Abstract
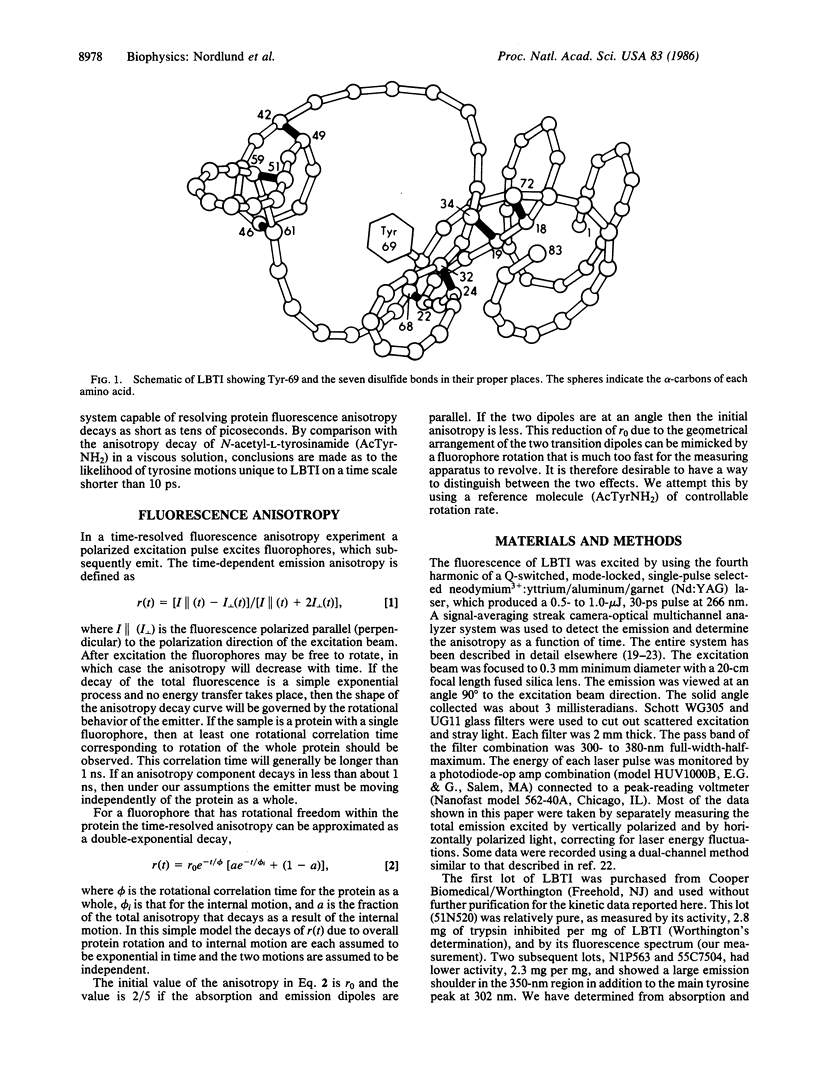
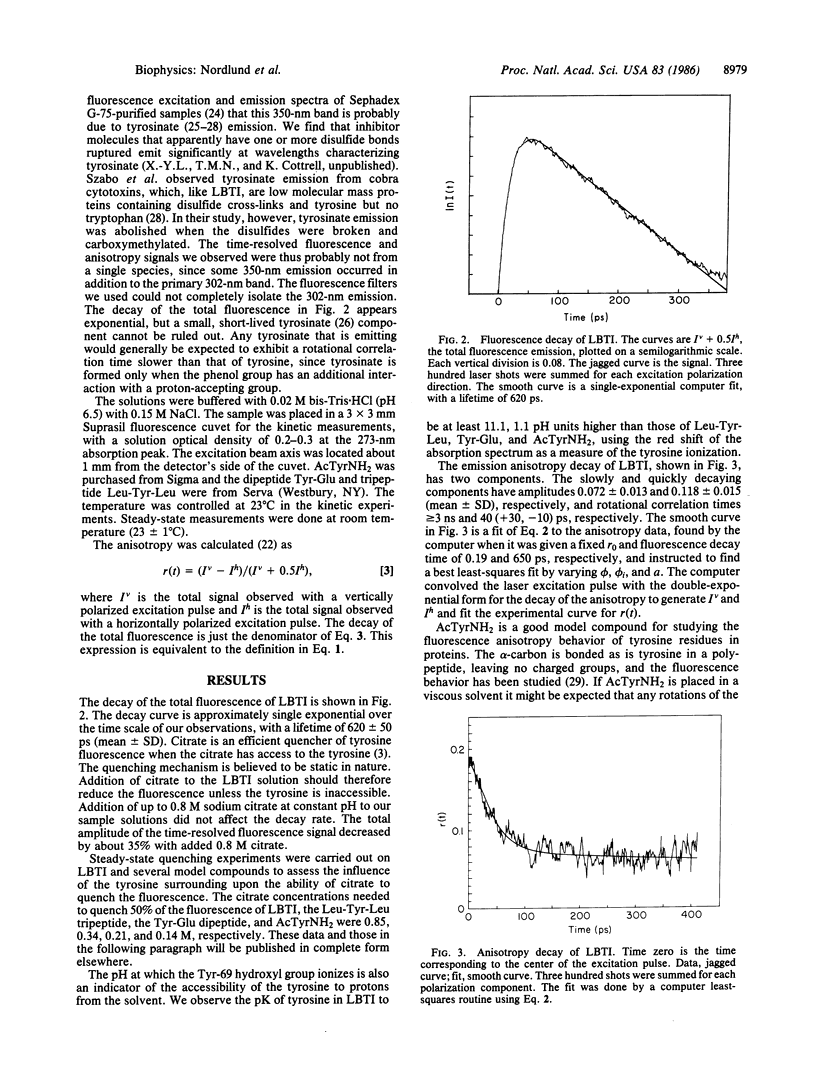
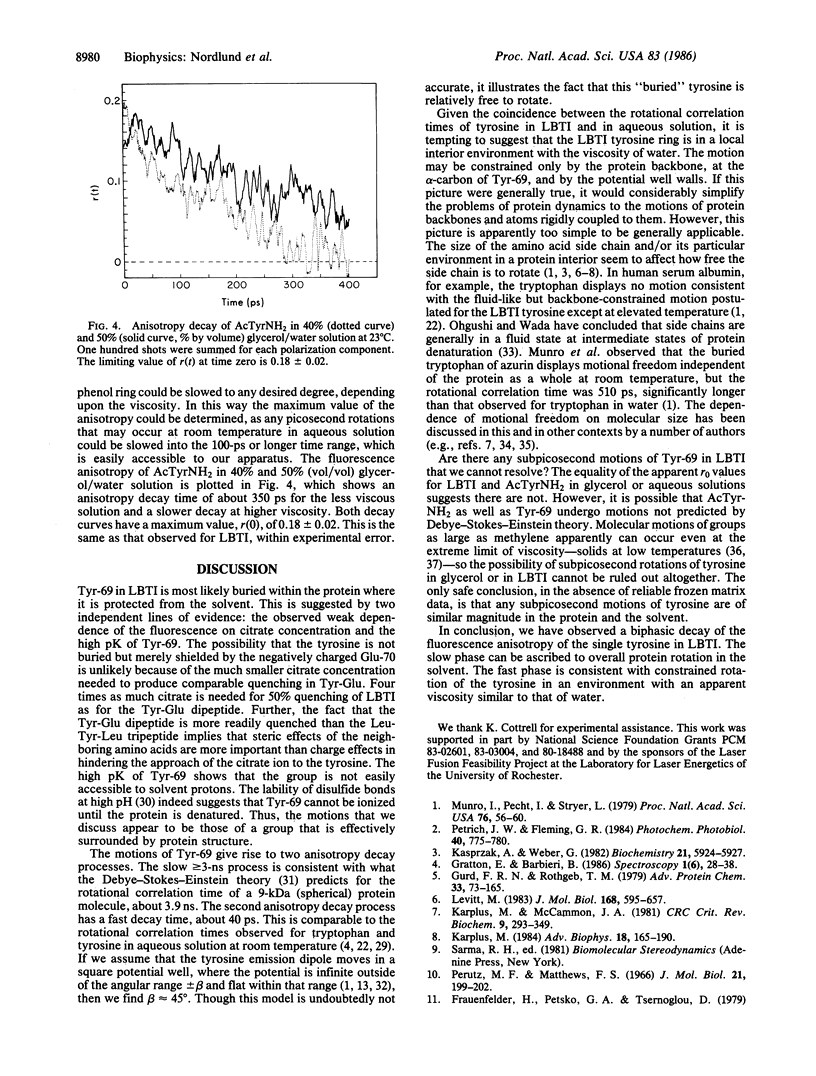
The fluorescence of lima bean trypsin inhibitor is due to a single tyrosine residue at position 69. The lifetime of this tyrosine fluorescence is 620 +/- 50 ps (mean +/- SD) and is little affected by addition of 0.88 M citrate, an efficient quencher of tyrosine fluorescence. The steady-state emission intensity is also only weakly reduced by the quencher. The tyrosine is thus not accessible to the citrate and is probably located in the interior of the protein. The high pK of the tyrosine supports this conclusion. The fluorescence anisotropy decay of the inhibitor's tyrosine can be fitted to a double exponential form, with time constants of about 40 ps and greater than or equal to 3 ns. The anisotropy at time zero is 0.19 +/- 0.015 (mean +/- SD), the same as for N-acetyl-L-tyrosinamide in viscous glycerol solution. The nanosecond component of the decay is consistent with rotation of the entire protein molecule. The 40-ps component demonstrates that the tyrosine has considerable freedom to move independently of the protein as a whole. This rotational correlation time is approximately what is observed for free tyrosine in aqueous solution. Since the polypeptide chain near tyrosine-69 is anchored by several disulfide bonds, the data argue that this interior portion of the protein consists of a rigid, immobile backbone embedded in fluid, mobile amino acid side chains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birk Y. The Bowman-Birk inhibitor. Trypsin- and chymotrypsin-inhibitor from soybeans. Int J Pept Protein Res. 1985 Feb;25(2):113–131. doi: 10.1111/j.1399-3011.1985.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Calhoun D. B., Vanderkooi J. M., Englander S. W. Penetration of small molecules into proteins studied by quenching of phosphorescence and fluorescence. Biochemistry. 1983 Mar 29;22(7):1533–1539. doi: 10.1021/bi00276a003. [DOI] [PubMed] [Google Scholar]
- Donovan J. W. Spectrophotometric titration of the functional groups of proteins. Methods Enzymol. 1973;27:525–548. doi: 10.1016/s0076-6879(73)27025-8. [DOI] [PubMed] [Google Scholar]
- Gaier J. R., Tulinsky A., Liener I. E. Formation, crystallization, and preliminary crystallographic data of the ternary complex of alpha-chymotrypsin, beta-trypsin, and the Bowman-Birk inhibitor. J Biol Chem. 1981 Nov 25;256(22):11417–11419. [PubMed] [Google Scholar]
- Gurd F. R., Rothgeb T. M. Motions in proteins. Adv Protein Chem. 1979;33:73–165. doi: 10.1016/s0065-3233(08)60459-3. [DOI] [PubMed] [Google Scholar]
- Karplus M. Dynamics of proteins. Adv Biophys. 1984;18:165–190. doi: 10.1016/0065-227x(84)90011-x. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Kasprzak A., Weber G. Fluorescence depolarization and rotational modes of tyrosine in bovine pancreatic trypsin inhibitor. Biochemistry. 1982 Nov 9;21(23):5924–5927. doi: 10.1021/bi00266a030. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Maliwal B. P. Oxygen quenching and fluorescence depolarization of tyrosine residues in proteins. J Biol Chem. 1983 Apr 25;258(8):4794–4801. [PubMed] [Google Scholar]
- Levitt M. Molecular dynamics of native protein. II. Analysis and nature of motion. J Mol Biol. 1983 Aug 15;168(3):621–657. doi: 10.1016/s0022-2836(83)80306-4. [DOI] [PubMed] [Google Scholar]
- Lipari G., Szabo A. Effect of librational motion on fluorescence depolarization and nuclear magnetic resonance relaxation in macromolecules and membranes. Biophys J. 1980 Jun;30(3):489–506. doi: 10.1016/S0006-3495(80)85109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro I., Pecht I., Stryer L. Subnanosecond motions of tryptophan residues in proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):56–60. doi: 10.1073/pnas.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgushi M., Wada A. Liquid-like state of side chains at the intermediate stage of protein denaturation. Adv Biophys. 1984;18:75–90. doi: 10.1016/0065-227x(84)90007-8. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Mathews F. S. An x-ray study of azide methaemoglobin. J Mol Biol. 1966 Oct 28;21(1):199–202. doi: 10.1016/0022-2836(66)90088-x. [DOI] [PubMed] [Google Scholar]
- Petrich J. W., Fleming G. R. Ultrafast processes in biology. Photochem Photobiol. 1984 Dec;40(6):775–780. doi: 10.1111/j.1751-1097.1984.tb04651.x. [DOI] [PubMed] [Google Scholar]
- Sommer J. H., Henry E. R., Hofrichter J. Geminate recombination of n-butyl isocyanide to myoglobin. Biochemistry. 1985 Dec 3;24(25):7380–7388. doi: 10.1021/bi00346a053. [DOI] [PubMed] [Google Scholar]
- Szabo A. G., Lynn K. R., Krajcarski D. T., Rayner D. M. Tyrosinate fluorescence maxima at 345 nm in proteins lacking tryptophan at pH 7. FEBS Lett. 1978 Oct 15;94(2):249–252. doi: 10.1016/0014-5793(78)80948-x. [DOI] [PubMed] [Google Scholar]
- Wei C. H. Crystallization of two cubic forms of soybean trypsin inhibitor E-I, a member of the Bowman-Birk inhibitor family. J Biol Chem. 1983 Aug 10;258(15):9357–9359. [PubMed] [Google Scholar]


