Abstract
Although the International Agency for Research on Cancer declared Helicobacter pylori (H. pylori) as a definite human carcinogen in 1994, the Japanese Society for Helicobacter Research only recently (February 2013) adopted the position that H. pylori infection should be considered as an indication for either amelioration of chronic gastritis or for decreasing gastric cancer mortality. Japanese researchers have found that H. pylori eradication halts progressive mucosal damage and that successful eradication in patients with non-atrophic gastritis most likely prevents subsequent development of gastric cancer. However, those who have already developed atrophic gastritis/gastric atrophy retain potential risk factors for gastric cancer. Because chronic perpetuated progression of H. pylori-associated gastric inflammation is associated with increased morbidity culminating in gastric carcinogenesis, a non-microbial approach to treatment that provides long-term control of gastric inflammation through nutrients and other interventions may be an effective way to decrease this morbidity. This non-microbial approach might represent a new form of prerequisite “rescue” therapy that provides a quicker path to the prevention of gastric cancer as compared to simple eradication.
Keywords: Helicobacter pylori, Gastric cancer, Prevention, Atrophic gastritis, Non-microbial approach
Core tip: Gastric cancer is a multi-factorial and multi-step disease associated with various risk factors including environmental and pathogenic microbial chronic inflammation. Pharmaceutical intervention and the eradication strategy can provide rapid relief of acute inflammation but fails to correct the underlying cause of chronic inflammation. A non-microbial approach for modulating Helicobacter pylori associated gastric inflammation may be an attractive and rapid alternative to optimize cancer prevention strategies and minimize adverse side effects associated with therapeutic regimens.
INTRODUCTION
Helicobacter pylori (H. pylori), a Gram-negative bacterial pathogen that infects approximately 50% of the world’s population, provokes chronic gastric inflammation which is considered a major risk factor for the development of gastric and duodenal ulcers, mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma[1]. In 1994, H. pylori was classified as a type I (definite) carcinogen by the International Agency for Research on Cancer[2]. Although the relationship between H. pylori and gastric cancer has been acknowledged by diverse forms of clinical evidence, it is still debatable as to whether eradication can lead to the prevention of gastric cancer[3-5]. Traditionally, treatment for H. pylori has focused primarily on eradicating the bacteria from the stomach using a combination of antibiotics, such as amoxicillin and clarithromycin, with a proton-pump inhibitor[6-10]. The eradication rate, however, has been declining due to the increasing prevalence of antibiotic resistance, especially clarithromycin resistance[11-15]. This increase in the prevalence of antibiotic resistance has diminished enthusiasm for the use of many popular H. pylori eradication therapies. To overcome this decline in the use of first-line treatment options, bismuth-containing quadruple and sequential therapies are emerging as second-line treatments for H. pylori infection[16-21]. Although newer treatments for eradicating H. pylori continue to be introduced, research on even more effective eradication regimens continues to be conducted. Unfortunately, literature from all over the world continues to document increases in H. pylori resistance to antibiotics and this major obstacle has prompted the introduction of new drugs and treatment schemes. It is also important to note that although removal or amelioration of gastric inflammation has been implicated in the prevention of gastric carcinogenesis, the persistent gastric inflammation observed in H. pylori-associated gastric carcinogenesis is not always amelioration by H. pylori eradication alone.
Because gastric cancer is a multi-step and multi-factorial disease, not all individuals infected with H. pylori will develop gastric cancer. In fact, the multi-factorial processes associated with the development of gastric cancer can give hope to some susceptible individuals that it may be prevented through the eradication of H. pylori. Conversely, in cases where chronic inflammation is caused by other environmental factors such as diet, eradication of H. pylori may only delay the development of gastric cancer rather prevent it. Importantly, there is no overt biomarker supporting the rationale of H. pylori eradication in clinic, although endoscopic findings might be recommended (Figure 1). Moreover, the nationwide cost associated with eradicating H. pylori in order to prevent gastric cancer would be prohibitive and represent a burden to socioeconomically challenged people in developing countries. Therefore, the strategy of cancer prevention through chemopreventive agents may be the most efficacious way to reduce the global burden of cancer.
Figure 1.
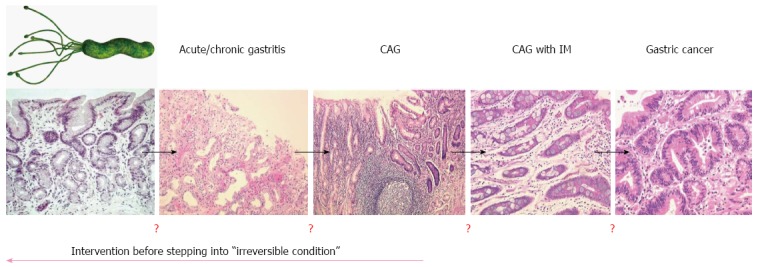
Point of no return in Helicobacter pylori infection. Helicobacter pylori (H. pylori) infection is responsible for acute and chronic gastritis, chronic atrophic gastritis, and intestinal metaplasia. The results of a few studies have shown that the eradication of H. pylori significantly reverted these gastric pathologies and promoted restoration of gastric function. H. pylori is also implicated in several extragastric manifestations including idiopathic thrombocytopenic purpura, iron deficiency anemia, atherosclerosis, and chronic urticaria. Because there are no biomarkers suggestive of a point of no return, the results of several large scale cohort studies continue to provide support for the strategy of H. pylori eradication in gastric cancer prevention.
Cancer chemoprevention was established by Dr. M. Sporn in 1976 and was defined as “the use of natural, synthetic, or nontoxic chemical substances to reverse, suppress, delay or prevent carcinogenic progression” by Dr. M. Sporn and Dr. W. K. Hong[22,23]. The results of several preclinical and clinical studies have indicated that diverse chemoprevention strategies can decrease gastrointestinal (GI) cancer incidence and mortality rates[24]. Essentially, the chemoprevention strategy involves interventions during all three stages of carcinogenesis, (initiation, promotion, and progression) using chemopreventive agents in order to interfere with tumor promotion or progression and reduce the risk of various cancers. All GI cancers have a unique etiology but share common mechanisms including oxidative stress-induced damage of genomic DNA, modification of cellular proteins and lipids, altered cell signaling, and persistent local tissue inflammation. Therefore, the combination of H. pylori eradication, anti-oxidant interventions, interventions to normalize aberrant cell signaling, and anti-inflammatory interventions may be an essential and anticipatory strategy for prevention of gastric cancer. Recently, numerous studies have investigated the potential therapeutic benefits of probiotics, phytochemicals, and antioxidant or vitamin supplementation as chemopreventive agents as well as adjuncts to increase the eradication rates of H. pylori infection. In this article, we discuss what is known currently about non-microbial preventive strategies for chronic infection with H. pylori which may represent a faster option for cancer prevention via enhancement of host adoptive responses as well as removal of inflammation responsible for mutagenesis (Figure 2).
Figure 2.
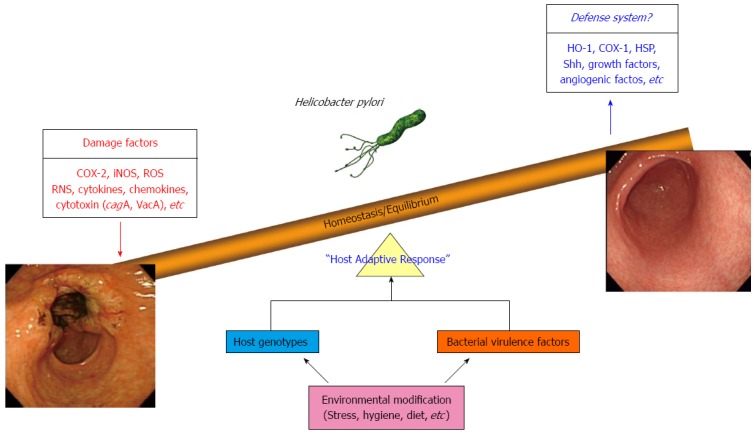
Host adoptive response through a non-microbial approach as the core defensive mechanism against Helicobacter pylori infection, especially gastric cancer prevention. Even though host genotype, environmental risk, and bacterial virulence factor are all implicated in Helicobacter pylori (H. pylori) infection, a non-microbial approach may provide the fastest means of cancer prevention as well as amelioration of H. pylori-associated gastric pathologies.
ANTICIPATING NON-MICROBIAL APPROACHES FOR PREVENTING H. PYLORI-ASSOCIATED GASTRIC CARCINOGENESIS
Cyclooxygenase and 5-lipoxygenase inhibition
H. pylori-induced inflammatory responses have been associated with high concentrations of phospholipase A2 (PLA2) which is an essential enzyme for the release of arachidonic acid (AA). AA metabolites, prostaglandins (PGs) or hydroxyl fatty acids (HETEs), are key mediators in inflammatory responses and are metabolized by cyclooxygenase (COX) and lipoxygenase (LOX)[25]. COX-1 and COX-2 are responsible for the production of inflammatory PGs and 5-LOX increases the release of gastrotoxic leukotrienes (LTs)[26]. Conversely, findings demonstrating that inflammatory responses were decreased by inhibiting COX and 5-LOX led research on COX and 5-LOX inhibitors as attractive medications for anti-inflammatory effects[27-29]. H. pylori infection induces higher levels of COX-2 expression, overexpression of which has been detected in various cancers including gastric cancer[30-36]. In this regard, nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for preventing cancers as well as reducing pain and inflammation by inhibiting both COX-1 and COX-2 or COX-2 only[37-39]. Long-term use of NSAIDs attenuated gastric mucosal chronic inflammation induced by H. pylori infection suggesting that NSAIDs may be preventive agents of the gastric carcinogenesis associated with H. pylori infection[40]. However, the adverse effects associated with the use of NSAIDs may present an obstacle to their use as chemopreventive agents. Traditional NSAIDs non-selectively inhibit both COX-1 and COX-2 and may cause GI toxicity, as COX-1 is a house keeping enzyme involved in the cytoprotection of gastric mucosa. Though selective COX-2 inhibitors (coxibs), such as celecoxib, rofecoxib, and valdecoxib, have been developed to improve the GI safety[41], coxibs also carry the risk of thromboembolic or cardiovascular complications[42-44]. 5-LOX inhibitors also suppressed H. pylori-induced proinflammatory mediators such as interleukin-8 and tumor necrosis factor-α in H. pylori-infected gastric epithelial cells, indicating that 5-LOX inhibitors can be preventive agents against H. pylori-associated gastric inflammation and carcinogenesis by inhibiting the 5-LOX signaling pathway and suppressing its expression[25].
Phytochemicals and phytonutrients
The results of several recent studies have shown that dietary phytochemicals can modulate key molecular signaling cascades by interacting with small molecules in cancer cells[45] and that phytochemicals present in foods can inhibit H. pylori-induced inflammation. Therefore, the combination of H. pylori eradication and the suppression of H. pylori-induced inflammation may represent a promising strategy for gastric cancer prevention. For instance, curcumin (diferuloylmethane), the yellow pigment of turmeric (Curcuma longa L.) possesses strong anti-inflammatory activities and has shown diverse suppressive actions against various cancers including gastric cancer. It has been reported that curcumin inhibits H. pylori-induced nuclear factor (NF)-κB activation, pro-inflammatory cytokines such as interleukin 8, matrix metalloproteinase-3 and -9, and the H. pylori-induced motogenic response[46,47]. In addition to these anti-inflammatory and anti-mutagenic actions, curcumin has showed anti-microbial effects in H. pylori-infected C57BL/6 mice as well as restorative actions following H. pylori-induced gastric damage[48]. Furthermore, curcumin inhibited the proliferation and invasion of gastric cancer cells by suppressing PAK1 activity and cyclin D1 expression[49]. Collectively, the results of these studies suggest that curcumin has potential as an antimicrobial compound and chemopreventive agent against H. pylori infection. The results of one recent study suggested that curcumin may prevent cancer therapy-induced oral mucositis due to its antibacterial and anti-inflammatory kinetics[50]. Further research has shown that foods such as broccoli sprouts and oils posses anti-H. pylori-associated inflammatory effects mediated by reducing the release of pro-inflammatory cytokines and suppressing the NF-κB pathway[51,52]. Additionally, daily intake of sulforaphane-rich broccoli sprouts was associated with anti-H. pylori activity and protection of the gastric mucosa against H. pylori-induced oxidative stress[53]. Broccoli sprouts contain high levels of glucoraphanin, a glucosinolate precursor of the isothiocynate sulforaphan known to suppress interleukin (IL)-8 via the NF-κB pathway[51,54]. Because H. pylori-induced inflammation has been associated with the expression IL-8, a potent neutrophil-attracting chemokine, via activation of the NF-κB pathway[55,56], reduction or disruption of this cascade or levels of this cytokine may be an appropriate strategy to intervene in H. pylori-induced inflammation.
Omega-3 polyunsaturated fatty acids
There is growing evidence that the diverse biological roles of n-3 polyunsaturated fatty acids (PUFAs) may contribute to their protective actions against chronic inflammatory disease[57]. In bacteria, n-3 PUFAs cause cell lysis, while in other cell types, n-3 PUFAs can be incorporated into membrane phospholipids that can cause a loss of membrane fluidity and may be associated with lipid raft assembly and function[58]. These lipid rafts are cholesterol-rich microdomains at the host cell surface and are required for NF-κB-dependent responses to H. pylori[59]. Recently, the results of several studies have suggested that n-3 PUFAs can be converted into bioactive mediators, including resolvins, that have inflammation-resolving properties via counter-regulation of lipid mediators such as pro-inflammatory LTs and PGs[52,57]. Correia et al[60] conducted experiments that showed that docosahexaenoic acid (DHA) significantly inhibited H. pylori growth both in vitro and in vivo in a dose-dependent manner and decreased mouse gastric mucosa inflammation. These results suggested that DHA could be used as an adjunct agent in H. pylori eradication treatment. In contrast, Meier et al[61] showed that an n-3 PUFA-containing eradication regimen failed to show any benefit when compared to a conventional eradication regimen. Thus, our group investigated the long-term treatment of n-3 PUFAs in an H. pylori-infected animal model and found that long-term administration of n-3 PUFAs ameliorated H. pylori-induced gastric inflammation, atrophied gastritis, and attenuated the incidence of H. pylori-associated gastric carcinogenesis. Kuriki et al[62] conducted a clinical investigation of the association between gastric cancer risk and the erythrocyte composition of DHA using 179 incident gastric cancer cases and 357 non-cancer controls (matched by age, sex, and season of sample collection). The study authors found that the erythrocyte composition of DHA was negatively associated with the risk of gastric cancer, especially of well-differentiated adenocarcinoma. Detailed, randomized, controlled trials should be conducted to obtain strong evidence for the incorporation of nutraceuticals, including n-3 PUFAs, into the therapeutic armamentarium in near future, as their use as therapeutic agents for GI disorders is moving rapidly into clinical settings and scientific studies are providing mechanisms of action to explain the therapeutic effects.
Probiotics and microbiota
Probiotics such as non-pathogenic microbial feed or food supplements are already being widely studied in the treatment of GI diseases including irritable bowel syndrome, inflammatory bowel disease, sever acute pancreatitis, and chronic liver diseases[63-66]. The use of probiotics in the treatment of GI infections is gaining traction as an alternative or complement to antibiotics due to their potential to decrease the use of antibiotics or reduce their side effects[67]. Results of clinical trials combining the use of agents for first-line eradication and adjunctive probiotics have been reported to increase the H. pylori eradication rate[68-70]. Moreover, emerging evidence shows that probiotics attenuate H. pylori infection rates and associated inflammation. The results of several in vitro studies have shown that Lactobacillus can ameliorate H. pylori-induced inflammation by modulating cytokine induction, activating suppressor of cytokine signaling (SOCS) expression, and inactivating the JAK2, Smad7 and NF-κB signaling pathways[71-73]. Twelve human studies have investigated the efficacy of combinations of antibiotics and probiotics, whereas 16 studies used probiotics alone as an alternative to antibiotics for the treatment of H. pylori infection. Most of the studies showed an improvement of H. pylori gastritis and decreases in H. pylori colonization after probiotic administration. None of the studies, however, could demonstrate complete eradication of H. pylori infection by probiotic treatment[67,74]. It should be noted, however, that one of the well-documented advantages of probiotic combinations was a reduction in adverse effects induced by H. pylori eradication treatment[75]. Since long-term intake of products containing probiotic strains may have a favorable effect on H. pylori infection in humans, particularly by reducing the risk of developing disorders associated with high degrees of gastric inflammation, it is possible that they contributed ultimately to chemoprevention. Recent advances in high throughput analysis technology have highlighted the importance of probiotics in H. pylori infection as well as other GI diseases involving “microbiota” as key controllers of H. pylori infection. The human organism is colonized by a large number of microorganisms that play important roles in several biochemical reactions. The microorganisms that colonize the human GI tract are collectively described as microbiota and a typical human may carry over 40 × 103 bacterial species in the intestinal microbiome[76]. The microbiota of the human stomach and its influence on H. pylori colonization has been characterized. Most phylotypes belong to the phyla Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes and Fusobacteria. Lactobacillus species are acid-resistant and commensal and their concentrations in the normal human stomach vary between 0 and 103 mL-1. The human microbiome co-evolved with mankind, is part of human physiology, and contributes to homeostasis. Although microbiota–host interactions through metabolic exchange and co-metabolism of substrates, or metabolome–metabolome interactions are still poorly understood, they may be implicated in the etiology of many human diseases including H. pylori infection. Therefore, the advantages attributed to probiotics in H. pylori infection, such as augmentation of the eradication rate, attenuation of side effects associated with eradication drugs, and some direct anti-inflammatory action, may represent only a small part of their involvement. Extensive investigation of the microbiota relevant to H. pylori infection will be required to elucidate additional mechanisms and relationships.
Mesenchymal stem cells
Although gastric epithelial stem cells have been localized, little is known about their molecular biology. Recent reports described the use of inducible Cre recombinase activity to indelibly label candidate stem cells and their progeny in the distal stomach[77]. H. pylori-induced chronic inflammation affects differentiation and promotes metaplasias, in which cellular and molecular mechanisms in spasmolytic polypeptide-expressing TFF2 pseudo-pyloric metaplasia predominates. The identification of signaling pathways and events that take place during embryonic development that eventually establish adult stem cells to maintain the specific features and functions of the stomach mucosa have elucidated how gastric epithelial stem cells contribute to either good regeneration, such as healing or rejuvenation, or bad regeneration, such as carcinogenesis. For example, because bone marrow-derived mesenchymal stem cells [BM-Mesenchymal stem cells (MSCs)] are known to play an important role in H. pylori-induced gastric carcinogenesis, Lin et al[78] transplanted BM-MSCs into the stomach of mice with a 44 wk mouse-adapted H. pylori infection. Study results revealed that transplantation of BM-MSCs into a chronic H. pylori-infected mouse led to an immunosuppressive environment such that stem cells fostered an environment compatible with the development of H. pylori-induced gastric cancer. Similarly, recent investigations into gastric stem cell or progenitor cell biology have uncovered valuable information for understanding gastric gland renewal and maintenance of homeostasis relevant to H. pylori infection. Ding and Zheng[79] provided clues for further defining the mechanisms by which gastric cancer may originate and progress. Using Lgr5, villin-promoter, TFF2-mRNA, and Mist, all of which are factors identified as gastric stem/progenitor cell markers, they explored how H. pylori or chronic inflammation affected gastric stem cells or their progenitors which give rise to mucus-, acid-, pepsinogen-, and hormone-secreting cell lineages. From their study results, they concluded that H. pylori infection induced oncogenic transformation and propagation into tumors based on the tumor microenvironment. In his recent publication, Peek stated that chronic H. pylori infection led to DNA damaged stem cells, a condition which could have severe negative consequences[80]. In detail, H. pylori-infected rodents that developed dysplasia harbored a subset of gastric epithelial cells in which levels of spermidine oxidase (SMO) production and DNA damage were high, but which were resistant to apoptosis, thereby representing a cellular population poised for neoplastic transformation targeted for gastric stem cells. In contrast to the results of these harmful interventions using gastric stem cells in H. pylori-associated gastric carcinogenesis, we found that exogenous stem cells could provide options for cancer prevention and intervention, as MSCs were able to rejuvenate atrophic gastritis into non-atropic condition and significantly ameliorate H. pylori-induced gastritis. Because gastric stem cells can have positive or negative effects dependent upon how they are used, further experimentation will be necessary to advance our understanding of stem cell properties in H. pylori infection, as well as the potential for rejuvenation of H. pylori-infection-associated chronic atrophic gastritis with or without intestinal metaplasia.
Antioxidants
H. pylori leads to chronic inflammation which in turn leads to oxidative stress derived from immune cells and gastric epithelial cells and is one of the main contributors to DNA damage associated with apoptosis and neoplastic transformation[81]. Both pathogen and host factors contribute directly to oxidative stress, including H. pylori virulence factors, and pathways involving DNA damage and repair, polyamine synthesis and metabolism, and oxidative stress responses. As previously mentioned, polyamine oxidation by SMO causes H2O2 release, DNA damage and apoptosis, and subsequent gastric transformation[82,83]. Since many studies reporting the potential contribution of oxidative stress and chronic inflammation to H. pylori-associated gastric carcinogenesis, antioxidants can provide enough hope for cancer prevention. H. pylori-associated inflammation can induce DNA damage due to oxygen radicals by persistent inflammatory cell infiltrations in the gastric mucosa, which may lead to alterations of the gene and result in the development of diffuse-type carcinoma. In order to elucidate the influence of H. pylori on changes in inflammation-related DNA damage, Hahm et al[84] measured the sequential changes of the 8-hydroxydeoxyguanosine (8-OHdG) content of DNA and changes of two biomarkers, inducible nitric oxide synthase (iNOS) and apoptosis, from human gastric mucosa according to the status of H. pylori. The increased levels of oxidative DNA damage, increased occurrences of apoptosis, and increased expressions of iNOS seemed to provide the mechanistic links between H. pylori infection and gastric carcinogenesis. In a subsequent study, we treated H. pylori-associated chronic atrophic gastritis with an antioxidative drug, rebamipide, and found that it contributed to either augmentation of the eradication rate or a significant decrement of 8-OHdG content[85]. Diseases associated with free radical overproduction are provoked by “blazed reactive oxygen species productions” far beyond the host’s capacity to quench. Free radicals have been implicated in the pathogenesis of diverse GI diseases including gastroesophageal reflux disease, gastritis, enteritis, colitis, and associated cancers, as well as pancreatitis and liver cirrhosis[86]. Antioxidants administered in a nutritional way or via pills will surely contribute to the amelioration of H. pylori-associated gastric catcinogenesis. However, additional proof of concept evidence is required.
CONCLUSION
Gastric cancer is a multi-factorial and multi-step disease associated with a variety of risk factors including environmental and pathogenic microbial chronic inflammation. In addition to life-style factors, especially diet, infection with the pathogenic microorganism H. pylori is a major concern for gastroenterologists because H. pylori infection causes chronic atrophic gastritis and peptic ulcer with an inflammatory response. Unfortunately, modern medicine cannot completely prevent gastric cancer and even eradication of H. pylori is problematic due to expense and antibiotic resistance, as well as insufficient evidence supporting a rationale for eradication. However, the Japanese government decided to take on the great challenge of H. pylori-associated chronic gastritis by including its eradication in their guideline this year in an attempt to decrease gastric cancer incidence and mortality. Until such time as proof emerges supporting the concept that H. pylori eradication is the fastest means of preventing gastric cancer, the attenuation or intervention of H. pylori-induced chronic inflammation may be alternative or complementary methods to achieve the prevention of gastric cancer. As shown in this review, the inhibitors of COX and LOX, a number of natural phytochemicals, including curcumin and broccoli sprouts (sulforaphane), oils such as omgega-3 PUFAs, probiotics, and stem cells have been shown to have anti-inflammatory and anti-microbial activities by targeting small molecules or regulating signaling cascades (Figure 3). Pharmacotherapy and the eradication strategy can provide rapid relief of acute inflammation but cannot correct the underlying cause of chronic inflammation. However, a non-microbial approach for modulating H. pylori-associated gastric inflammation may be an attractive and fast way to optimize cancer preventive strategies and minimize adverse side effects associated with therapeutic regimens.
Figure 3.
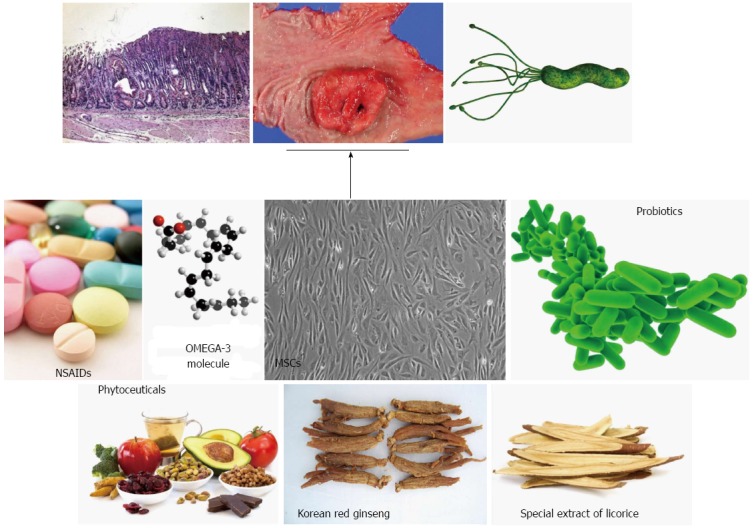
A non-microbial approach for Helicobacter pylori-associated gastritis as well as gastric cancer. Simply removing Helicobacter pylori (H. pylori) can contribute to gastric cancer prevention in some patients. For example, H. pylori eradication suppressed the metachronous occurrence of gastric cancer in patients who underwent endoscopic submucosal dissection, whereas insignificant outcomes were noted in general eradication. Supplementation or treatment with long-term phytoceuticals or other agents were proven to be very efficacious in the prevention of H. pylori-associated gastric carcinogenesis. These treatment strategies are supported by the clear mechanisms of anti-inflammation, anti-oxidation, and anti-mutagenesis associated with their use.
Footnotes
Supported by A Grant from the Ministry of Education and Science Technology, No. 2009-0081758, South Korea
P- Reviewers: Aurello P, Chow WK, Xu WX S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [Google Scholar]
- 3.Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 6.Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther. 2011;34:1255–1268. doi: 10.1111/j.1365-2036.2011.04887.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka T, Aoyama N, Sakai K, Miwa Y, Kudo M, Kawashima J, Matsubara Y, Miwa J, Yakabi K. A large-scale nationwide multicenter prospective observational study of triple therapy using rabeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in Japan. J Gastroenterol. 2012;47:276–283. doi: 10.1007/s00535-011-0487-6. [DOI] [PubMed] [Google Scholar]
- 8.Dai WQ, Zhou YQ, Xu L, Wang BF, Fan XM, Wu JY, Wang CY, Xu XF, Guo CY. The eradicating Helicobacter pylori infection in duodenal ulcer patients by three short-term triple therapies in China: a multicenter clinical comparative study. Hepatogastroenterology. 2012;59:296–299. doi: 10.5754/hge11241. [DOI] [PubMed] [Google Scholar]
- 9.Karatapanis S, Georgopoulos SD, Papastergiou V, Skorda L, Papantoniou N, Lisgos P, Kouvidou C, Fragkou P, Mentis A. “7, 10 and 14-days rabeprazole-based standard triple therapies for H. pylori eradication: are they still effective? A randomized trial”. Acta Gastroenterol Belg. 2011;74:407–412. [PubMed] [Google Scholar]
- 10.Mansour NM, Hashash JG, El-Halabi M, Ghaith O, Maasri K, Sukkarieh I, Malli A, Sharara AI. A randomized trial of standard-dose versus half-dose rabeprazole, clarithromycin, and amoxicillin in the treatment of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2011;23:865–870. doi: 10.1097/MEG.0b013e3283496502. [DOI] [PubMed] [Google Scholar]
- 11.Calvet X, García N, López T, Gisbert JP, Gené E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:603–609. doi: 10.1046/j.1365-2036.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim BG, Lee DH, Ye BD, Lee KH, Kim BW, Kim SG, Kim SW, Kim SK, Kim JJ, Kim HY, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007;12:31–35. doi: 10.1111/j.1523-5378.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 13.Abadi AT, Taghvaei T, Mobarez AM, Carpenter BM, Merrell DS. Frequency of antibiotic resistance in Helicobacter pylori strains isolated from the northern population of Iran. J Microbiol. 2011;49:987–993. doi: 10.1007/s12275-011-1170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamade M, Sugimoto M, Uotani T, Nishino M, Kodaira C, Furuta T. Resistance of Helicobacter pylori to quinolones and clarithromycin assessed by genetic testing in Japan. J Gastroenterol Hepatol. 2011;26:1457–1461. doi: 10.1111/j.1440-1746.2011.06815.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Kim N, Park HK, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, et al. [Primary antibiotic resistance of Helicobacter pylori strains and eradication rate according to gastroduodenal disease in Korea] Korean J Gastroenterol. 2011;58:74–81. doi: 10.4166/kjg.2011.58.2.74. [DOI] [PubMed] [Google Scholar]
- 16.Lee BH, Kim N, Hwang TJ, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010;15:38–45. doi: 10.1111/j.1523-5378.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- 17.Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33–45. doi: 10.1159/000350719. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol. 2013;25:1134–1140. doi: 10.1097/MEG.0b013e3283633b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zullo A, De Francesco V, Hassan C, Ridola L, Repici A, Bruzzese V, Vaira D. Modified sequential therapy regimens for Helicobacter pylori eradication: a systematic review. Dig Liver Dis. 2013;45:18–22. doi: 10.1016/j.dld.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Liou JM, Chen CC, Chang CY, Chen MJ, Fang YJ, Lee JY, Chen CC, Hsu SJ, Hsu YC, Tseng CH, et al. Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2013;68:450–456. doi: 10.1093/jac/dks407. [DOI] [PubMed] [Google Scholar]
- 21.Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205–213. doi: 10.1016/S0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 22.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–2702. [PubMed] [Google Scholar]
- 23.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 24.Chun KS, Kim EH, Lee S, Hahm KB. Chemoprevention of gastrointestinal cancer: the reality and the dream. Gut Liver. 2013;7:137–149. doi: 10.5009/gnl.2013.7.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S, Han SU, Lee KM, Park KH, Cho SW, Hahm KB. 5-LOX inhibitor modulates the inflammatory responses provoked by Helicobacter pylori infection. Helicobacter. 2007;12:49–58. doi: 10.1111/j.1523-5378.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 26.Wallace JL, McKnight GW, Keenan CM, Byles NI, MacNaughton WK. Effects of leukotrienes on susceptibility of the rat stomach to damage and investigation of the mechanism of action. Gastroenterology. 1990;98:1178–1186. doi: 10.1016/0016-5085(90)90331-t. [DOI] [PubMed] [Google Scholar]
- 27.Gyömber E, Vattay P, Szabo S, Rainsford KD. Effect of lipoxygenase inhibitors and leukotriene antagonists on acute and chronic gastric haemorrhagic mucosal lesions in ulcer models in the rat. J Gastroenterol Hepatol. 1996;11:922–927. [PubMed] [Google Scholar]
- 28.Fiorucci S, Meli R, Bucci M, Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol. 2001;62:1433–1438. doi: 10.1016/s0006-2952(01)00747-x. [DOI] [PubMed] [Google Scholar]
- 29.Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–509. doi: 10.1136/ard.62.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendum D, Masliah J, Trugnan G, Fléjou JF. Cyclooxygenase-2 and its role in colorectal cancer development. Virchows Arch. 2004;445:327–333. doi: 10.1007/s00428-004-1105-2. [DOI] [PubMed] [Google Scholar]
- 31.Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ, Mukherjee P. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol. 2004;11:328–339. doi: 10.1245/aso.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Patel S, Chiplunkar S. Role of cyclooxygenase-2 in tumor progression and immune regulation in lung cancer. Indian J Biochem Biophys. 2007;44:419–428. [PubMed] [Google Scholar]
- 33.Ozuysal S, Bilgin T, Ozgur T, Celik N, Evrensel T. Expression of cyclooxygenase-2 in ovarian serous carcinoma: correlation with angiogenesis, nm23 expression and survival. Eur J Gynaecol Oncol. 2009;30:640–645. [PubMed] [Google Scholar]
- 34.Chen Z, Liu M, Liu X, Huang S, Li L, Song B, Li H, Ren Q, Hu Z, Zhou Y, et al. COX-2 regulates E-cadherin expression through the NF-κB/Snail signaling pathway in gastric cancer. Int J Mol Med. 2013;32:93–100. doi: 10.3892/ijmm.2013.1376. [DOI] [PubMed] [Google Scholar]
- 35.Tseng YC, Tsai YH, Tseng MJ, Hsu KW, Yang MC, Huang KH, Li AF, Chi CW, Hsieh RH, Ku HH, et al. Notch2-induced COX-2 expression enhancing gastric cancer progression. Mol Carcinog. 2012;51:939–951. doi: 10.1002/mc.20865. [DOI] [PubMed] [Google Scholar]
- 36.Bartchewsky W, Martini MR, Masiero M, Squassoni AC, Alvarez MC, Ladeira MS, Salvatore D, Trevisan M, Pedrazzoli J, Ribeiro ML. Effect of Helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand J Gastroenterol. 2009;44:153–161. doi: 10.1080/00365520802530853. [DOI] [PubMed] [Google Scholar]
- 37.Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- 38.Tan VP, Wong BC. Gastric cancer chemoprevention: the current evidence. Gastroenterol Clin North Am. 2013;42:299–316. doi: 10.1016/j.gtc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 40.Tanigawa T, Watanabe T, Higuchi K, Tominaga K, Fujiwara Y, Oshitani N, Tarnawski AS, Arakawa T. Long-term use of nonsteroidal anti-inflammatory drugs normalizes the kinetics of gastric epithelial cells in patients with Helicobacter pylori infection via attenuation of gastric mucosal inflammation. J Gastroenterol. 2009;44 Suppl 19:8–17. doi: 10.1007/s00535-008-2287-1. [DOI] [PubMed] [Google Scholar]
- 41.Futagami S, Kawagoe T, Horie A, Shindo T, Hamamoto T, Suzuki K, Kusunoki M, Miyake K, Gudis K, Tsukui T, et al. Celecoxib inhibits apurinic/apyrimidinic endonuclease-1 expression and prevents gastric cancer in Helicobacter pylori-infected mongolian gerbils. Digestion. 2008;78:93–102. doi: 10.1159/000167978. [DOI] [PubMed] [Google Scholar]
- 42.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 43.Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071–1073. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 44.Andersohn F, Schade R, Suissa S, Garbe E. Cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs and the risk of ischemic stroke: a nested case-control study. Stroke. 2006;37:1725–1730. doi: 10.1161/01.STR.0000226642.55207.94. [DOI] [PubMed] [Google Scholar]
- 45.Lee KW, Bode AM, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer. 2011;11:211–218. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]
- 46.Foryst-Ludwig A, Neumann M, Schneider-Brachert W, Naumann M. Curcumin blocks NF-kappaB and the motogenic response in Helicobacter pylori-infected epithelial cells. Biochem Biophys Res Commun. 2004;316:1065–1072. doi: 10.1016/j.bbrc.2004.02.158. [DOI] [PubMed] [Google Scholar]
- 47.Kundu P, De R, Pal I, Mukhopadhyay AK, Saha DR, Swarnakar S. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS One. 2011;6:e16306. doi: 10.1371/journal.pone.0016306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai XZ, Wang J, Li XD, Wang GL, Liu FN, Cheng MS, Li F. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther. 2009;8:1360–1368. doi: 10.4161/cbt.8.14.8720. [DOI] [PubMed] [Google Scholar]
- 50.Lüer S, Troller R, Aebi C. Antibacterial and antiinflammatory kinetics of curcumin as a potential antimucositis agent in cancer patients. Nutr Cancer. 2012;64:975–981. doi: 10.1080/01635581.2012.713161. [DOI] [PubMed] [Google Scholar]
- 51.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2009;2:353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 54.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 55.Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, Peek RM, Blanke SR, Chen LF. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–1249. doi: 10.1038/embor.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 57.Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 58.Siddiqui RA, Harvey KA, Zaloga GP, Stillwell W. Modulation of lipid rafts by Omega-3 fatty acids in inflammation and cancer: implications for use of lipids during nutrition support. Nutr Clin Pract. 2007;22:74–88. doi: 10.1177/011542650702200174. [DOI] [PubMed] [Google Scholar]
- 59.Hutton ML, Kaparakis-Liaskos M, Turner L, Cardona A, Kwok T, Ferrero RL. Helicobacter pylori exploits cholesterol-rich microdomains for induction of NF-kappaB-dependent responses and peptidoglycan delivery in epithelial cells. Infect Immun. 2010;78:4523–4531. doi: 10.1128/IAI.00439-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Correia M, Michel V, Matos AA, Carvalho P, Oliveira MJ, Ferreira RM, Dillies MA, Huerre M, Seruca R, Figueiredo C, et al. Docosahexaenoic acid inhibits Helicobacter pylori growth in vitro and mice gastric mucosa colonization. PLoS One. 2012;7:e35072. doi: 10.1371/journal.pone.0035072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meier R, Wettstein A, Drewe J, Geiser HR. Fish oil (Eicosapen) is less effective than metronidazole, in combination with pantoprazole and clarithromycin, for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2001;15:851–855. doi: 10.1046/j.1365-2036.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 62.Kuriki K, Wakai K, Matsuo K, Hiraki A, Suzuki T, Yamamura Y, Yamao K, Nakamura T, Tatematsu M, Tajima K. Gastric cancer risk and erythrocyte composition of docosahexaenoic acid with anti-inflammatory effects. Cancer Epidemiol Biomarkers Prev. 2007;16:2406–2415. doi: 10.1158/1055-9965.EPI-07-0655. [DOI] [PubMed] [Google Scholar]
- 63.Penner R, Fedorak RN, Madsen KL. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr Opin Pharmacol. 2005;5:596–603. doi: 10.1016/j.coph.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Capurso G, Marignani M, Piciucchi M, Merola E, Delle Fave G. Probiotics and severe acute pancreatitis. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 1:S148–S151. doi: 10.1097/MCG.0b013e318169e935. [DOI] [PubMed] [Google Scholar]
- 65.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel A, Shah N, Prajapati JB. Clinical appliance of probiotics in the treatment of Helicobacter pylori infection-A brief review. J Microbiol Immunol Infect. 2013:Jun 8; Epub ahead of print. doi: 10.1016/j.jmii.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Kim MN, Kim N, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Kim JS, Jung HC, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13:261–268. doi: 10.1111/j.1523-5378.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 69.Franceschi F, Cazzato A, Nista EC, Scarpellini E, Roccarina D, Gigante G, Gasbarrini G, Gasbarrini A. Role of probiotics in patients with Helicobacter pylori infection. Helicobacter. 2007;12 Suppl 2:59–63. doi: 10.1111/j.1523-5378.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 70.Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther. 2006;23:1077–1086. doi: 10.1111/j.1365-2036.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- 71.Ryan KA, O’Hara AM, van Pijkeren JP, Douillard FP, O’Toole PW. Lactobacillus salivarius modulates cytokine induction and virulence factor gene expression in Helicobacter pylori. J Med Microbiol. 2009;58:996–1005. doi: 10.1099/jmm.0.009407-0. [DOI] [PubMed] [Google Scholar]
- 72.Lee JS, Paek NS, Kwon OS, Hahm KB. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: a novel mechanism. J Gastroenterol Hepatol. 2010;25:194–202. doi: 10.1111/j.1440-1746.2009.06127.x. [DOI] [PubMed] [Google Scholar]
- 73.Yang YJ, Chuang CC, Yang HB, Lu CC, Sheu BS. Lactobacillus acidophilus ameliorates H. pylori-induced gastric inflammation by inactivating the Smad7 and NFκB pathways. BMC Microbiol. 2012;12:38. doi: 10.1186/1471-2180-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilhelm SM, Johnson JL, Kale-Pradhan PB. Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother. 2011;45:960–966. doi: 10.1345/aph.1Q104. [DOI] [PubMed] [Google Scholar]
- 75.Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25:155–168. doi: 10.1111/j.1365-2036.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 76.Vítor JM, Vale FF. Alternative therapies for Helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol. 2011;63:153–164. doi: 10.1111/j.1574-695X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 77.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin R, Ma H, Ding Z, Shi W, Qian W, Song J, Hou X. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev. 2013;22:2836–2848. doi: 10.1089/scd.2013.0166. [DOI] [PubMed] [Google Scholar]
- 79.Ding SZ, Zheng PY. Helicobacter pylori infection induced gastric cancer; advance in gastric stem cell research and the remaining challenges. Gut Pathog. 2012;4:18. doi: 10.1186/1757-4749-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peek RM. Chronic Helicobacter pylori infection and DNA-damaged stem cells: a recipe for disaster. Dig Dis Sci. 2013;58:13–16. doi: 10.1007/s10620-012-2466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT. Chronic inflammation and oxidative stress: The smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes. 2013:Jun 28; Epub ahead of print. doi: 10.4161/gmic.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 83.Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141:1696–708.e1-2. doi: 10.1053/j.gastro.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hahm KB, Lee KJ, Kim JH, Cho SW, Chung MH. Helicobacter pylori infection, oxidative DNA damage, gastric carcinogenesis, and reversibility by rebamipide. Dig Dis Sci. 1998;43:72S–77S. [PubMed] [Google Scholar]
- 85.Hahm KB, Song YJ, Oh TY, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Han SU, Nahm KT, et al. Chemoprevention of Helicobacter pylori-associated gastric carcinogenesis in a mouse model: is it possible? J Biochem Mol Biol. 2003;36:82–94. doi: 10.5483/bmbrep.2003.36.1.082. [DOI] [PubMed] [Google Scholar]
- 86.Kim YJ, Kim EH, Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol. 2012;27:1004–1010. doi: 10.1111/j.1440-1746.2012.07108.x. [DOI] [PubMed] [Google Scholar]


