Abstract
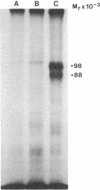
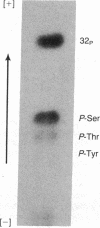
Varicella zoster virus glycoprotein I (VZV gpI; Mr 98,000) was phosphorylated in virus-infected human cell monolayers, while two other major VZV glycoproteins (gpII and gpIII) were not similarly modified. Phosphorylation of VZV gpI was not blocked by inhibitors of glycosylation, nor were the phosphoryl groups enzymatically removed by endoglycosidases. Phosphoamino acid analysis revealed the presence of phosphoserine and phosphothreonine residues on the polypeptide backbone. The selective nature of the phosphorylation event was further demonstrated in vitro by a protein kinase (Mr 50,000), which was present in virus-infected cells but absent from uninfected cells or purified virions. The enzyme catalyzed the transfer of 32Pi from [gamma-32P]ATP to gpI but not to gpII and gpIII. Like VZV gpI, this virus-induced protein kinase was also a constituent of the plasma membrane of live VZV-infected cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso F. V., Compans R. W. Differential effect of monensin on enveloped viruses that form at distinct plasma membrane domains. J Cell Biol. 1981 Jun;89(3):700–705. doi: 10.1083/jcb.89.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavanandan V. P., Umemoto J., Davidson E. A. Characterization of an endo-alpha-N-acetyl galactosaminidase from Diplococcus pneumoniae. Biochem Biophys Res Commun. 1976 Jun 7;70(3):738–745. doi: 10.1016/0006-291x(76)90654-9. [DOI] [PubMed] [Google Scholar]
- Blue W. T., Stobbs D. G. Isolation of a protein kinase induced by herpes simplex virus type 1. J Virol. 1981 Apr;38(1):383–388. doi: 10.1128/jvi.38.1.383-388.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Edson C. M., Ellis R. W., Forghani B., Gilden D., Grose C., Keller P. M., Vafai A., Wroblewska Z., Yamanishi K. New common nomenclature for glycoprotein genes of varicella-zoster virus and their glycosylated products. J Virol. 1986 Mar;57(3):1195–1197. doi: 10.1128/jvi.57.3.1195-1197.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M., Darai G. Protein kinase and specific phosphate acceptor proteins associated with tupaia herpesvirus. J Virol. 1982 Aug;43(2):410–415. doi: 10.1128/jvi.43.2.410-415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs W. E., Grose C. Varicella-zoster virus p32/p36 complex is present in both the viral capsid and the nuclear matrix of the infected cell. J Virol. 1986 Jan;57(1):155–164. doi: 10.1128/jvi.57.1.155-164.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Grose C., Friedrichs W. E., Smith G. C. Purification and molecular anatomy of the varicella-zoster virion. Biken J. 1983 Mar;26(1):1–15. [PubMed] [Google Scholar]
- Grose C., Perrotta D. M., Brunell P. A., Smith G. C. Cell-free varicella-zoster virus in cultured human melanoma cells. J Gen Virol. 1979 Apr;43(1):15–27. doi: 10.1099/0022-1317-43-1-15. [DOI] [PubMed] [Google Scholar]
- Grose C. The synthesis of glycoproteins in human melanoma cells infected with varicella-zoster virus. Virology. 1980 Feb;101(1):1–9. doi: 10.1016/0042-6822(80)90478-x. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Keenan R. W., Elbein A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979 May 29;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T., Takaki K., Hinuma Y., Watanabe Y. Protein kinase activity associated with Epstein-Barr virus-determined nuclear antigen. Virology. 1981 Sep;113(2):512–520. doi: 10.1016/0042-6822(81)90179-3. [DOI] [PubMed] [Google Scholar]
- Katan M., Stevely W. S., Leader D. P. Partial purification and characterization of a new phosphoprotein kinase from cells infected with pseudorabies virus. Eur J Biochem. 1985 Oct 1;152(1):57–65. doi: 10.1111/j.1432-1033.1985.tb09163.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaster S., Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980 Sep;35(3):798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S., Tardy-Panit M., Bârzu O. Properties of a human cytomegalovirus-induced protein kinase. Virology. 1984 Apr 30;134(2):259–268. doi: 10.1016/0042-6822(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Montalvo E. A., Grose C. Neutralization epitope of varicella zoster virus on native viral glycoprotein gp118 (VZV glycoprotein gpIII). Virology. 1986 Mar;149(2):230–241. doi: 10.1016/0042-6822(86)90124-8. [DOI] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985 Mar;53(3):761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Varicella-zoster viral glycoprotein envelopment: ultrastructural cytochemical localization. J Histochem Cytochem. 1986 Feb;34(2):281–284. doi: 10.1177/34.2.3003184. [DOI] [PubMed] [Google Scholar]
- Randall C. C., Rogers H. W., Downer D. N., Gentry G. A. Protein kinase activity in equine herpesvirus. J Virol. 1972 Feb;9(2):216–222. doi: 10.1128/jvi.9.2.216-222.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Gravell M., Darlington R. Protein kinase in enveloped herpes simplex virions. Virology. 1972 Oct;50(1):287–290. doi: 10.1016/0042-6822(72)90374-1. [DOI] [PubMed] [Google Scholar]
- Stevely W. S., Katan M., Stirling V., Smith G., Leader D. P. Protein kinase activities associated with the virions of pseudorabies and herpes simplex virus. J Gen Virol. 1985 Apr;66(Pt 4):661–673. doi: 10.1099/0022-1317-66-4-661. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J Infect Dis. 1983 Oct;148(4):630–638. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Molecular dissection of the humoral immune response to individual varicella-zoster viral proteins during chickenpox, quiescence, reinfection, and reactivation. J Infect Dis. 1984 May;149(5):741–749. doi: 10.1093/infdis/149.5.741. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]