Abstract
AIM: To investigate the safety and effectiveness of combined 131I-metuximab and transcatheter arterial chemoembolization (TACE) for hepatocellular carcinoma (HCC).
METHODS: One hundred and eighty-five patients (159 men and 26 women) with advanced HCC were enrolled in this study from February 2009 to July 2011. There were 95 patients in the combined metuximab and TACE group, and 90 patients in the TACE only group. The patients were followed for 12 mo. Clinical symptoms, blood cell counts, Karnofsky Performance Score (KPS) evaluation and therapeutic effects according to the Response Evaluation Criteria in Solid Tumors were recorded and evaluated.
RESULTS: The 1-mo effective rates (complete response + partial response + stable disease) of the test group and control group were 71.23% and 38.89%, respectively (P < 0.001). The 6-, 9- and 12-mo survival rates were 86.42%, 74.07% and 60.49% for the test group and 60.0%, 42.22% and 34.44% for the control group (P < 0.001). The incidence of adverse events (gastrointestinal symptoms, fever and pain) and blood cell toxicity were significantly higher for the test group than for the control group (P < 0.001). No severe 131I-metuximab-related complications were identified. With respect to efficacy, patients in the test group had greater improvement in tumor-related pain (P = 0.014) and increase in KPS (P < 0.001) than those in the control group.
CONCLUSION: Combination of 131I-metuximab and TACE prolonged the survival time in patients with HCC compared with TACE alone. The combination treatment was safe and effective.
Keywords: Hepatocellular carcinoma, 131I-metuximab, Transcatheter arterial chemoembolization, Radioimmunotherapy
Core tip: 131I-metuximab has high affinity with a target antigen highly expressed on hepatocellular carcinoma (HCC) cells and a limited area of action. The combination of metuximab and transcatheter arterial chemoembolization had a synergistic effect in the treatment of HCC. It may represent a promising treatment modality for patients with advanced HCC, especially for those patients with multiple nodules who have a heavy tumor burden.
INTRODUCTION
Hepatocellular carcinoma (HCC) has traditionally been regarded as a radioresistant tumor because external beam radiation does great harm to the surrounding normal tissue. On the contrary, since the 1980s, radioimmunotherapy has become a promising treatment modality for HCC, due to the specificity of the antibodies and the killing power of the radionuclides, resulting in improvement of clinical efficacy with fewer side effects.
A therapeutic anti-HCC radioimmunological agent, 131I-metuximab, generated by 131I labeling of the murine monoclonal antibody (mAb) fragment HAb18 F(ab’)2 derived from HAb18G/CD147, has been approved for the treatment of primary HCC by the China State Food and Drug Administration (Registration No. S20050039).
Transcatheter arterial chemoembolization (TACE) is currently one of the widely used treatment modalities for unresectable advanced HCC. However, the long-term survival rate of such patients remains low, with a reported 5-year survival rate of 17%[1]. Although 131I-metuximab monotherapy has been shown to be effective, both in the treatment of HCC and in the prevention of HCC recurrence after orthotopic liver transplantation[2], its efficacy in combination with other established treatment modalities such as TACE has seldom been tested. Theoretically, the TACE can enhance the antitumor effects of 131I-metuximab, because of substantially reduced blood flow to the tumor that prolongs retention of 131I-metuximab in the tumor tissues. Radioimmunotherapy combined with TACE may provide a new concept in radiotherapy for patients with HCC.
In this study, the safety and efficacy of 131I-metuximab in combination with TACE were evaluated in patients with advanced HCC to demonstrate that the combination of 131I-metuximab with TACE could produce better results than TACE alone.
MATERIALS AND METHODS
131I-metuximab injection
131I-metuximab injection (Licartin; Chengdu Hoist Hitech Co. Ltd., Chengdu, China) is an 131I-labeled HAb18 F(ab’)2 fragment of murine mAb against the HCC-associated antigen HAb18G/CD147. Before metuximab therapy, 0.5 mL of iodine solution should be taken orally tid for 3 d and continued for 7 d after treatment for thyroid protection. A vial of 131I-metuximab injection solution that had been prepared at the standard dose of 0.75 mCi/kg was removed from a lead box containing ice at a temperature of 0 °C. The thawed solution was diluted with 1 mL of saline and sucked into a 5-mL syringe for arterial injection.
Patient cohort
One hundred and eighty-five patients (159 men and 26 women, aged 12-87 years) with advanced unresectable HCC were enrolled in this study from February 2009 to July 2011. Patients with a Karnofsky Performance Score (KPS) < 60 or severe heart, kidney of hematological disease were excluded to ensure at least a 3-mo lifespan in the enrolled patients, so as to have enough time for follow-up. Patients with a history of allergy to biological products, pregnant or breast-feeding women, or patients receiving other therapies within 4 wk of the clinical trial were also excluded. All patients in our study gave written informed consent. Patients in the test group underwent 131I-metuximab therapy and TACE while those in the control group received TACE only. The patients received local ethanol injection, microwave coagulation, resection or liver transplantation before and after TACE or 131I-metuximab therapy if needed. All tumors were diagnosed according to pathological examination or distinctive findings on computed tomography (CT), conventional angiography, magnetic resonance imaging (MRI), or serum tumor markers [α-fetoprotein (AFP)].
Procedure of TACE and 131I-metuximab intra-arterial injection
TACE and 131I-metuximab injection were performed through the femoral artery using the Seldinger technique with local anesthesia. Arteriography of the celiac trunk and superior mesenteric artery was performed to visualize the arterial vascularization of the liver and evaluate portal vein patency, and anticancer drugs were injected. The angiographic catheter was superselected into the hepatic artery where the target tumor was located. An embolic agent (mainly Lipiodol) was continuously injecting through the artery until the rate of blood flow to the tumor mass fell below 25%, or minimal hepatic vein appeared to protect the liver tissues around the tumor so that Licartin is more liable to stay in tumor tissues by minimizing the effect of quick or slow blood flow. Patients in the test group underwent 131I-metuximab therapy immediately after TACE. At each injection, 131I-metuximab was administered at a dose of 0.75 mCi/kg according to the patient’s weight and the intra-arterial injection usually lasted 1-2 min. Patients in the control group received TACE only. In both groups, the dose of Lipiodol, ranging from 3-20 mL, was determined according to the size and number of tumors and functional hepatic reserve. Anticancer drugs for each patient enrolled in this trial were 5-fluorouracil (800-1000 mg) and epirubicin-adriamycin (30-40 mg) according to the body surface area. Therapy for patients in both groups was repeated according to the patient’s clinical condition and the iconography exams at a 1-6-mo interval.
Follow-up protocol and efficiency evaluation
Clinical symptoms, blood cell counts and KPS evaluation were recorded before and after treatment. After treatment, ultrasound, CT scan or MRI was performed every 1-3 mo, with or without contrast enhancement, to evaluate the features of Lipiodol deposit and the therapeutic effect according to the Response Evaluation Criteria in Solid Tumors (RECIST). If elevated tumor marker (AFP), diminished Lipiodol, enlarged lesions or new nodules were observed, the patients were readmitted for angiography and treatment. The starting point of survival analysis was regulated as the day of initial treatment. The Kaplan-Meier method was used to analyze the survival rates in the two groups.
Statistical analysis
The primary endpoint of this study was overall survival and the secondary endpoint was short-term (1 mo) treatment response. Survival analysis was estimated by the Kaplan-Meier method. Survival probabilities were estimated using the life-table method, and between-group differences in survival rates were compared using the log-rank test. All statistical analyses were carried out with SPSS version 17.0 (SPSS, Chicago, IL, United States). All reported P values were two-sided, with P < 0.05 considered statistically significant.
RESULTS
Patient population
The patients were divided into the test group (n = 95) with a mean age of 50.2 years (range: 22-80 years) and the control group (n = 90) with a mean age of 51.4 years (range: 12-87 years). All the patients in this trial were classified as Barcelona Clinic Liver Cancer Stage C. Both the test group and control group had a high percentage of patients (89.47% and 85.56%, respectively) with a tumor/liver volume ratio > 50%. Thus, the patients enrolled in this clinical trial had advanced HCC. Although this was a nonrandomized prospective cohort study, no significant difference was observed in baseline characteristics between the two groups (Table 1).
Table 1.
Baseline characteristics
| Characteristics | Test group (n = 95) | Control group (n = 90) | Statistical analysis |
| Age (yr) | 50.2 (22-80) | 51.4 (12-87) | NS |
| Sex (M/F) | 83/12 | 76/14 | NS |
| Child–Pugh classification | NS | ||
| Child class A | 91 | 88 | |
| Child class B | 4 | 2 | |
| BCLC stage | NS | ||
| C | 95 | 90 | |
| Size of main tumors | |||
| ≥ 5 cm | 80 | 81 | NS |
| < 5 cm | 15 | 9 | NS |
| Tumor/liver volume ratio | |||
| 0%–50% | 10 | 13 | NS |
| ≥ 50% | 85 | 77 | NS |
| Hepatitis B/C | 70 | 76 | NS |
| KPS | 75.16 ± 7.42 | 73.89 ± 11.39 | NS |
BCLC: Barcelona clinic liver cancer; KPS: Karnofsky performance status; NS: Not significant.
The patients were followed for 12 mo. At the time of analysis, 46 and 80 patients had died while 14 and zero were lost to follow-up in the test and control groups, respectively. Causes of death in the test and control groups included tumor progression in 46 and 72 patients, digestive tract hemorrhage in zero and four, tumor rupture in zero and one, acute renal failure in zero and one, and other causes in zero and two, respectively; none was possibly related to treatment.
Two hundred and forty-one (mean: 2.56 procedures) and 261 (mean: 2.90 procedures) procedures of interventional therapy were performed in the test and control groups, respectively. Arterial portal vein shunt (AVS), arterial hepatic vein shunt (APS) and/or portal vein involvement, which indicate high invasion and poor prognosis were found in 35.79% (34/95) of patients in the test group and 33.3% (30/90) of patients in the control group. No difference was observed in the time of therapy and the incidence of malignancy signs such as AVS, APS or portal vein involvement between the two groups.
Safety
The clinical symptoms were carefully recorded after treatment (Table 2). Overall, although 131I-metuximab in combination with TACE was well tolerated, the patients in the test group obviously suffered more frequent adverse events than those in the control group. The most frequent adverse event in the test group was abdominal pain. Of the 95 patients in the test group, 93 (97.89%) suffered from abdominal pain, 90 (94.74%) had fever of 37.2 °C-40 °C, which usually occurred 0.5-10 h after 131I-metuximab injection and lasted for 1-14 d, and 74 (77.89%) had anorexia and/or vomiting, which often faded away in several days. The corresponding numbers of patients in the control group were 19 (21.11%), 28 (31.11%) and 16 (17.78%) (P < 0.001). The changes in blood cell count and liver function before and 1 mo after treatment were evaluated. Statistical analysis showed that the changes in leukocytes and platelets were significant. Changes in total bilirubin, albumin, aspartate aminotransferase, alanine transaminase and hemoglobin were not significant (Table 3).
Table 2.
Clinical symptoms immediately after treatment n (%)
| Group | Fever1 | Gastrointestinal1 symptoms | Pain1 | Sudden death |
| Test group | 90 (94.74) | 74 (77.89) | 93 (97.89) | 0 |
| Control group | 28 (31.11) | 16 (17.78) | 19 (21.11) | 1 (1.11) |
P < 0.001.
Table 3.
Changes in blood cells and liver function before and 1 mo after treatment
| Group | Test group | Control group | P value |
| Leukocytes | –1.25 ± 1.79 | 2.04 ± 11.51 | 0.0270 |
| Platelets | –36.69 ± 49.62 | 12.74 ± 52.59 | < 0.001 |
| TB | 1.7 | 0.95 | 0.860 |
| Alb | –0.86 ± 6.89 | –1.30 ± 5.36 | 0.6708 |
| AST | 5 | 7.5 | 0.631 |
| ALT | –1 | 0 | 0.5137 |
| HGB | –5.86 ± 16.42 | –7.98 ± 20.26 | 0.515 |
| KPS | < 0.001 | ||
| Mass-associated pain | 0.014 |
Total bilirubin (TB), Aspertate aminotransferase (AST), Glutamic-pyruvic transaminase (ALT), Karnofsky and pain (Wilcoxon rank sum test); others (t test). Alb: Albumin; HGB: Hemoglobin; KPS: Karnofsky performance score.
One patient in the test group had hypothyroidism and was prescribed oral thyroxin, and one sudden death occurred in the control group, possibly because of liver rupture. No human anti-murine antibody immune responses, anaphylactic reaction and changes in myocardial zymograms were observed.
Efficacy
The palliative rate of mass-associated pain 1 mo after treatment was 71.1% (27/38) for patients in the test group, which was higher than that in the control group (31.6%, 24/76) (P = 0.014). For changes in KPS, the patients in the test group had a greater increase than those in the control group (P < 0.001, Table 3). The therapeutic effect was evaluated following the RECIST after treatment. Rates of complete response (CR), partial response (PR), stable disease (SD) and progressive disease in the two groups are listed in Table 4. The total effective rates (CR + PR + SD) were 71.23% and 38.89% for the test group and control group, respectively. Wilcoxon rank sum test showed that the therapeutic effects in the two groups were significantly different (P < 0.001). The survival rates at 6, 9 and 12 mo after treatment were 86.42%, 74.07% and 60.49% in the test group, and 60.0%, 42.22% and 34.44% in the control group, suggesting that the survival rates for the test group were significantly higher than for the control group (P < 0.001, Figure 1).
Table 4.
Therapeutic effect evaluated according to Response Evaluation Criteria in Solid Tumors at 1 mo after treatment n (%)
| Group | CR | PR | SD | PD | Effective rate (CR + PR + SD) |
| Test group | 4 (4.11) | 38 (39.73) | 26 (27.40) | 27 (28.77) | 68 (71.23) |
| Control group | 1 (1.11) | 14 (15.56) | 20 (22.22) | 55 (61.11) | 35 (38.89) |
P < 0.001. CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
Figure 1.
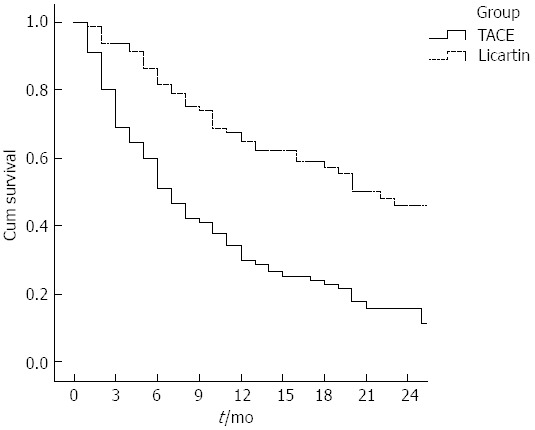
Kaplan-Meier curves of survival of patients in the test group receiving combination therapy (Licartin) and those in the control group receiving transcatheter arterial chemoembolization only (P < 0.001). TACE: Transcatheter arterial chemoembolization.
DISCUSSION
HCC is a highly malignant tumor with high morbidity and mortality rates. Although TACE, as a palliative treatment for unresectable HCC, has become one of the most common interventional therapies[3-6], its effect is limited due to the lack of appropriate and reliable embolic agents, and the infiltrative or hypovascular nature, too large or small in size[7-9]. Another limitation of TACE is the need for repeated treatment, thus resulting in deterioration of liver function[10]. Therefore, many efforts have been made to explore other new therapies in order to achieve better efficacy. Percutaneous ethanol injection, radiofrequency ablation, targeted molecular therapies, and gene therapy in combination with TACE have been found to improve survival in patients with advanced HCC[11-13] and decrease the risk of liver failure[14-16]. However, in spite of all the above, advanced HCC with wide metastasis, especially in patients with multiple lesions who always have a high tumor/liver volume ratio, still has a poor prognosis and lack of efficient therapeutic modalities.
The target antigen for 131I-metuximab, HAb18G/CD147, a member of the CD147 family, is highly expressed on HCC cells. The binding rate of HAb18 to human 7721 hepatoma cells, determined by flow cytometry, is up to 99.55%[17,18]. Immunohistochemistry performed with HAb18 showed that the positive rate of HCC staining was 75% and had no cross-reaction to normal tissues[17,19]. Moreover, the results of drug safety studies showed that 131I-metuximab injection caused no impairment to cardiovascular, respiratory, or nervous systems[2].
The mechanism by which 131I-metuximab may benefit patients with HCC has been investigated both in vitro and in vivo, as well as in clinical trials[2,17,20]. 131I-metuximab is specific to and has high affinity for a target antigen highly expressed on HCC cells. This allows for concentration of conjugated 131I in HCC tissues, both in the liver and metastatic nodules, which kills tumor cells directly. In addition, the target antigen, HAb18G/CD147, is a cell adhesion molecule with multiple functions and is closely related to tumor metastasis. This antigen is involved in the adhesion and motion of tumor cells, angiogenesis, and signal transduction and can induce fibroblasts to produce matrix metalloproteinases (MMPs), including MMP-1, MMP-2, and MMP-9. These MMPs can degrade the extracellular matrix and promote the metastasis of HCC cells. Injection of 131I-metuximab into HCC cells inhibits oncogenesis and metastasis within and outside the liver, blocking and destroying cells carrying HAb18G/CD147 and inhibiting HCC metastasis[1].
The combination of 131I-metuximab and TACE ought to have a synergistic effect in the treatment of HCC. First, TACE may enhance the efficacy of 131I-metuximab due to its arterial embolization effect, substantially reducing blood flow to the HCC and resulting in prolonged retention of 131I-metuximab in the tumor. Second, retention of the anticancer drug in the tumor may have a radiosensitizing effect on 131I-metuximab. Third, 131I-metuximab can eliminate residual cancer cells after TACE for its continuous radiation. Taken together, these mechanisms may explain, at least in part, the ability of combination therapy to enhance survival, compared with conventional TACE alone, in patients with advanced HCC. Of course, they are the reasons why the test group suffered more blood cell toxicity and adverse events.
In the present study, the results for the control group were similar to those of earlier trials of TACE in patients with HCC. In those studies, the tumor response rate according to WHO criteria ranged from 12%-57.9%[1,21-23], median survival ranged from 7-19 mo (mean: 13.63 ± 6.10 mo), and 1- and 2-year survival rates ranged from 42%-72% (mean: 58.30 ± 10.14%) and from 0%-55% (mean: 28.74% ± 15.88%), respectively[24-28]. Moreover, treatment of patients with HCC with 131I-metuximab alone resulted in 6-mo and 12-mo survival rates of 82.63% and 58.68%, respectively, with a median survival time of 19 mo and an objective response rate (CR + PR) of 15.53% according to World Health Organization (WHO) criteria[1]. In comparison, patients receiving combination therapy in our study showed 6- and 12-mo survival rates of 86.42% and 60.49%, respectively; a median survival time of 20.0 mo; and an objective response rates of 43.84% according to WHO criteria. All of these studies were performed in patients staged as Child-Pugh class A/B, and their baseline characteristics were similar to those of our patients, therefore, it can be suggested that the combination of 131I-metuximab and TACE tested here exhibited better clinical efficacy than either treatment alone.
Given the high level of expression of the high-affinity target antigen on HCC cells and the limited area of action of 131I-metuximab, we thought we would observe more advantages in our clinical trial because most of the enrolled patients had multiple nodules and a high tumor/liver volume ratio. And we did find that the median survival of patients in the test group was significantly longer than that in the control group (20.0 mo vs 7.0 mo). This improvement was more marked than that in two similar earlier trials (21.15 mo vs 17.73 mo and 26.7 mo vs 20.6 mo). The most notable difference between the present and previous studies is that our patients had more advanced HCC and a greater tumor burden (tumor/liver volume ratio ≥ 50%; 87.56% vs < 29%)[29,30].
It is commonly accepted that HCC patients with countable nodules often have more treatment choices, better treatment efficacy, and longer lifespan than those with countless nodules. Our clinical trial proved that 131I-metuximab combined with TACE had an extensive range of therapeutic function, especially for advanced liver cancer with wide metastasis and multiple lesions. The combination of 131I-metuximab and TACE may greatly improve the treatment efficacy in these patients and extend their poor life expectancy.
The present study had several limitations. First, the relatively short follow-up period may have resulted in underestimation of survival. Second, most patients were treated with combination therapy only once, a few twice and followed by TACE again. Repeated combination therapy may have a more significant effect on survival. Third, the effects of Chinese traditional medicine, which the patients used when discharged from the hospital, were uncertain. These were hard to control and might have affected the final results.
In conclusion, our findings indicate that combination of 131I-metuximab and TACE was safe and more effective than TACE alone. It may represent a promising treatment modality for patients with advanced HCC. Nevertheless, caution should be exercised, and a few questions remain. What is the best method for delivering 131I-metuximab with the TACE procedure? Does the 25% be the best point to administer Licartin? Whether and how much could the Licartin injected into the hepatic artery concentrate in the metastasis outside the liver? All these questions require well-designed, prospective randomized controlled trials.
COMMENTS
Background
Transcatheter arterial chemoembolization (TACE) is currently one of the widely used treatment modalities for unresectable advanced hepatocellular carcinoma (HCC). However, the long-term survival rate of such patients remains poor. At the same time, 131I-metuximab monotherapy has been shown to be effective, both in the treatment of HCC and in the prevention of HCC recurrence after orthotopic liver transplantation. However, its efficacy in combination with TACE has seldom been tested. Theoretically, TACE can enhance the antitumor effects of 131I-metuximab, because of substantially reducing blood flow to the tumor, which prolongs retention of 131I-metuximab in the tumor tissues.
Research frontiers
131I-metuximab (Licartin) has high affinity with a target antigen highly expressed on HCC cells and has a limited area of action. The combination of 131I-metuximab and chemoembolization has a synergistic effect in the treatment of HCC. It may especially benefit patients with multiple nodules who always have a high tumor/liver volume ratio.
Innovations and breakthroughs
It is commonly accepted that HCC patients with countable nodules often have better outcome than patients with countless lesions. At present, patients with multiple lesions still have a poor prognosis and lack of efficient therapeutic modalities. However, in our trial, they managed to treat the same types of patients with 131I-metuximab combined with TACE and prolonged their lifespan significantly. This improvement was more notable than in earlier similar trials. The combination of 131I-metuximab and TACE had an extensive range of therapeutic function, especially for advanced liver cancer with wide metastasis and multiple lesions. Combination therapy may greatly improve the treatment efficacy in these patients and extend their poor life expectancy.
Applications
Radioimmunotherapy combined with TACE may provide a new concept in radiotherapy for patients with HCC.
Peer review
The authors performed a study on 185 patients (159 men and 26 women) with advanced HCC. The data indicate that combination of 131I-metuximab with TACE was safe and more effective than TACE alone. The title of the paper accurately reflects the content of the article. The abstract contains a short description of the study. The abstract and article are written in accordance with the journal requirements. The introduction gives sufficient information about the research objectives. It is important that authors state their motivation to conduct this investigation. The design of the study is simple and understandable.
Footnotes
P- Reviewers: Reshetnyak VI, Vogel A S- Editor: Ma YJ L- Editor: Wang T E- Editor: Wang CH
References
- 1.Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574–1581. [PubMed] [Google Scholar]
- 2.Chen ZN, Mi L, Xu J, Song F, Zhang Q, Zhang Z, Xing JL, Bian HJ, Jiang JL, Wang XH, et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: clinical phase I/II trials. Int J Radiat Oncol Biol Phys. 2006;65:435–444. doi: 10.1016/j.ijrobp.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511–512. doi: 10.3748/wjg.v4.i6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizoe A, Yamaguchi J, Azuma T, Fujioka H, Furui J, Kanematsu T. Transcatheter arterial embolization for advanced hepatocellular carcinoma resulting in a curative resection: report of two cases. Hepatogastroenterology. 2000;47:1706–1710. [PubMed] [Google Scholar]
- 6.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 7.Qian J, Truebenbach J, Graepler F, Pereira P, Huppert P, Eul T, Wiemann G, Claussen C. Application of poly-lactide-co-glycolide-microspheres in the transarterial chemoembolization in an animal model of hepatocellular carcinoma. World J Gastroenterol. 2003;9:94–98. doi: 10.3748/wjg.v9.i1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33–37. doi: 10.3748/wjg.v4.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319–S328. doi: 10.1111/j.1440-1746.1997.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahrar K, Gupta S. Hepatic artery embolization for hepatocellular carcinoma: technique, patient selection, and outcomes. Surg Oncol Clin N Am. 2003;12:105–126. doi: 10.1016/s1055-3207(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Guan YS, Liu Y, He Q, Li X, Yang L, Hu Y, La Z. p53 gene therapy in combination with transcatheter arterial chemoembolization for HCC: one-year follow-up. World J Gastroenterol. 2011;17:2143–2149. doi: 10.3748/wjg.v17.i16.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885–1891. doi: 10.3748/wjg.v9.i9.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XM, Luo PF, Lin HH, Zhou ZJ, Shao PJ, Fu L, Li WK. Long-term result of combination of transcatheter arterial chemoembolization and percutaneous ethanol injection for treatment of hepatocellular carcinoma. Ai Zheng. 2004;23:829–832. [PubMed] [Google Scholar]
- 16.Guo WJ, Yu EX, Liu LM, Li J, Chen Z, Lin JH, Meng ZQ, Feng Y. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J Gastroenterol. 2003;9:1697–1701. doi: 10.3748/wjg.v9.i8.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Bian H, Feng Q, Mi L, Mo T, Kuang A, Tan T, Li Y, Lu W, Zhang Y, et al. Biodistribution and localization of iodine-131-labeled metuximab in patients with hepatocellular carcinoma. Cancer Biol Ther. 2006;5:318–322. doi: 10.4161/cbt.5.3.2431. [DOI] [PubMed] [Google Scholar]
- 18.Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003;54:371–389. doi: 10.1016/s0070-2153(03)54015-7. [DOI] [PubMed] [Google Scholar]
- 19.Jin C, Yang W, Bai L, Wang J, Dou K. Preparation and characterization of targeted DOX-PLGA-PEG micelles decorated with bivalent fragment HAb18 F(ab’)2 for treatment of hepatocellular carcinoma. J Control Release. 2011;152 Suppl 1:e14–e15. doi: 10.1016/j.jconrel.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 20.Raoul JL, Guyader D, Bretagne JF, Heautot JF, Duvauferrier R, Bourguet P, Bekhechi D, Deugnier YM, Gosselin M. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 21.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 23.Yamada R, Kishi K, Sonomura T, Tsuda M, Nomura S, Satoh M. Transcatheter arterial embolization in unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:135–139. doi: 10.1007/BF02575464. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura H, Hashimoto T, Oi H, Sawada S, Furui S, Mizumoto S, Monden M. Treatment of hepatocellular carcinoma by segmental hepatic artery injection of adriamycin-in-oil emulsion with overflow to segmental portal veins. Acta Radiol. 1990;31:347–349. [PubMed] [Google Scholar]
- 25.Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74–78. doi: 10.3748/wjg.v8.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, Inaba Y, Aramaki T, Kwon SH, Yamamoto S, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24:490–500. doi: 10.1016/j.jvir.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, Van Steenbergen W, Buffet C, Rougier P, Adler M, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–134. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer. 2006;106:1653–1663. doi: 10.1002/cncr.21811. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Yang YF, Ge NJ, Shen SQ, Liang J, Wang Y, Zhou WP, Shen F, Wu MC. Hepatic arterial iodine-131-labeled metuximab injection combined with chemoembolization for unresectable hepatocellular carcinoma: interim safety and survival data from 110 patients. Cancer Biother Radiopharm. 2010;25:657–663. doi: 10.1089/cbr.2010.0801. [DOI] [PubMed] [Google Scholar]
- 30.Wu L, Yang YF, Ge NJ, Shen SQ, Liang J, Wang Y, Zhou WP, Shen F, Wu MC. Hepatic artery injection of ¹³¹I-labelled metuximab combined with chemoembolization for intermediate hepatocellular carcinoma: a prospective nonrandomized study. Eur J Nucl Med Mol Imaging. 2012;39:1306–1315. doi: 10.1007/s00259-012-2145-5. [DOI] [PubMed] [Google Scholar]


