induced mutations. This process allows for the transmission of genetic alterations to daughter cells, thereby favoring liver neoplastic progression. Alternatively, autocrine secretion of transforming growth factor (TGF)-β by hepatocytes induces cell survival and proliferation in the absence of liver damage and independent of NPC-mediated secretion of hepato-mitogens. Increased proliferation is then followed by dysplasia, adenoma, and HCC formation[8,9]. In conclusion, NF-κB activation-associated carcinogenesis most likely depends on downstream hepato-mitogen release and death-driven compensatory proliferation[9,10].
HBV X (HBx) protein is encoded by the smallest HBV open reading frame and is 154 amino acids in size, with a molecular weight of approximately 17.5 kDa. HBx can localize to the mitochondria where it acts as an adaptor or kinase activator to influence signal transduction pathways such as: protein kinase C, Janus kinase/signal transducer and activator of transcription (JAK/STAT), phosphoinositide 3-kinase, stress-activated protein kinase/Jun N-terminal kinase (SAPK/JNK), Ras-Raf-mitogen-activated protein kinase (Ras-Raf-MAPK), and extracellular signal-regulated kinase (ERK). This may provide a unified mechanism by which HBx exerts many of its pleiotropic activities, including transcription, cell cycle control, and apoptosis[11-14]. It is also reported that HBx can activate NF-κB directly, which could be partially via upregulated inhibitor of NF-κB kinase activity and the mammalian target of rapamycin (mTOR) pathway[15]. As mentioned above, various inflammatory cytokines, including TNF-α, IL-1α, IL-1β, IL-6 and IL-8, which play an important role in the inflammation-carcinogenesis axis of the liver, are NF-κB activation-mediated, and IL-6 is thought to be one of the most important[16]. Recently, our group found that IL-22 could also promote HCC via STAT3 activation, suggesting that inflammatory cytokines have also attracted considerable attention as mediators of the association between inflammation and hepatocarcinogenesis[17].
Furthermore, HBx could interfere with the anti-tumor immune response via other inflammatory cells. Infected intrahepatic natural killer cells are also known to induce cytolytic activity without IFN-γ production, suggesting that hepatocellular killing occurs without virus clearance[18]. Dendritic cells may be infected with HBV, which will cause defective chronic HBV infection, resulting in poor adaptive immunity[19]. CD4+ CD25+ FOX3P+ regulatory T cells could be induced by HBx-stimulated production of TGF-β1, and their crosstalk with Th17 cells may contribute to an immune tolerance-clearance balance in the liver[20].
MECHANISMS OF HBV ONCOGENESIS
Increasing evidence suggests that HBV contributes to HCC by directly modulating pathways that may promote the malignant transformation of hepatocytes. Firstly, HBV insertions are associated with host large genetic alterations: deletions, duplications and chromosomal translocations. These events could either induce chromosome changes or act “in cis” on the expression or function of nearby cellular genes that contribute to chromosome instability[21,22]. For instance, integration of HBx gene fragments (316-462/262-462 bp) could directly transform human immortalized normal liver L02 cells in studies using a cell model. Further, these integrations could be detected in five of 44 clinical HBV-positive HCC tissues[23]. Integration at specific sites in host genes may contribute to a growth advantage in a clonal cell population but subsequent additional mutations will eventually accumulate. Evidence was first provided in two independent HCCs, with retinoic acid receptors and cyclin A being targeted by HBV integration in tumors[24]. Recently, more genes involved in cell survival, proliferation and immortalization were also reported as the HBV integration target, such as human telomerase reverse transcriptase (hTERT, a regulator of telomerase), platelet-derived growth factor receptor, calcium signaling-related genes, and ribosomal protein genes[25].
Although it is suggested that upregulated expression of HBx and HBV S proteins is associated with hepatocarcinogenesis in transgenic mouse models, the exact mechanism remains unclear[26]. It is worth noting that hepatocytes in cirrhotic livers display decreased proliferation rates with a dominant replicative senescence phenotype characterized by critically shortened telomeres and permanent cell cycle arrest[1]. However, during hepatocyte proliferation, low or absent telomerase activity in cirrhotic liver is associated with upregulated HBx or pre-S2 protein[27]. In a study of 55 HCC and 17 chronic hepatitis patients, hTERT was positive in 81% of HCCs, and the mean telomere length in HCC was significantly shorter compared with that in chronic hepatitis[28].
HBx is also suggested to have the ability to induce direct chromosomal instability by interfering with the mitotic checkpoints[29,30]. HBx induces epigenetic changes, including DNA methylation aberration, histone modification and miRNA expression. Jiang et al[31] reported that increased miR-22 is associated with HCC development in male patients. Xu et al[32] also suggested that suppression of miR-148a upon HBx activation can enhance tumorigenesis. Moreover, HBx binds and inactivates p53, and interacts with DNA damage-binding protein 1 (DDB1, the DNA repair protein), which may affect repair functions and allow the accumulation of genetic changes, and also confer resistance against nucleolar stress and anticancer drugs[33].
CURRENT OPINION IN ANTIVIRAL THERAPY
Despite dramatic improvements in the treatment of patients against HBV over the past decade, treatment of chronic HBV infection is currently based on two different strategies: (1) IFN-α or thymosin-a1 (T-a1) aimed at inducing a sustained antiviral response; and (2) oral anti-HBV Nucs to achieve long-term complete suppression of HBV replication[34].
The first strategy is typically used in patients with less advanced liver disease, with high alanine aminotransferase (ALT) and not too high HBV DNA replication. It is particularly successful in younger patients and in those infected with HBV genotype A or B. Since the first introduction of IFN-α in 1976, the long-term benefit of IFN therapy has included a sustained and cumulative immune response. T-a1 is an immunomodulator that triggers maturational events in lymphocytes and T-cell function. It can promote reconstitution of immune defects and promote disease remission and cessation of HBV replication in patients with hepatitis B e antigen (HBeAg)-positive chronic hepatitis B, without significant side effects[35,36]. Eighteen patients with HBeAg-positive and serum HBV DNA-positive chronic hepatitis B received 6 mo of treatment with 1.6 mg subcutaneous T-a1 twice weekly[37]. They achieved better HBV loss and seroconversion than 30 patients receiving 6 mo of 3-5 MU subcutaneous IFN-α (injection daily for 15 d, then three times weekly). The results of this trial indicate that T-a1 is of potential interest in patients with anti-HBe- and HBV DNA-positive chronic hepatitis B.
The introduction of 12-kDa linear polyethylene glycol (PEG) for IFN-α2b and 40-kDa branched PEG for IFN-α2a has allowed weekly rather than daily or three times weekly injection[34]. This has had a significant impact on the tolerability and ease of use. In addition, for patients with HBeAg-positive chronic hepatitis B, Pegylated IFN (PEG IFN)-α2a offers superior efficacy over lamivudine, on the basis of HBeAg seroconversion, HBV DNA suppression, and hepatitis B surface antigen (HBsAg) seroconversion[37]. Overall, PEG IFN-α is an ideal treatment strategy in selected patients with HBeAg-negative chronic hepatitis B, because of its well-recognized and predictable safety profile and unique mechanism of antiviral activity leading to long-lasting immune control.
For high HBV DNA levels, Nucs are typically adopted for patients with more advanced liver disease, and for those who have failed or cannot tolerate IFN therapy. However, the main limitation is the development of resistance: for example, after 5 years of therapy with lamivudine (L-nucleoside), 76% of patients developed resistance. Telbivudine, another L-nucleoside, is more potent than lamivudine but resistance still developed in 25% of HBeAg-positive and 11% of HBeAg-negative patients after 2 years. Adefovir, an acyclic phosphonate, is relatively weak, but is effective against lamivudine- and telbivudine-resistant mutations, and it should be used in combination rather than substituted. Resistance to adefovir develops relatively slowly, rising to 29% for HBeAg-negative patients after 5 years, but more rapidly when used alone for lamivudine-resistant HBV. Currently, the two first-line Nucs are entecavir and tenofovir. Entecavir, a cyclopentane (D-nucleoside), is very potent, with 94% of patients having undetectable HBV DNA after 5 years. Resistance develops in only 1.2% of treatment-naïve patients. Tenofovir, another acyclic nucleotide, is more potent with less renal toxicity compared to adefovir. It is effective against lamivudine-resistant mutations when used alone. No resistance to tenofovir has been described after its use for 3 years or longer, often for patients with human immunodeficiency virus/HBV co-infection[38].
In conclusion, for patients with HBeAg-positive chronic hepatitis B, PEG IFN-α offers superior efficacy on the basis of HBeAg seroconversion, HBV DNA suppression, and HBsAg seroconversion. As a result of these features, new therapeutic regimens based on combinations of PEG IFN-α and third-generation Nucs such as entecavir and tenofovir are being developed to increase the rate of HBsAg seroclearance, which remains the ideal end-point in all HBeAg-negative chronic hepatitis B patients.
ANTIVIRAL THERAPY SUPPRESSES THE CHRONIC INFLAMMATION-CANCER TRANSITION
A prospective cohort study with 11 years follow-up showed that HBV DNA concentration > 104 copies/mL is an especially strong predictor of risk of developing HCC in individuals aged ≥ 30 years, independent of the level of serum ALT[39]. It is accepted that anti-HBV therapy can improve the outcome of chronic HBV infection in terms of HCC incidence.
In a randomized controlled trial of 101 male patients in the Taiwan region, cumulative incidence of HCC development was significantly decreased in the IFN-α-treated group (1 of 67 patients) than in the control group (4 of 34 patients), at 1.1-11.5 years after the end of therapy[40]. In addition, a retrospective study suggested that natural lymphoblastoid IFN-α (IFN-α nl) and IFN therapy may provide better long-term beneficial effects than placebo in terms of HBV clearance, reduction of HCC, and prolonged survival. HCC was detected in 1.5% of the IFN-α nl group, 3.7% of the IFN-α2a group and 14.7% of the control group.
As for the long-term benefits of Nucs, in a randomized control trial, HCC occurred in 3.9% of patients treated with lamivudine and 7.4% of those in the placebo group in a total of 651 patients (HR = 0.49, P = 0.047)[41]. A retrospective multicenter study of 377 Japanese patients receiving lamivudine treatment for up to 96 (23.1 ± 19.0) mo showed a marked reduction in the incidence of HCC compared with a historical control group matched for age, sex, liver fibrosis score, albumin level and platelet count (0.4% per year vs 2.5% per year, P < 0.001)[42]. In another study of 656 HBeAg-negative patients (54% had chronic hepatitis, 30% had cirrhosis), lamivudine (median 22 mo, range 1-66) was highly effective in reducing viral load in HBeAg-negative patients, and HBV suppression reduced the development of HCC and disease worsening in patients with cirrhosis[43]. A Korean study also showed a reduced incidence of HCC in patients with compensated cirrhosis who received lamivudine therapy (4.9%) compared to untreated patients or patients treated with lamivudine who had viral breakthrough (11.8%) or a suboptimal response (19.4%)[44]. In a recent systemic review, Papatheodoridis et al[45] reviewed 21 studies that included 3881 treated and 534 untreated patients and found that HCC developed less frequently in Nuc-treated patients (2.8% vs 6.4%, P < 0.003).
In a systematic review of 11 studies of the effect of IFN and Nuc therapy on the outcome of HBV infection over the past 10 years, Sung et al[46] indicated that IFN-α or Nuc treatment significantly reduced the risk of HCC. Although IFN benefited patients with cirrhosis, Nucs benefited those with non-cirrhosis and HBeAg-positive infection. From the experiences mentioned above, sustained HBV suppression induced by IFN-α and Nuc therapy may be necessary to reduce the development of HCC in HBV-infected patients.
EFFECT OF ANTIVIRAL THERAPY ON HCC RECURRENCE
Does anti-HBV therapy decrease the risk of HCC recurrence after the most effective methods to reduce tumor burden: partial hepatectomy or liver transplantation? As suggested previously, the 5-year overall survival for all early HCC patients was 58% (transplantation: 63%; resection: 53%)[47]. Huang et al[48] reported that patients with HBV reactivation after liver resection have a higher liver failure rate, lower 3-year disease-free survival rate, and lower overall survival rate than those without reactivation (11.8% vs 6.4%, P = 0.002, 34.1% vs 46.0%, P = 0.009, and 51.6% vs 67.2%, P < 0.001, respectively).
Exploratory subset analysis showed that adjuvant IFN-α had no survival benefit for pTNM stage I/II tumor (5-year survival 90% in both groups; P = 0.917) but prevented early recurrence and improved the 5-year survival of patients with stage III/IVA tumor from 24% to 68% (P = 0.038)[49]. Lee et al[50] also reported that metastasis-associated protein 1-positive HCC recurred postoperatively in 26 of 93 patients (28%), although the PEG IFN group had significantly lower overall cumulative recurrence rates than the control group (7% and 14% vs 24% and 34% at 1 and 2 years, respectively; P < 0.05). In addition, the 1- and 2-year cumulative survival rates were higher in the PEG IFN group compared with the control group (100% vs 93% and 100% vs 87%, respectively; P < 0.05). In a report of 237 HCC patients after hepatectomy treated with IFN-α or placebo within comparable clinicopathological parameters, the median overall survival was 63.8 mo in the IFN-α group and 38.8 mo in the placebo group (P = 0.0003), and the median disease-free survival period was 31.2 vs 17.7 mo (P = 0.142)[51]. Chen et al[52] showed that adjuvant IFN-α2b treatment was associated with a significantly higher incidence of leukopenia and thrombocytopenia and did not reduce postoperative recurrence of viral hepatitis-related HCC.
Regarding the effect of Nucs on HCC recurrence, Anselmo et al[53] suggested that hepatitis B immunoglobulin (HBIg) and lamivudine treatment markedly reduced HBV recurrence rate and significantly improved 1- and 3-year recurrence-free survival rates after liver transplantation. Chan et al[54] also reported that the 1-, 3- and 5-year disease-free survival rates in patients treated with lamivudine or entecavir were 66.5%, 51.4% and 51.4% compared with 48.9%, 33.8% and 33.8%, respectively, in the control group. Kubo et al[55] reported that the tumor-free survival rate after hepatectomy was significantly higher in the lamivudine than the control group. Recently, multivariate analysis showed that HCC recurrence after transplantation was markedly associated with HBV reinfection. However, HBIg was associated with worse survival as well as HBV reinfection and HCC recurrence (P = 0.002, P < 0.001 and P < 0.001, respectively)[56].
In our center (Liver Transplantation Center, The First Affiliated Hospital of Nanjing Medical University), we suggest that HBsAg-positive patients who have > 104/mL or 103-104/mL HBV DNA copies with impaired liver function, must take lamivudine after curative surgery. Moreover, for those who have HBV YMDD mutation during initial treatment, entecavir and/or adefovir dipivoxil should be used as the replacement. If drug resistance occurs, tenofovir disoproxil fumarate could be used instead. In our randomized controlled clinical study, we verified that standardized anti-HBV therapy could significantly improve the outcome and decrease the recurrence of patients who underwent partial hepatectomy and liver transplantation (Figure 1).
Figure 1.
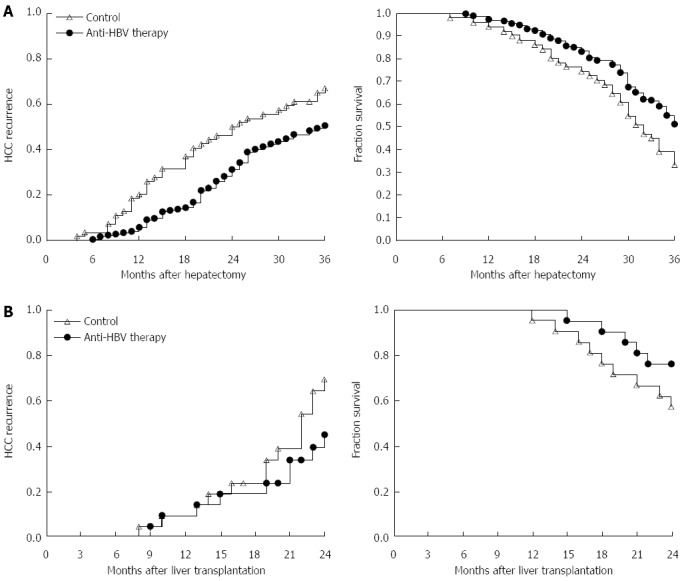
Comparison of hepatocellular carcinoma recurrence and outcome in patients who received anti-hepatitis B virus therapy or placebo after hepatectomy or liver transplantation. A: From September 2009 to May 2010, 224 HCC patients who received partial hepatectomy due to HBV-related HCC were enrolled. HCC recurrence and 3-year overall survival rate in patients with anti-HBV treatment (n = 173) and patients without standardized anti-HBV treatment (n = 51) were monitored for at least 3 years. Left: log-rank test, P = 0.013; right: log-rank test, P = 0.006; B: From January 2010 to August 2011, 42 HCC patients within Milan criteria who received liver transplantation were enrolled. HCC recurrence and 2-year overall survival rate in patients with anti-HBV treatment (n = 28) and patients without standardized anti-HBV treatment (n = 14) are shown. Left: log-rank test, P = 0.031; right: log-rank test, P = 0.045. HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus.
CONCLUSION
This literature review describes two different aspects of the tumorigenesis of chronic HBV infection: the direct mechanism by which HBV DNA and its main product HBx induce host DNA instability; and HBV infection-associated liver inflammation and imbalanced immunoregulation. We also briefly introduce the current strategy against HBV infection and show that timely usage of Nucs and immunomodulatory agents can eventually prevent further disease progression, including HCC, in patients with chronic HBV infection. Long-term studies will probably confirm that new antiviral drugs such as entecavir, tenofovir and telbivudine can offer even more opportunities for reducing disease progression than lamivudine therapy does.
For patients who undergo hepatectomy or liver transplantation as curative treatment for HCC, tumor recurrence must be monitored by ultrasound and α-fetoprotein assay. More importantly, from our experience, HBV replication should also be monitored because sustained HBV activation or relapse is significantly related to HCC development and recurrence. Standardized anti-HBV treatment can ultimately delay HCC recurrence and benefit survival.
Since PEG-IFN, as the newly introduced IFN, offers a better opportunity to suppress HBV replication in patients who do not have cirrhosis or fibrosis, it should provide promising prospects in reducing HCC development and recurrence. While in many third world countries, lamivudine is still the first-line drug, mass usage of newly developed IFN could be used more frequently in the future and show better prospects.
In conclusion, developing safe and affordable agents, as well as management strategies to improve sustained or maintained HBV suppression, should be the ultimate goals in the management of chronic HBV infection.
Footnotes
P- Reviewer: Wu ZJ S- Editor: Cui XM L- Editor: Logan S E- Editor: Ma S
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Sherman M. Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment. Cleve Clin J Med. 2009;76 Suppl 3:S6–S9. doi: 10.3949/ccjm.76.s3.02. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–1629. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- 5.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 6.Sun BS, Pan J, Clayton MM, Liu J, Yan X, Matskevich AA, Strayer DS, Gerber M, Feitelson MA. Hepatitis C virus replication in stably transfected HepG2 cells promotes hepatocellular growth and tumorigenesis. J Cell Physiol. 2004;201:447–458. doi: 10.1002/jcp.20083. [DOI] [PubMed] [Google Scholar]
- 7.Broering R, Lu M, Schlaak JF. Role of Toll-like receptors in liver health and disease. Clin Sci (Lond) 2011;121:415–426. doi: 10.1042/CS20110065. [DOI] [PubMed] [Google Scholar]
- 8.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012;61 Suppl 1:i6–17. doi: 10.1136/gutjnl-2012-302056. [DOI] [PubMed] [Google Scholar]
- 9.Sun B, Karin M. Inflammation and liver tumorigenesis. Front Med. 2013;7:242–254. doi: 10.1007/s11684-013-0256-4. [DOI] [PubMed] [Google Scholar]
- 10.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 11.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chirillo P, Falco M, Puri PL, Artini M, Balsano C, Levrero M, Natoli G. Hepatitis B virus pX activates NF-kappa B-dependent transcription through a Raf-independent pathway. J Virol. 1996;70:641–646. doi: 10.1128/jvi.70.1.641-646.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weil R, Sirma H, Giannini C, Kremsdorf D, Bessia C, Dargemont C, Bréchot C, Israël A. Direct association and nuclear import of the hepatitis B virus X protein with the NF-kappaB inhibitor IkappaBalpha. Mol Cell Biol. 1999;19:6345–6354. doi: 10.1128/mcb.19.9.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benn J, Su F, Doria M, Schneider RJ. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996;70:4978–4985. doi: 10.1128/jvi.70.8.4978-4985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen CJ, Lin YJ, Yen CS, Tsai HW, Tsai TF, Chang KY, Huang WC, Lin PW, Chiang CW, Chang TT. Hepatitis B virus X protein upregulates mTOR signaling through IKKβ to increase cell proliferation and VEGF production in hepatocellular carcinoma. PLoS One. 2012;7:e41931. doi: 10.1371/journal.pone.0041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, Wang X, Sun B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–909. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhang S, Zou Z, Shi J, Zhao J, Fan R, Qin E, Li B, Li Z, Xu X, et al. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology. 2011;53:73–85. doi: 10.1002/hep.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J, Janssen HL. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Qiu SJ, She WM, Wang FP, Gao H, Li L, Tu CT, Wang JY, Shen XZ, Jiang W. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One. 2012;7:e39307. doi: 10.1371/journal.pone.0039307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol. 2011;17:4853–4857. doi: 10.3748/wjg.v17.i44.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki H, Kajino K, Arakawa Y, Hino O. Molecular cloning of a rat chromosome putative recombinogenic sequence homologous to the hepatitis B virus encapsidation signal. Proc Natl Acad Sci USA. 1996;93:7300–7304. doi: 10.1073/pnas.93.14.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, You X, Li N, Zhang W, Gagos S, Wang Q, Banos A, Cai N, Zhang H, Zhang H, et al. Involvement of hepatitis B virus X gene (HBx) integration in hepatocarcinogenesis via a recombination of HBx/Alu core sequence/subtelomeric DNA. FEBS Lett. 2012;586:3215–3221. doi: 10.1016/j.febslet.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Chenivesse X, Henglein B, Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- 25.Fallot G, Neuveut C, Buendia MA. Diverse roles of hepatitis B virus in liver cancer. Curr Opin Virol. 2012;2:467–473. doi: 10.1016/j.coviro.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Terradillos O, Billet O, Renard CA, Levy R, Molina T, Briand P, Buendia MA. The hepatitis B virus X gene potentiates c-myc-induced liver oncogenesis in transgenic mice. Oncogene. 1997;14:395–404. doi: 10.1038/sj.onc.1200850. [DOI] [PubMed] [Google Scholar]
- 27.Kojima H, Kaita KD, Xu Z, Ou JH, Gong Y, Zhang M, Minuk GY. The absence of up-regulation of telomerase activity during regeneration after partial hepatectomy in hepatitis B virus X gene transgenic mice. J Hepatol. 2003;39:262–268. doi: 10.1016/s0168-8278(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 28.Saini N, Srinivasan R, Chawla Y, Sharma S, Chakraborti A, Rajwanshi A. Telomerase activity, telomere length and human telomerase reverse transcriptase expression in hepatocellular carcinoma is independent of hepatitis virus status. Liver Int. 2009;29:1162–1170. doi: 10.1111/j.1478-3231.2009.02082.x. [DOI] [PubMed] [Google Scholar]
- 29.Ng SA, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol. 2011;46:974–990. doi: 10.1007/s00535-011-0415-9. [DOI] [PubMed] [Google Scholar]
- 30.Forgues M, Difilippantonio MJ, Linke SP, Ried T, Nagashima K, Feden J, Valerie K, Fukasawa K, Wang XW. Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol Cell Biol. 2003;23:5282–5292. doi: 10.1128/MCB.23.15.5282-5292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang R, Deng L, Zhao L, Li X, Zhang F, Xia Y, Gao Y, Wang X, Sun B. miR-22 promotes HBV-related hepatocellular carcinoma development in males. Clin Cancer Res. 2011;17:5593–5603. doi: 10.1158/1078-0432.CCR-10-1734. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng X, Zhu Z, Jiao H, Lin J, Jiang K, et al. Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis. J Clin Invest. 2013;123:630–645. doi: 10.1172/JCI64265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapoor NR, Ahuja R, Shukla SK, Kumar V. The HBx protein of hepatitis B virus confers resistance against nucleolar stress and anti-cancer drug-induced p53 expression. FEBS Lett. 2013;587:1287–1292. doi: 10.1016/j.febslet.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Alberti A, Caporaso N. HBV therapy: guidelines and open issues. Dig Liver Dis. 2011;43 Suppl 1:S57–S63. doi: 10.1016/S1590-8658(10)60693-7. [DOI] [PubMed] [Google Scholar]
- 35.Low TL, Goldstein AL. Thymosins: structure, function and therapeutic applications. Thymus. 1984;6:27–42. [PubMed] [Google Scholar]
- 36.Chien RN, Liaw YF, Chen TC, Yeh CT, Sheen IS. Efficacy of thymosin alpha1 in patients with chronic hepatitis B: a randomized, controlled trial. Hepatology. 1998;27:1383–1387. doi: 10.1002/hep.510270527. [DOI] [PubMed] [Google Scholar]
- 37.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 38.Yuen MF, Lai CL. Treatment of chronic hepatitis B: Evolution over two decades. J Gastroenterol Hepatol. 2011;26 Suppl 1:138–143. doi: 10.1111/j.1440-1746.2010.06545.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol. 2006;101:1797–1803. doi: 10.1111/j.1572-0241.2006.00647.x. [DOI] [PubMed] [Google Scholar]
- 40.Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999;29:971–975. doi: 10.1002/hep.510290312. [DOI] [PubMed] [Google Scholar]
- 41.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–184. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Di Marco V, Marzano A, Lampertico P, Andreone P, Santantonio T, Almasio PL, Rizzetto M, Craxì A. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40:883–891. doi: 10.1002/hep.20381. [DOI] [PubMed] [Google Scholar]
- 44.Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118–125. doi: 10.1016/j.jhep.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 46.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–1077. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 47.Dhir M, Lyden ER, Smith LM, Are C. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB (Oxford) 2012;14:635–645. doi: 10.1111/j.1477-2574.2012.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang G, Lai EC, Lau WY, Zhou WP, Shen F, Pan ZY, Fu SY, Wu MC. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257:490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 49.Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee D, Chung YH, Kim JA, Park WH, Jin YJ, Shim JH, Ryu SH, Jang MK, Yu E, Lee YJ. Safety and efficacy of adjuvant pegylated interferon therapy for metastatic tumor antigen 1-positive hepatocellular carcinoma. Cancer. 2013;119:2239–2246. doi: 10.1002/cncr.28082. [DOI] [PubMed] [Google Scholar]
- 51.Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458–465. doi: 10.1007/s00432-006-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, Wu CC, Mok KT, Chen CL, Lee WC, et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg. 2012;255:8–17. doi: 10.1097/SLA.0b013e3182363ff9. [DOI] [PubMed] [Google Scholar]
- 53.Anselmo DM, Ghobrial RM, Jung LC, Weaver M, Cao C, Saab S, Kunder G, Chen PW, Farmer DG, Yersiz H, et al. New era of liver transplantation for hepatitis B: a 17-year single-center experience. Ann Surg. 2002;235:611–619; discussion 619-620. doi: 10.1097/00000658-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–681. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- 55.Kubo S, Tanaka H, Takemura S, Yamamoto S, Hai S, Ichikawa T, Kodai S, Shinkawa H, Sakaguchi H, Tamori A, et al. Effects of lamivudine on outcome after liver resection for hepatocellular carcinoma in patients with active replication of hepatitis B virus. Hepatol Res. 2007;37:94–100. doi: 10.1111/j.1872-034X.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 56.Campsen J, Zimmerman M, Trotter J, Hong J, Freise C, Brown R, Cameron A, Ghobrial M, Kam I, Busuttil R, et al. Liver transplantation for hepatitis B liver disease and concomitant hepatocellular carcinoma in the United States With hepatitis B immunoglobulin and nucleoside/nucleotide analogues. Liver Transpl. 2013;19:1020–1029. doi: 10.1002/lt.23703. [DOI] [PubMed] [Google Scholar]


