Abstract
The establishment of hormone target breast cells in the 1970's resulted in suitable models for the study of hormone control of cell proliferation and gene expression using two-dimensional (2D) cultures. However, to study mammogenesis and breast tumor development in vitro, cells must be able to organize in three-dimensional (3D) structures like in the tissue. We now report the development of a hormone-sensitive 3D culture model for the study of mammogenesis and neoplastic development. Hormone-sensitive T47D breast cancer cells respond to estradiol in a dose-dependent manner by forming complex epithelial structures. Treatment with the synthetic progestagen promegestone, in the presence of estradiol, results in flat epithelial structures that display cytoplasmic projections, a phenomenon reported to precede side-branching. Additionally, as in the mammary gland, treatment with prolactin in the presence of estradiol induces budding structures. These changes in epithelial organization are accompanied by collagen remodeling. Collagen is the major acellular component of the breast stroma and an important player in tumor development and progression. Quantitative analysis of second harmonic generation of collagen fibers revealed that collagen density was more variable surrounding budding and irregularly shaped structures when compared to more regular structures; suggesting that fiber organization in the former is more anisotropic than in the latter. In sum, this new 3D model recapitulates morphogenetic events modulated by mammogenic hormones in the breast, and is suitable for the evaluation of therapeutic agents.
Introduction
Mammary gland morphogenesis is mediated by reciprocal interactions between the mesenchyme/stroma and epithelial compartments throughout life. The complexity of these interactions makes it difficult to elucidate their underlying mechanisms. To overcome this hindrance, culture models were developed. Conventional two-dimensional (2D) cultures of epithelial cells are excellent models to study hormonal regulation of cell proliferation and of gene expression.1–3 However, morphogenetic processes such as the formation of ducts and acini, which are three-dimensional (3D) structures, require appropriate substrates that allow cells to proliferate, move, and organize in a 3D space.
During fetal life and prepubertal stages, mammary gland development is not estrogen dependent, as shown by the phenotypes in estrogen receptor (ER)α4 and ERβ5 null mutants. On the contrary, peri-pubertal development is hormone dependent and consists of ductal elongation and branching of the epithelium. Thereafter, the mammary gland undergoes subtle changes during each estrus/menstrual cycle and massive ones during pregnancy, lactation, and involution. Reproductive and metabolic hormones act in the presence of estrogen throughout all of these stages.6 Several 3D cell culture models have been developed using estrogen receptor-negative (ER−) breast cells; they allow for the study of some aspects of mammary gland development pertaining to the hormone-independent isometric growth such as the formation of anatomically correct structures. These models have increased the understanding of matrix–epithelial cell interactions7 and the role of stromal cells and collagen fiber organization on epithelial morphogenesis.8,9 For example, our previous work using MCF10A cells in type I collagen showed that collagen fiber organization is important for the development of elongated (ductal) structures.8 Additionally, we observed that basement membrane proteins (Matrigel) hinder fiber organization and result in the formation of round (acinar) structures.10 Others have shown the importance of matrix rigidity in determining lumen formation.11 Despite the fact that ER+ epithelial cell lines such as MCF712 and T47D13 have been used in 3D cultures, these studies have not explored the role of hormones in epithelial organization.
The purpose of this study was to develop a 3D model in which the effects of hormones on breast epithelium morphogenesis could be studied. This culture model is suitable to study the regulation of the neoplastic phenotype and it could therefore be employed for the testing of therapeutic agents.14
T47D cells express ER, progesterone receptor (PR), and prolactin receptor (PrlR), are responsive to hormones in 2D culture,15,16 and form duct-like structures when they are cultured in collagen gels for 7 days.13 Although these cells do not secrete basement membrane components collagen IV17 or laminin V (our own observations), they do form organized structures in 3D culture.13,17,18 The rationale for using T47D breast cancer cells is based on the following considerations: (i) the fact that there are no established normal human breast cell lines that are estrogen sensitive regarding the control of cell proliferation,19 (ii) that cells in primary cultures rapidly lose ER expression,20 and (iii) that of all established estrogen-sensitive cell lines, T47D cells seem to form the most normal-like structures among those tested.13,21 Here we examine the effect of the mammotrophic hormones 17 β-estradiol (E2), the progestagen promegestone, and prolactin in a 3D culture model and conclude that overall, cells in this model respond to these hormones in a comparable fashion to that which occurs in vivo with respect to cell proliferation, morphology, and matrix organization.
Materials and Methods
Cell maintenance
The T47D cells used in this study were cloned from a population obtained from Dr. G. Green (U. Chicago). The cells used in these experiments were tested for estrogen sensitivity before they were used.3 They were grown in the Dulbecco's Modified Eagle's medium (DMEM) containing 5% fetal bovine serum (FBS) (propagation medium). For experiments aimed at examining hormonal effects in both 2D and 3D cultures, a medium without phenol red was used containing 75% DMEM, 25% Ham's F12, 7.5% charcoal dextran stripped-FBS (CD-FBS), 2 mM L-glutamine, and 106 U/mL penicillin (heretofore CD-FBS). In all cases, cells were incubated at 37°C in 6% CO2.
Chemicals
The synthetic progestagen promegestone (R5020) (PerkinElmer) and E2 (Calbiochem) were dissolved in ethanol. Prolactin (Sigma) was dissolved in distilled de-ionized water. Antiestrogen ICI 182,780 (Tocris Bioscience) was dissolved in DMSO. E2 and promegestone stocks were prepared at a concentration of 3×10–3 M. Prolactin stock was prepared at 1 mg/mL and used at a 10−7 M final concentration. ICI 182,780 stock was prepared at a concentration of 10−2M.
Three-dimensional cultures
Rat tail collagen type I (BD Biosciences) was used at a final concentration of 1 mg/mL in accordance with Paszek et al.11 Collagen was neutralized with 1N NaOH according to the manufacturer's instructions. T47D cells were seeded at a density of 75,000 per gel. Cells were suspended in a final volume of 1.5 mL of collagen and poured into 12-well plates (Becton Dickinson). The mixture was allowed to congeal for 30 min at 37°C and 1.5 mL of the CD-FBS or CD-FBS medium containing E2, promegestone, or prolactin was added to each well. Gels were detached as described by Dhimolea et al.8 Cultures were maintained for 2 weeks for cell organization analysis and the medium was changed every 2 days. For the sequential hormonal treatment, after a 1-week incubation with E2 10−10 M, the medium was removed and replaced by E2 10−10 M+promegestone 10−10 M+prolactin 10−7 M for one additional week. Gels were harvested and processed for whole mount and histology as described below.
Dose–response curves to estradiol, promegestone, and prolactin
T47D cells between passages 39 and 61 were used. For the dose–response curves in 2D culture, T47D cells were seeded at a density of 35,000 per well in 12-well plates. Two days later, the propagation medium was removed and substituted by the CD-FBS or CD-FBS medium containing hormones. Controls were treated with vehicle. After 6 days, cells were counted using a Coulter particle counter (Becton Dickinson). For the dose–response curves in 3D culture, T47D cells were seeded at a density of 75,000 per gel. Two milliliters of the CD-FBS or CD-FBS medium containing hormones was added immediately after the gel had congealed. After 6 days, cells were extracted from the gels by collagenase treatment and counted.
Extraction of cells from gels
The method was adapted from Phillips & Brown.22 Gels were rinsed with phosphate-buffered saline (PBS) and transferred to 2 mL of a 0.125% collagenase type 3 (from Clostridium histolyticum) (Worthington) solution in the DMEM/F12. Gels were mechanically disrupted by gently pipetting up and down and the suspension was incubated at 37°C for 25 min. Collagenase was inhibited by adding 2 mL of the complete propagation medium and cells were collected by centrifugation at 1000 RPM for 5 min. The supernatant was discarded and the cell pellet was resuspended in 2 mL of the lysing solution. After 5 min, 18 mL of isoton was added and nuclei were counted using a Coulter particle counter (Becton Dickinson).
Gel processing
On the day of harvest, gels were cut into two pieces. One half was fixed overnight in 10% phosphate-buffered formalin, paraffin embedded, and used for histological analysis. The second piece was whole mounted onto a slide and fixed overnight in 10% phosphate-buffered formalin for morphometric analysis and second harmonic generation (SHG) and confocal microscopy. The whole-mounted gels were stained with Carmine Alum overnight as described previously.23 After staining, the whole mounts (WMs) were progressively dehydrated in 70%, 95%, and 100% ethanol, cleared in xylene, and mounted with Permount™ (Fisher Scientific).
Immunohistochemistry and F-actin staining
Immunohistochemistry was performed in 3D cultures. Paraffin sections were treated with xylene to remove paraffin and rehydrated through a series of alcohols and PBS. Antigen retrieval was achieved by microwave treatment in a 10 mM citrate buffer, pH6. Sections were then blocked in normal goat serum in 1.5% milk for 1 h before being incubated overnight at 4°C in a humid chamber with a primary antibody. Specific antibodies for ERα (1:50 dilution; Novocastra), PR A+B isoforms (1:50 dilution; Affinity Bioreagents), and E-cadherin (1:75 dilution; Novocastra) were used. Biotinylated secondary antibodies were applied to sections for 1 h in a humidified chamber at room temperature the following day. Slides were rinsed with PBS and detection of positive cells was accomplished using DAB (Sigma). Samples were counterstained with Harris' hematoxylin, dehydrated, and mounted with a permanent mounting medium. qRT-PCR was performed on cells isolated from 2D cultures (Supplementary Materials and Methods and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tec).
F-actin was visualized by staining the gels with rhodamine-labeled phalloidin (Cytoskeleton, Inc.). Briefly, gels were harvested and washed with PBS and fixed with 2% paraformaldehyde in PBS for 15 min, permeabilized with 0.2% Triton X-100 in PBS for 30 min, and washed with PBS before staining with 1/100 rhodamine-labeled phalloidin in PBS for 1 h at room temperature. After washing with PBS, cells were counterstained with DAPI (protocol adapted from Bott24). Preparations were observed using confocal microscopy.
Analysis of epithelial structures
For shape analysis, images of WMs were acquired at 5×magnification using a Zeiss stereoscope. A digital grid with horizontal and vertical lines spaced every 100 μm was randomly superimposed onto the image and every structure falling on the cross hair was counted and measured. Structures were categorized as follows: rounded, if minor and major axis were equal in length±5 μm; elongated, if the major axis was at least twice the length of the minor axis±5 μm; irregular, if the structure fell outside of the rounded or elongated criteria. A total of three WMs per treatment from three individual experiments were analyzed. The analysis of thickness of cell structures was performed using confocal microscopy. Structures with a major axis between 60–80 μm were selected. A total of 30 structures per treatment from 3 individual experiments were analyzed. WMs were imaged using a Zeiss LSM 510 system (Carl Zeiss MicroImaging, Inc.). Samples were excited with the HeNe 633 nm laser as the Carmine dye fluoresces at this wavelength. Images were acquired with a 40×oil immersion objective. Z-stacks were acquired using a frame size of 1700×1700 pixels and z-step of 1 μm. Three-dimensional reconstructions of the epithelial structures from the z-stacks were produced using Volocity 3D analysis software (PerkinElmer).
Analysis of collagen organization by SHG
SHG images of the WMs were acquired on a Leica TCS SP2 confocal microscope (Wetzlar, Germany) equipped with a Ti:sapphire laser (Spectra Physics). Samples were excited with 800 nm light using a 63x/1.2 NA water immersion objective as 12-bit, 1024×1024 pixel images with 238×238 μm2 field of view. Carmine dye fluorescence images were acquired by a nondescanned PMT with a filter cube containing a 700 nm short pass filter, a 495 nm dichroic mirror, and 525 (±25) nm bandpass filter. SHG images were recorded in the forward direction through a 400 (±10) nm bandpass filter. A total of 10–17 structures per treatment from three individual experiments were collected.
Automated analysis of cell structure morphology and the spatial organization of collagen fibers surrounding the cell structures were performed with Matlab using custom-written software (Mathworks). To define cell structure morphology, Carmine dye fluorescence intensity was segmented using a threshold of 1/3 the max intensity within the image. The largest segmented object based on pixel connectivity was dilated, and then eroded by 40 pixels to produce the final segmented cell structure. The area (A) and perimeter (P) of the structure was calculated and the shape form factor was defined as 4πA/P2.
Collagen fiber density with respect to the distance to the cell structure was also defined. For each pixel within the SHG image, collagen was defined when the SHG intensity exceeded 25% of the maximum value within the image. To produce a map of regional collagen fiber density with respect to the cell structure, a grid of points spaced 40 pixels (9.3 μm) apart radiating outward from the cell structure edge was generated, and a meshed grid was created via Delaunay triangulation. Fiber density based on the thresholded SHG image was calculated within each triangular element. To measure variability in fiber density surrounding the cell structure, the mean and standard deviation of fiber density from triangular elements within 20 μm of the cell structure edge were calculated. Similar to techniques used to smooth and display full-field strain maps,25 the average fiber density at each point was computed from all elements defined using the point. The fiber density field was displayed with the fluorescence and SHG images.
Statistics
SPSS software package 15.0 (SPSS) was used for all statistical analyses. ANOVA followed by Bonferroni post hoc tests were used to determine differences in the dose–response curves and differences in the percentage of structures in each of the treatments. Differences in the thickness of structures between E2 and promegestone + E2 groups were determined using independent sample t-tests for equality of means. ANCOVA was used to control for the variance of the size of each structure in the case of form factor measurements. For all statistical tests, results were considered significant at p<0.05. Results are presented as mean±standard error of the mean.
Results
Effects of hormones on cell proliferation
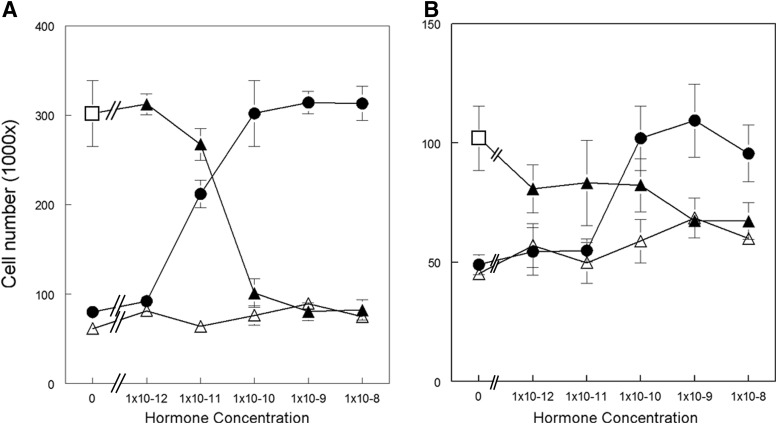
T47D cells treated with E2 showed an increase in cell number in a dose-dependent manner in 2D culture (Fig. 1A).1,3 In 3D culture (Fig. 1B), cell numbers increased with increasing hormone concentration in a dose-dependent manner and, like in 2D culture, reached its maximal cell yield at 10−10M E2. However, the cell yield ratio between E2 and CD-FBS was larger when cells were grown in 2D culture (3.75-fold) than when they were grown in 3D culture (2.5-fold). The effect of E2 on T47D proliferation was abrogated by treatment with ICI 182,780 both in 2D (data not shown) and in 3D cultures (Supplementary Fig. S1). Promegestone alone did not affect T47D cell proliferation in 2D culture in the range of concentrations tested (Fig. 1A). In contrast, an increase in cell number was observed at 10−9M promegestone when cells were grown in 3D culture (p=0.034) (Fig. 1B). The effect of E2 on cell proliferation was diminished in the presence of 10−10 to 10−8M promegestone in 2D culture (Fig. 1A). In 3D culture, this effect was apparent at a concentration of promegestone one order of magnitude higher (10−9M) (p=0.002) than in 2D cultures (Fig. 1B). Prolactin did not affect cell proliferation at any of the concentrations tested either in 2D or 3D culture alone or in the presence of E2 (data not shown).
FIG. 1.
Dose–response curves to E2 (circle), promegestone (open triangle), and both hormones, E2 (at a constant concentration of 10−10M) (open square) together with variable concentrations of promegestone (filled triangle) of T47D cells growing in (A) two-dimensional (2D) and in (B) three-dimensional (3D) culture. Bars: SEM.
Hormone receptor expression
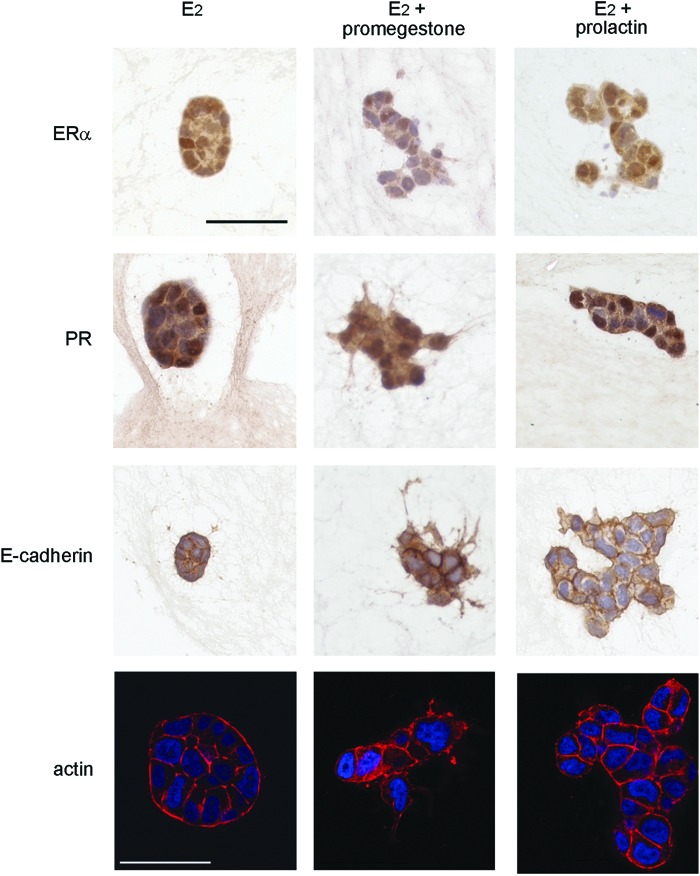
In 2D culture, T47D cells expressed ERα, PR, and PrlR when grown in CD-FBS as shown by qRT-PCR. The expression of hormone receptors was also affected by the concentration of E2 in the medium. ERα mRNA expression was downregulated at concentrations of E2 higher than 10−11M, while PR was induced by E2 reaching maximal levels at 10−11M. PrlR remained constant at the range of E2 concentrations tested (Supplementary Fig. S2). In 3D culture, the expression of hormone receptors was assessed by immunohistochemistry. At 10−9 M E2, T47D cells stained positive for PR and ERα regardless of the shape of the epithelial structures. The addition of promegestone to E2-containing medium resulted in downregulation of the expression of ERα as shown by the weak nuclear staining and a decrease of positively stained cells (Fig. 2).
FIG. 2.
ERα, PR, E-cadherin, and F-actin in T47D cells growing in 3D culture. Scale bar: 50 μm. Color images available online at www.liebertpub.com/tec
Effect of hormones on epithelial organization patterns
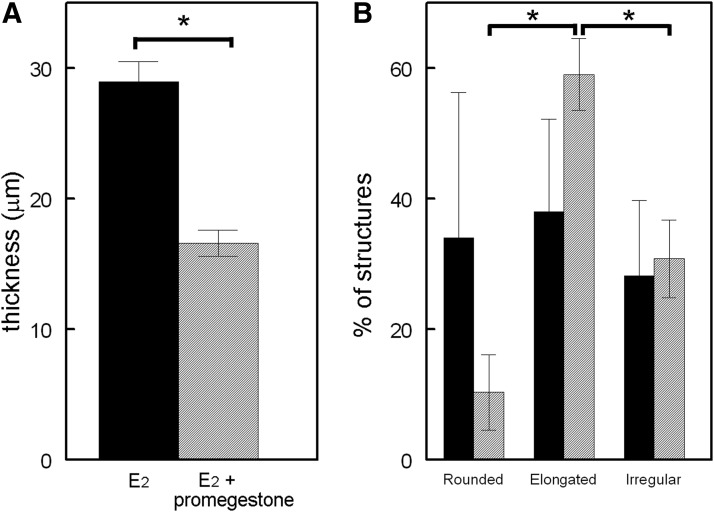
Different patterns of epithelial organization were observed in 3D culture. In the presence of E2, T47D cells formed rounded (Figs. 3A and 4A), elongated, and irregular structures. Cells within these structures showed basolateral expression of E-cadherin and F-actin (Fig. 2). In contrast, when cultured in the hormone-depleted CD-FBS medium, only clusters of 2–3 cells were observed (Fig. 3B). Promegestone also affected the shape of the structures formed in the presence of E2. Addition of promegestone (10−10 M) to the medium containing 10−10 M E2, induced flattening of epithelial structures (Fig. 3C). Confocal imaging confirmed that the cells were clustered together forming flat structures with cytoplasmic projections (Fig. 4B). The average thickness of the epithelial structures was significantly reduced (p<0.001) by 50% when compared to those observed in 10−10 M E2 alone as measured by confocal z-stacks (Fig. 5A). E-cadherin expression confirmed the existence of cell–cell contacts (Fig. 2). The pattern of E-cadherin expression, however, was different from that found in the epithelial structures formed in the presence of E2 alone. In the presence of promegestone+E2, cells at the center of the structures showed strong staining at cell boundaries, which was attenuated toward the periphery of the structures. F-actin was also localized differently from the structures formed in E2 as it was stronger toward the lateral surface (Fig. 2).
FIG. 3.
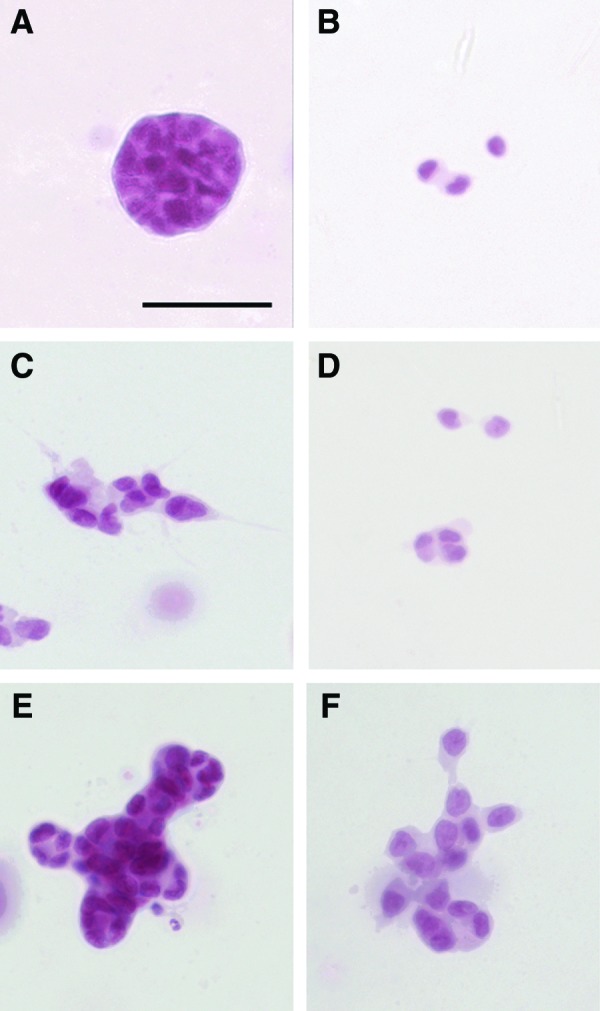
T47D cells growing in 3D culture in (A) E2 (10−10 M), (B) CD-FBS, (C) E2 (10−10 M)+promegestone (10−10 M), (D) promegestone (10−10 M), (E) E2 (10−10 M)+prolactin (10−7 M), and (F) a sequential treatment of E2 (10−10 M) followed by E2 (10−10 M)+promegestone (10−10 M)+prolactin (10−7 M). Scale bar: 50 μm. Color images available online at www.liebertpub.com/tec
FIG. 4.
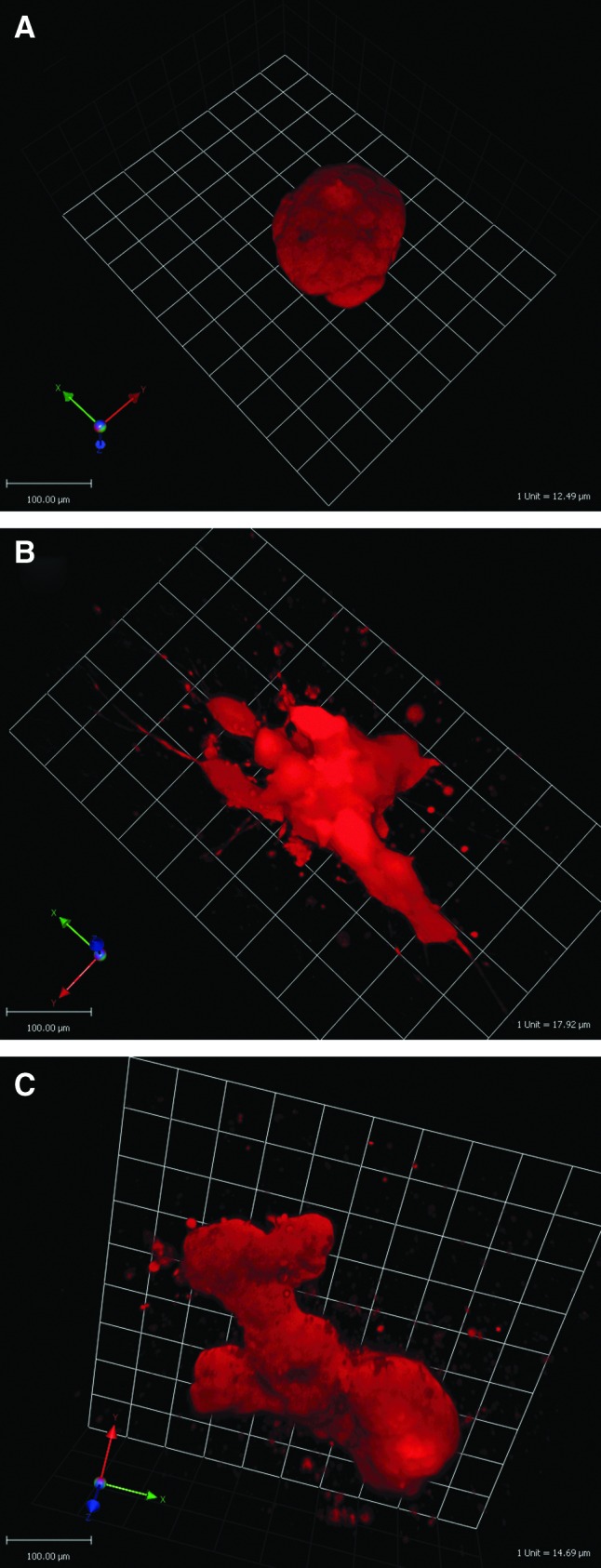
T47D structures after treatment with (A) E2 (10−10 M), (B) E2 (10−10 M)+promegestone (10−10 M), and (C) E2 (10−10 M)+prolactin (10−7 M) for 2 weeks in 3D culture. Three-dimensional projections were reconstructed from z stack images using Volocity software. Color images available online at www.liebertpub.com/tec
FIG. 5.
(A) Thickness of epithelial structures was measured on carmine stained whole mounts by confocal microscopy. Average length of E2 and E2+promegestone structures was 68±6 and 69±8 μm, respectively. (B) Percentage of structures observed in 3D culture after treatment with E2 (black bars) or E2+prolactin (gray bars). *Statistical significance. Bars: SEM.
Prolactin modified the pattern of epithelial cell organization when added to E2, resulting in the formation of budding structures (Figs. 3E and 4C) showing E-cadherin and F-actin expression (Fig. 2). Additionally, significant changes in the distribution of rounded, elongated, and irregular structures were observed when comparing the effect of prolactin+E2 to that of E2 alone (Fig. 5B).
A sequential hormonal treatment consisting of 1 week of exposure to E2 followed by 1 week of exposure to the three mammogenic hormones (Fig. 3F) resulted in morphologically similar structures to those obtained with promegestone+E2 (Fig. 3C). However, the nuclei were more rounded in the former.
Effect of hormones on collagen organization patterns
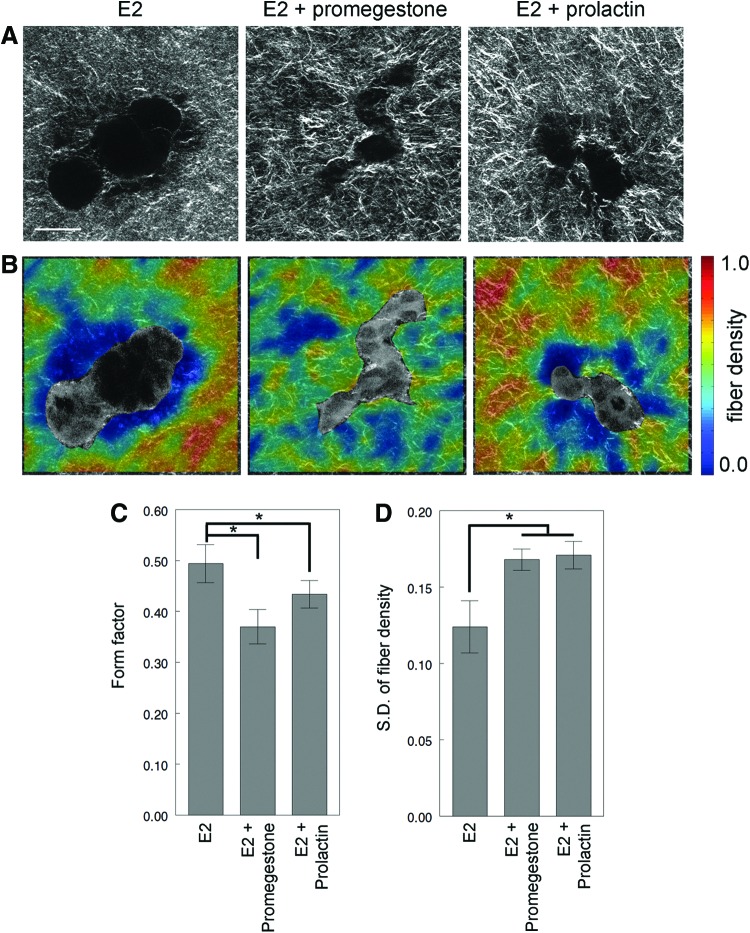
To study collagen fiber organization, structures were arbitrarily selected and imaged (Fig. 6A). The elongated structures resulting from E2 treatment had a significantly higher form factor when compared to combined treatments with promegestone (p=0.0147) or prolactin (p=0.0346) (Fig. 6C). This suggests that the structures in the combined treatment with prolactin or promegestone were more irregular in shape than elongated structures in E2 alone. The mean fiber density was lower in E2 structures compared with that in the combined treatments, although this difference was not statistically significant (data not shown). However, combined hormone treatment resulted in higher collagen density variability within 20 μm from the epithelial structure (E2 vs. prolactin+E2, p=0.015; E2 vs. promegestone+E2, p=0.028) (Fig. 6D). This was consistent with the finding that the structures in the combined treatments, promegestone or prolactin, were more irregular in shape and branched when compared to those observed in E2 alone as evidenced by their lower form factor (Fig. 6B, C).
FIG. 6.
Collagen organization analysis of T47D cells growing in 3D cultures in the presence of E2 (10−10 M), E2 (10−10 M)+promegestone (10−10 M) and E2 (10−10 M)+prolactin (10−7 M) for 2 weeks. (A) Second harmonic generation (SHG) images, (B) fiber density maps on merged SHG and two-photon excited fluorescence channels, (C) form factor of epithelial structures, and (D) standard deviation of fiber density. *, statistical significance. Scale bar: 50 μm. Color images available online at www.liebertpub.com/tec
Discussion
Here we have described the first hormone-sensitive 3D culture model of breast cancer suitable for the development and testing of novel therapeutic agents. This model recapitulates the effect of mammogenic hormones on the mammary epithelium, thus providing a reliable 3D culture model to study hormone action and morphogenesis. It exhibits clear morphogenic responses to E2, promegestone, and prolactin, which are comparable to the effects described in vivo. Namely, (i) E2 induced the growth of epithelial structures, (ii) promegestone produced elongated structures suggestive of branching, and (iii) prolactin produced budding structures. This suggests that the 3D model we describe provides a more realistic representation of the in vivo situation for the study of hormone action on tissue organization than 2D culture models.
Regarding cell proliferation, the pattern of response to E2 was similar in 2D and in 3D; in both cultures, it was inhibited by the antiestrogen ICI 182,780. In contrast to 2D culture, promegestone increased epithelial cell number in 3D. Previous studies utilizing fresh breast organoids growing in Matrigel have also shown a modest proliferative effect of promegestone.26 In contrast, promegestone in the presence of E2 inhibited the proliferative effect of E2 in a dose-dependent manner in both 2D and 3D, a result previously reported in 2D culture.27,28 Prolactin did not have an effect on cell proliferation in 2D or 3D culture in the conditions tested, which reinforces previous findings that prolactin has no proliferative effect on T47D cells growing in 2D culture after a 3-day treatment.15,29
Mammogenic hormones directly and/or indirectly modify the architecture of the epithelium and of the surrounding extracellular matrix. Similar to breast tissue, T47D cells in 3D culture established cell–cell interactions comparable to those exerted in mammary gland morphogenesis. At puberty, development of the mammary gland is mainly due to the effect of 17β estradiol produced by the ovaries, which promotes ductal growth and invasion (reviewed in30). The stunted mammary gland phenotype of ovariectomized31 and ERα-KO mice32 suggests that this effect is mediated through ERα. Administration of E2 to ovariectomized mice induced ductal growth and proliferation of the epithelial cells that form those ducts.31
Consistent with the effect observed in vivo, in our 3D model, T47D cells exhibited a low proliferative activity in the absence of E2, and the effects of estrogen were obliterated by estrogen antagonists. This is an important feature of our model that contrasts with that reported for the MCF-12A cells. The latter, which were derived from a normal breast epithelium and do not form tumors when transplanted into animals, respond to estrogens by upregulating PR and by inhibiting apoptosis. However, MCF-12A cells do not exhibit estrogen-sensitive proliferation. Although the net effect of estrogen administration was the defective formation of acini in an ECM, these effects were not obliterated by exposure to estrogen antagonists, a central feature of effects mediated by ERs.19
A detailed understanding of the processes underlying ductal elongation during puberty has yet to be achieved; however, it is known that for elongation to happen, ERα should be expressed in the epithelial cells, and that the effect of estrogens involves cell–cell and paracrine interactions involving both the epithelium and the stroma.33 The fact that the effect of estradiol can be observed in our model suggests that it could be further exploited to unravel steps of morphogenesis as other elements of the stroma are added (i.e., fibroblasts, adipocytes).
At present, there are no culture models that show side branching with similar characteristics to those observed in vivo. Promegestone, like progesterone, induce side branching in the mammary gland in the presence of E2.34 A model consisting of organoids of normal epithelial and myoepithelial cells freshly isolated from the mouse, responds to progesterone with blunting of ductal morphogenesis, a phenomenon that the authors associated with side branching.35 In the conditions of our 3D model, promegestone induced the formation of chain-like structures, which are considered an early step of ductal formation according to Meyer et al.35 The fact that side branching is only partially recapitulated in our model containing epithelial cells and in the model containing epithelial and myoepithelial cells,34,35 suggests that stromal components such as fibroblasts and/or adipocytes may significantly contribute to this morphogenetic event, as shown in the MCF10A model.9 We have previously shown that mammary fibroblasts normalize the structures formed by the ER+/PR+ MCF7 cells.12
The stimulation of budding by prolactin was successfully reproduced in our culture model. Prolactin induces lobuloalveolar development during pregnancy and lactation. Knockout mice for prolactin36 and PrlR,37 had arrested the development of the mammary gland at an early pubertal stage. Lobuloalveolar budding was impaired and side branching was reduced compared to wild-type mice.
The structures that we observed in the sequential hormonal treatment (E2 alone for 1 week followed by E2, promegestone, and prolactin for 1 week) were not as developed as those resulting from E2+prolactin treatment. This is consistent with the fact that in vivo, completion of alveolar morphogenesis requires high prolactin levels and a decrease of progesterone levels,6 which occurs right before parturition.
In our studies of hormone insensitive MCF10A cells in 3D culture, we observed that fiber organization was a main determinant of morphogenesis.8,9 In our current study, we observed that hormonal treatment influenced collagen fiber organization. Quantitative analysis showed that the changes in epithelial organization induced by mammogenic hormones were accompanied by differences in collagen organization. Fiber density was more variable surrounding budding structures displaying irregular shapes, which were quantitatively measured as having a low-form factor. These results suggest that hormones affect epithelial cell behavior, which in turn affects the matrix organization. Collagen organization in the mammary gland also varied according to hormonal status. For example, during pregnancy, collagen type I was abundant surrounding the ducts and decreased when alveoli began to form.38 Here we observed that collagen organized differently in breast epithelial structures that resulted from treatment with E2 alone or in combination with prolactin or promegestone (Fig. 6). Organization of such structures in the tissue was also greatly influenced by mechanical forces. Transmission of forces through collagen fibers facilitate the interaction between epithelial cell structures39 and mechanical forces acting upon cells altered gene expression and cytoskeletal organization. Mammogenic hormones require the presence of receptors in the epithelium, where they exert their effect through paracrine loops and stromal–epithelial interactions. In our 3D culture model, these hormones have, in addition, affected the organization of the matrix by influencing the epithelial cells. This suggests a loop between fiber organization and the forces they exert on cells, which contribute to morphogenesis. Thus, this model has the basic elements for studying mammogenesis because it can be modified by adding cellular components of the stroma as well as myoepithelial cells to progressively construct a more comprehensive anatomo-physiological model of the mammary gland.
On the technical side, the data reported herein show that this model provides the opportunity to observe hormone action on the dynamics of tissue formation in real time. This could be achieved by the use of noninvasive imaging modalities, such as SHG, which allows monitoring dynamic changes on fiber organization. When performed in combination with other modalities, such as two-photon excited fluorescence to monitor the cellular components of these models, there is additional potential to dynamically assess epithelial–stromal interactions.40
Finally, it is worth highlighting that despite their neoplastic origin, T47D cells were able to respond to mammogenic hormones in a fashion comparable to that of primary epithelial cells isolated from a normal mammary gland.26,35 The plasticity of the neoplastic phenotype and its normalization by induction of proper tissue organization has been reported multiple times bringing into question the existence of a neoplastic identity at the cellular level.41,42 In this context, it is likely that the addition of normal stromal cells and myoepithelial cells would incrementally contribute to a more anatomically and functionally normal phenotype, whereas the addition of cancer-associated fibroblasts would produce a more abnormal one. Indeed, the T47D model could be used to understand cancer in the context of tissue architecture and hormone action.
Conclusion
The 3D culture model described here reproduces the effects of estradiol, promegestone, and prolactin in a fashion consistent with what it is observed in vivo. Collagen fiber rearrangement accompanied hormone-directed morphogenesis of the epithelial cells. Thus, this hormone-responsive model of the breast tissue provides a physiologically relevant context to study cell–cell and cell–stroma interactions at the level of cell proliferation and organization, while opening the possibility of observing hormone action on the dynamics of tissue formation in real time.
Supplementary Material
Acknowledgments
We greatly appreciate the technical and editorial contributions by Michael Borrero, Cheryl Schaeberle and Vyvyan Howard. This research was supported by Avon Grant #02-2009-093 and 02-2011-095 to AMS. Additional support was provided by NIAMS/NIH Grant F32AR061933 to KPQ, American Cancer Society Grant RSG-09-174-01-CCE and NIH/NIBIB grant R01EB00542 to IG, and NC3R Grant 94744, which supports CB and NIEHS/NIH ES 08314 to AMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chalbos D., Vignon F., Keydar I., and Rochefort H.Estrogens stimulate cell proliferation and induce secretory proteins in a human breast cancer cell line (T47D). J Clin Endocrinol Metab 55,276, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Vignon F., Bardon S., Chalbos D., and Rochefort H.Antiestrogenic effect of R5020, a synthetic progestin in human breast cancer cells in culture. J Clin Endocrinol Metab 56,1124, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Soto A.M., Murai J.T., Siiteri P.K., and Sonnenschein C.Control of cell proliferation: evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res 46,2271, 1986 [PubMed] [Google Scholar]
- 4.Bocchinfuso W.P., and Korach K.S.Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia 2,323, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Krege J.H., Hodgin J.B., Couse J.F., Enmark E., Warner M., Mahler J.F., Sar M., Korach K.S., Gustafsson J.A., and Smithies O.Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Nat Acad Sci USA 95,15677, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neville M.C., McFadden T.B., and Forsyth I.Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7,49, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Petersen O.W., Rønnov-Jessen L., Howlett A.R., and Bissell M.J.Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Nat Acad Sci USA 89,9064, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhimolea E., Maffini M.V., Soto A.M., and Sonnenschein C.The role of collagen reorganization on mammary epithelial morphogenesis in a 3D culture model. Biomaterials 31,3622, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Krause S., Jondeau-Cabaton A., Dhimolea E., Soto A.M., Sonnenschein C., and Maffini M.V.Dual regulation of breast tubulogenesis using extracellular matrix composition and stromal cells. Tissue Eng Part A 18,520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause S., Maffini M.V., Soto A.M., and Sonnenschein C.A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng 14,261, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., Reinhart-King C.A., Margulies S.S., Dembo M., Boettiger D., Hammer D.A., and Weaver V.M.Tensional homeostasis and the malignant phenotype. Cancer Cell 8,241, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Krause S., Maffini M.V., Soto A.M., and Sonnenschein C.The microenvironment determines the breast cancer cells' phenotype: organization of MCF7 cells in 3D cultures. BMC Cancer 10,263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wozniak M.A., and Keely P.J.Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol Proced Online 7,144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver V.M., Lelievre S., Lakins J.N., Chrenek M.A., Jones J.C., Giancotti F., Werb Z., and Bissell M.J.b4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2,205, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen L.M., Frederiksen K.S., Din N., Glasgaard E., Christensen L., Berchtold M.W., and Panina S.Prolactin and oestrogen synergistically regulate gene expression and proliferation of breast cancer cells. Endocr Relat Cancer 17,809, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Xu J., Zhang Y., Berry P.A., Jiang J., Lobie P.E., Langenheim J.F., Chen W.Y., and Frank S.J.Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol Endocrinol 25,597, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keely P.J., Fong A.M., Zutter M.M., and Santoro S.A.Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense alpha 2 integrin mRNA in mammary cells. J Cell Sci 108,595, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Barcus C.E., Keely P.J., Eliceiri K.W., and Schuler L.A.Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J Biol Chem 288,12722, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchese S., and Silva E.Disruption of 3D MCF-12A breast cell cultures by estrogens—an in vitro model for ER-mediated changes indicative of hormonal carcinogenesis. PLoS One 7,e45767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novaro V., Roskelley C.D., and Bissell M.J.Collagen-IV and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. J Cell Sci 116,2975, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny P.A., Lee G.Y., Myers C.A., Neve R.M., Semeiks J.R., Spellman P.T., Lorenz K., Lee E.H., Barcellos-Hoff M.H., Petersen O.W., Gray J.W., and Bissell M.J.The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 1,84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips J.B., and Brown R.Micro-structured materials and mechanical cues in 3D collagen gels. Methods Mol Biol 695,183, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Maffini M.V., Ortega H., Stoker C., Giardina R., Luque E.H., and Munoz de Toro M.M.Bcl-2 correlates with tumor ploidy and nuclear morphology in early stage prostate carcinoma. Path Res Pract 197,487, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Bott K., Upton Z., Schrobback K., Ehrbar M., Hubbell J.A., Lutolf M.P., and Rizzi S.C.The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 31,8454, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Quinn K.P., and Winkelstein B.A.Full field strain measurements of collagenous tissue by tracking fiber alignment through vector correlation. J Biomechanics 43,2637, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Graham J.D., Mote P.A., Salagame U., Balleine R.L., Huschtscha L.I., and Clarke C.L.Hormone-responsive model of primary human breast epithelium. J Mammary Gland Biol Neoplasia 14,367, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Rochefort H., Chalbos D., Capony F., Garcia M., Veith F., Vignon F., and Westley B.Effect of estrogen in breast cancer cells in culture: released proteins and control of cell proliferation. Prog Clin Biol Res 142,37, 1984 [PubMed] [Google Scholar]
- 28.Iwasaki K., Underwood B., Herman M., Dinda S., Kodali S., Kloosterboer H.J., Hurd C., and Moudgil V.K.Effects of antiprogestins on the rate of proliferation of breast cancer cells. Mol Cell Biochem 198,141, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Huang K., Chen K.E., and Walker A.M.Prolactin and estradiol utilize distinct mechanisms to increase serine-118 phosphorylation and decrease levels of estrogen receptor alpha in T47D breast cancer cells. Breast Cancer Res Treat 120,369, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Fendrick J.L., Raafat A.M., and Haslam S.Z.Mammary gland growth and development from the postnatal period to postmenopause: Ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia 3,7, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Daniel C.W., Silberstein G.B., and Strickland P.Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res 47,6052, 1987 [PubMed] [Google Scholar]
- 32.Couse J.F., and Korach K.S.Exploring the role of sex steroids through studies of receptor deficient mice. J Mol Med 76,497, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Mallepell S., Krust A., Chambon P., and Brisken C.Paracrine signaling through the epithelial estrogen receptor a is required for proliferation and morphogenesis in the mammary gland. Proc Nat Acad Sci USA 103,2196, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raafat A.M., Li S., Bennett J.M., Hofseth L.J., and Haslam S.Z.Estrogen and estrogen plus progestin act directly on the mammary gland to increase proliferation in a postmenopausal mouse model. J Cell Physiol 187,81, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Meyer G., Leipprandt J., Xie J., Aupperlee M.D., and Haslam S.Z.A potential role of progestin-induced laminin-5/á6-integrin signaling in the formation of side branches in the mammary gland. Endocrinology 153,4990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horseman N.D.Prolactin and mammary gland development. J Mammary Gland Biol Neoplasia 4,79, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Brisken C., Kaur S., Chavarria T., Binart N., Sutherland R.L., Weinberg R.A., Kelly P.A., and Ormandy C.J.Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol 210,96, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Keely P.J., Wu J.E., and Santoro S.A.The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation 59,1, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Guo C.L., Ouyang M., Yu J.Y., Maslov J., Price A., and Shen C.Y.Long-range mechanical force enables self-assembly of epithelial tubular patterns. Proc Nat Acad Sci USA 109,5576, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xylas J., Alt-Holland A., Garlick J., Hunter M., and Georgakoudi I.Intrinsic optical biomarkers associated with the invasive potential of tumor cells in engineered tissue models. Biomed Opt Express 1,1387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maffini M.V., Soto A.M., Calabro J.M., Ucci A.A., and Sonnenschein C.The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci 117,1495, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Tanner K., Mori H., Mroue R., Bruni-Cardoso A., and Bissell M.J.Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc Nat Acad Sci USA 109,1973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.