Abstract
Despite an increased interest in the use of hydrogel encapsulation and cellular self-assembly (often termed “self-aggregating” or “scaffold-free” approaches) for tissue-engineering applications, to the best of our knowledge, no study to date has been undertaken to directly compare both approaches for generating functional cartilaginous grafts. The objective of this study was to directly compare self-assembly (SA) and agarose hydrogel encapsulation (AE) as a means to engineer such grafts using passaged chondrocytes. Agarose hydrogels (5 mm diameter × 1.5 mm thick) were seeded with chondrocytes at two cell seeding densities (900,000 cells or 4 million cells in total per hydrogel), while SA constructs were generated by adding the same number of cells to custom-made molds. Constructs were either supplemented with transforming growth factor (TGF)-β3 for 6 weeks, or only supplemented with TGF-β3 for the first 2 weeks of the 6 week culture period. The SA method was only capable of generating geometrically uniform cartilaginous tissues at high seeding densities (4 million cells). At these high seeding densities, we observed that total sulphated glycosaminoglycan (sGAG) and collagen synthesis was greater with AE than SA, with higher sGAG retention also observed in AE constructs. When normalized to wet weight, however, SA constructs exhibited significantly higher levels of collagen accumulation compared with agarose hydrogels. Furthermore, it was possible to engineer such functionality into these tissues in a shorter timeframe using the SA approach compared with AE. Therefore, while large numbers of chondrocytes are required to engineer cartilaginous grafts using the SA approach, it would appear to lead to the faster generation of a more hyaline-like tissue, with a tissue architecture and a ratio of collagen to sGAG content more closely resembling native articular cartilage.
Introduction
Cartilage damage can arise from degenerative diseases such as osteoarthritis or due to physical trauma to the articular surface. A large number of tissue-engineering strategies have been proposed to repair such cartilaginous defects. Typical approaches involve the use of a scaffold or hydrogel for supporting and organizing the cells in a three-dimensional environment. Agarose hydrogels are commonly used for cartilage tissue-engineering applications, as they have been found to support the chondrogenic phenotype and the synthesis of cartilaginous extracellular matrix (ECM).1–16 When seeded with primary chondrocytes, such hydrogels can be used to engineer tissues, attaining native levels of compressive moduli and sulphated glycosaminoglycan (sGAG) content.17 However, as with many scaffolds or hydrogels, such an approach raises the issues of scaffold degradation products, inflammatory responses to the implanted materials, stress shielding of cells, and a reduction in cell-to-cell communication.18,19 This has motivated research into scaffold-free techniques as a potential method for generating functional cartilage tissue.
One of the first reported uses of a scaffold-free or self-assembly (SA) (or self-aggregating) approach for engineering cartilage-like tissue involved directly seeding chondrocytes onto plastic dishes precoated with poly(2-hydroxyethyl methacrylate),20–24 which leads to the development of a graft with a hyaline cartilage phenotype in terms of the expression of collagen type II and aggrecan.22 Alternative SA approaches involve aliquoting chondrocytes into an agarose mold or similar, and allowing these cells to self-assemble over time.18 After 12 weeks of culture, this SA approach has been shown to support the generation of a hyaline-like cartilaginous tissue with biochemical and mechanical properties approaching those of native articular cartilage. Numerous other studies have investigated the SA of chondrocytes,25–33 with determination of the initial cell seeding number identified as a key parameter to successfully engineer a cartilaginous graft using the SA approach. Researchers have also investigated the potential of generating cartilage grafts through SA of mesenchymal stem cells (MSCs),19,34 with some success reported in repairing chondral defects in vivo using this approach.35,36 Furthermore, in terms of chondrogenic differentiation of human bone marrow-derived MSCs, the SA method has demonstrated benefits compared with the traditional pellet culture system.37
Despite the extensive research into scaffold-free cartilage tissue engineering, particularly in the area of chondrocyte SA, to the best of our knowledge, no study to date has been undertaken to directly compare the SA approach with hydrogel encapsulation for engineering functional cartilaginous grafts. The objective of this study was to directly compare SA with agarose hydrogel encapsulation (AE) as a means to engineer such grafts. Passaged chondrocytes were encapsulated into agarose hydrogels at different cell seeding densities and maintained in a chemically defined media. The properties of these engineered tissues were then compared with those generated using a SA approach. Since it is known that articular chondrocytes dedifferentiate after they attach to cell culture plastic,3 the cells were allowed to self-assemble on an agarose bed that prevents cell attachment. Previous studies have shown that the SA of chondrocytes on a nonadhesive agarose coating leads to the development of a more smooth, flat, and hyaline-like construct, when compared with those assembled on culture-treated plastic.18 Constructs were seeded at two seeding densities: first, a typical AE seeding density (∼900,000 cells per construct or 30 million cells/mL for a 5 mm diameter × 1.5 mm thick construct), and second, a typical SA seeding density (4 million cells per construct). Finally, since transient transforming growth factor (TGF)-β3 stimulation has been shown to enhance chondrogenesis in chondrocyte-seeded agarose hydrogels,17 we compared the effect of such media supplementation conditions on the development of cartilaginous grafts engineered using both SA and AE.
Materials and Methods
Cell isolation and expansion
Articular cartilage was aseptically harvested from porcine femoral condyles (4 months old), and the cartilage slices were rinsed thoroughly with Dulbecco's phosphate-buffered saline (Sigma-Aldrich, Dublin, Ireland; PBS) containing penicillin (200 U/mL)-streptomycin (100 μg/mL) (GIBCO, Invitrogen, Dublin, Ireland), and amphotericin B (2.5 μg/mL) (Sigma-Aldrich, Dublin, Ireland). Chondrocytes were isolated from cartilage slices via digestion with high-glucose Dulbecco's modified Eagle's medium GlutaMAX (4.5 mg/mL D-Glucose, 200 mM L-Glutamine; hgDMEM) (GIBCO, Invitrogen, Dublin, Ireland) containing collagenase type II (315 U/mg) (Worthington, Langanbach Services, Ireland) for 12–14 h under constant rotation at 37°C. The resulting cell suspension was passed through a 40 μm pore-size cell sieve (Fisher Scientific), and the filtrate was centrifuged and rinsed with PBS twice. Cell number and viability were determined using a hemocytometer and 0.4% trypan blue staining (Sigma-Aldrich), and the chondrocytes were then frozen in hgDMEM supplemented with 10% v/v foetal bovine serum (GIBCO, Invitrogen; FBS) and 10% dimethyl sulphoxide (Sigma-Aldrich; DMSO) and stored in liquid nitrogen. Before experiments were initiated, cells were thawed and counted. Chondrocytes were plated at a seeding density of 5×103 cells/cm2 in 500 cm2 triple flasks (Thermo Fisher Scientific) and expanded to passage two (P2) in a humidified atmosphere at 37°C and 5% CO2. Chondrocytes were maintained in DMEM GlutaMAX supplemented with 10% v/v FBS, penicillin (100 U/mL)-streptomycin (100 μg/mL) and 5 ng/mL human fibroblast growth factor-2 (FGF-2; Prospec) during the expansion phase.
Formation and culture of SA and agarose hydrogel constructs
At P2 cells were trypsinized, counted, and suspended in basic chondrogenic medium (basic CDM) consisting of hgDMEM supplemented with penicillin (100 U/mL)-streptomycin (100 μg/mL) (both from GIBCO, Invitrogen), 100 μg/mL sodium pyruvate, 40 μg/mL L-proline, and 1.5 mg/mL bovine serum albumin (all Sigma-Aldrich). A custom-built polydimethylsiloxane mold was used to create sterile, 3% agarose wells (Type VII; Sigma-Aldrich) of 5 mm diameter, and 3 mm thickness. SA constructs were formed by adding either 900,000 cells (low seeding density; 46,000 cells/mm2) or 4 million cells (high seeding density; 204,000 cells/mm2) in 40 μL aliquots of defined CDM to the 5 mm diameter agarose wells, seated in either 12-well plates (low seeding density constructs) or 6-well plates (high seeding density constructs) (Fisher Scientific). Defined CDM consisted of basic CDM supplemented with 0.25 μg/mL amphotericin B, 1 × insulin-transferrin-selenium, 4.7 μg/mL linoleic acid, 50 μg/mL L-ascorbic acid-2-phosphate, and 100 nM dexamethasone (all Sigma-Aldrich). SA constructs were initially not supplemented with TGF-β3 to minimize cell contraction. Cells self-assembled within 12 h, upon which defined CDM supplemented with 10 ng/mL of TGF-β3 (ProSpec-Tany TechnoGene Ltd.) was added to each well; t=0 was defined at this time point.
Chondrocyte encapsulated agarose hydrogel constructs were formed by mixing the chondrocyte cell suspension in basic CDM with 4% agarose in sterile PBS. This solution was mixed at a ratio of 1:1 at ∼40°C, to yield a final gel concentration of 2% and a cell density of either 30 million cells/mL or 136 million cells/mL. The agarose/cell suspensions were cast in a stainless steel mold, allowed to cool for 30 min, and solid construct cylinders (5 mm diameter × 1.5 mm thick) were removed using a biopsy punch. Constructs were then placed in 6- or 12-well plates corresponding to their cell number, and immersed in defined CDM supplemented with 10 ng/mL of TGF-β3. The low and high agarose cell seeding densities correspond to the SA seeding density of 900,000 and 4 million cells, respectively. The high seeding density of 4 million cells was chosen as this has previously been shown to be the optimal initial seeding number for chondrocyte SA constructs.31 The low seeding density of 900,000 cells (30 million cells/mL) was chosen to enable comparisons to be made with other chondrocyte agarose hydrogel studies previously undertaken in our laboratory. Typical values for SA thickness range from 0.88 to 1.4 mm19; therefore, we chose a thickness value of 1.5 mm for our agarose constructs in order to generate similarly sized constructs to the SA approach at the end of the experiment.
Constructs at low seeding density were maintained in 2.5 mL fully supplemented CDM, with high seeding density constructs maintained in 11 mL (hence maintaining a constant ratio of media to cells). Medium was fully exchanged every 3 or 4 days, with 500 μL samples taken from wells for each group (n=3) at each medium exchange for biochemical analysis (as described below). All agarose and SA constructs were maintained for 2 weeks in fully supplemented CDM, upon which TGF-β3 was withdrawn from half the samples of all experimental groups for the remaining 4 weeks. In addition, all SA constructs were removed from their agarose molds after 2 weeks of in vitro culture, as this has been shown to enhance aggregate moduli and collagen organization in SA constructs.28
Biochemical analysis
The biochemical content of constructs (n=3–4) was assessed at each time point (0, 21, and 42 days). To gain an appreciation of the spatial accumulation of sGAG and collagen, the core of high seeding density constructs was removed using a 3 mm biopsy punch and analyzed separately from the annulus. On removal from culture, construct diameter was measured, the wet mass of both the core and annulus was recorded, and all samples were subsequently frozen at −85°C for later analyses. Samples were digested with papain (125 μg/mL) in 0.1 M sodium acetate, 5 mM L-cysteine-HCL, 0.05 M EDTA, and pH 6 (all Sigma-Aldrich) under constant rotation at 60°C for 18 h. DNA content was quantified using the Hoechst Bisbenzimide 33258 dye assay as previously described.38 Proteoglycan content was estimated by quantifying the amount of sGAG in each hydrogel core/annulus using the dimethylmethylene blue dye binding assay (Blyscan, Biocolor Ltd.), with a shark chondroitin sulphate standard. sGAG secreted to culture media at each media exchange was also analyzed for each group (n=3). Total collagen content was determined by measuring the hydroxyproline content,39 using a hydroxyproline to collagen ratio of 1:7.69.40
Histology and immunohistochemistry
At each time point, two or more samples per group were fixed in 4% paraformaldehyde (Sigma-Aldrich), dehydrated with a graded series of alcohol, and embedded in paraffin. Five micrometer sections were produced of the cross section perpendicular to the construct face. Sections were stained with 1% alcian blue 8GX (Sigma-Aldrich) in 0.1 M HCL for sGAG accumulation. Collagen type II deposition was identified by immunohistochemical analysis. Briefly, sections were treated with peroxidase, and then rinsed with PBS before treatment with chondroitinase ABC (Sigma-Aldrich) in a humidified environment at 37°C to enhance permeability of the ECM. The sections were rinsed in PBS, and then incubated with goat serum to block nonspecific sites, before the primary antibody was applied to the sections for 1 h. A mouse monoclonal anti-collagen type II antibody (1:100; 1 mg/mL; Abcam) was used as the primary antibody for collagen type II. Next, an anti-mouse IgG biotin conjugate secondary antibody (1:133; 2 mg/mL; Sigma-Aldrich) was applied for 1 h, followed by incubation with ABC reagent (Vectastain PK-4000; Vector Labs) for 45 min. Finally, the samples were developed with DAB peroxidase (Vector Labs) for 5 min. Positive and negative controls (porcine cartilage and ligament respectively) were included.
Statistical analysis
Statistical analyses were performed using the software package MINITAB 15.1 (Minitab Ltd.). Groups were analyzed for significant differences using a general linear model for analysis of variance with factors of time point, scaffold type, culturing conditions, construct region, and interactions between these factors examined. Tukey's test for multiple comparisons was used to compare conditions. Significance was accepted at a level of p<0.05. Numerical and graphical results are presented as mean±standard deviation (n=3–4 for each group at each time point), with graphical results produced using GraphPad Prism (Version 4.03).
Results
SA using large numbers of chondrocytes leads to the development of a tissue with a more articular cartilage-like composition compared with hydrogel encapsulation
The morphology of SA constructs seeded at low and high seeding densities varied dramatically (Fig. 1). By week 6, constructs formed with 4 million cells were firm, smooth, and flat with a hyaline-like appearance. This was in stark contrast to SA constructs engineered at the lower seeding density (900,000 cells), which were uneven in their appearance, with a significantly reduced diameter (Table 1). Hence, it would appear that 900,000 cells was too low a cell number to generate a satisfactory SA construct. Agarose constructs seeded with 4 million cells were found to significantly increase in thickness over 42 days, with evidence of bulging at the top and bottom surfaces. These constructs were found to weigh substantially more than SA constructs (Fig. 2A, B).
FIG. 1.
Constructs formed through self-assembly (SA) at day 42. Scale bar=5 mm. Color images available online at www.liebertpub.com/tec
Table 1.
Construct Physical Parameters of Diameter (mm) and Thickness (mm) for Chondrocyte Encapsulated Agarose and Self-Assembled Constructs for Both Low and High Seeding Densities
| |
|
Low seeding density |
High seeding density |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
D21 |
D42 |
|
D21 |
D42 |
||||
| Construct type | Parameter | D0 | Transient TGF-β3 | Continuous TGF-β3 | Transient TGF-β3 | Continuous TGF-β3 | D0 | Transient TGF-β3 | Continuous TGF-β3 | Transient TGF-β3 | Continuous TGF-β3 |
| Agarose |
Diameter (mm) |
4.97±0.08 |
5.04±0.1 |
5.08±0.04 |
5.08±0.03 |
5.05±0.08 |
5.06±0.18 |
5.53±0.05a,b |
5.39±0.19a |
5.52±0.06a,b |
5.54±0.1a,b |
| |
Thickness (mm) |
1.56±0.02 |
1.57±0.06 |
1.64±0.01 |
1.62±0.05 |
1.69±0.06a |
1.61±0.01b |
2.86±0.23a,c,b |
2.32±0.16a,b |
3.86±0.47a–d |
3.08±0.03a,b,d |
| Self-Assembly |
Diameter (mm) |
3.62±0.1e |
3.03±0.39e |
2.31±0.75a,e |
3.24±0.3e |
3.1±0.18e |
4.67±0.29b |
5.13±0.04a,b,e |
5.04±0.05a,b,e |
5.1±0.36b,e |
5.18±0.14a,b,e |
| Thickness (mm) | 0.13±0.01e | 0.99±0.27a,e | 0.69±0.59a,e | 1.1±0.13a,e | 1.11±0.2a,e | 0.23±0.02b,e | 1.47±0.38a,e | 1.31±0.06a,e | 1.35±0.14a,e | 1.38±0.1a,e | |
p<0.05 versus day 0.
p<0.05 versus corresponding group in low seeding density.
p<0.05 versus continuous TGF-β3 (same time point).
p<0.05 versus day 21 with same culturing conditions.
p<0.05 versus corresponding agarose group.
TGF, transforming growth factor.
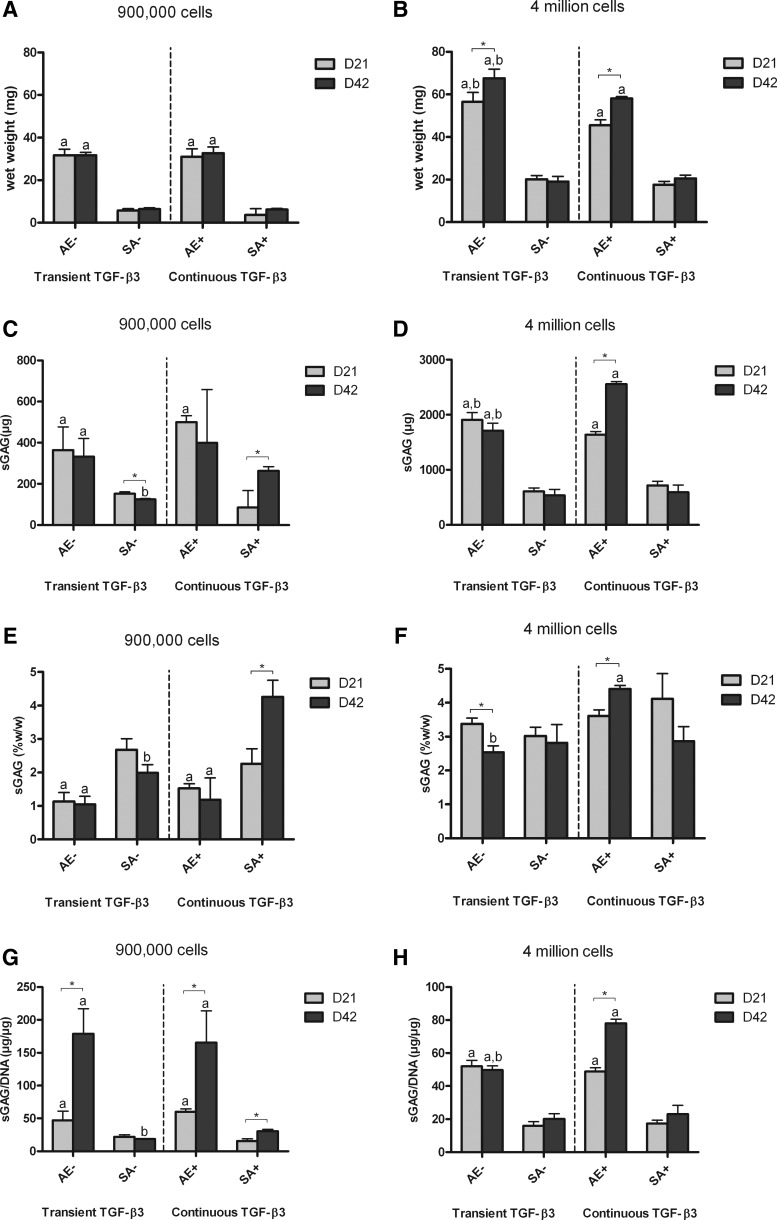
FIG. 2.
Wet weight and sulphated glycosaminoglycan (sGAG) content of agarose encapsulation (AE) and SA constructs for both transient (AE−, SA−) and continuous (AE+, SA+) transforming growth factor (TGF)-β3 supplementation. (A) wet weight for low seeding density constructs (mg); (B) wet weight for high seeding density constructs (mg); (C) sGAG content for low seeding density constructs (μg); (D) sGAG content for high seeding density constructs (μg); (E) sGAG content normalized to wet weight for low seeding density constructs (%w/w); (F) sGAG content normalized to wet weight for high seeding density constructs (%w/w); (G) sGAG content normalized to DNA content for low seeding density constructs (μg/μg); (H) sGAG content normalized to DNA content for high seeding density constructs (μg/μg). ap<0.05 versus SA with same culturing conditions at same time point; bp<0.05 versus continuous TGF-β3 with same scaffold at same time point. *Denotes significant difference with p<0.05.
By day 42 in culture, agarose constructs seeded with 4 million chondrocytes accumulated higher levels of sGAG (2555.21±46.05 μg) compared with any other group (Fig. 2C, D). For both transient and continuous TGF-β3 supplementation, AE led to greater amounts of absolute sGAG accumulation (measured in μg) compared with SA. However, when normalized to wet weight, SA constructs were found to accumulate comparable levels of sGAG to agarose hydrogels (Fig. 2E, F). Finally, when normalized to DNA content (Fig. 2G, H), sGAG accumulation was found to be significantly greater in agarose gels compared with SA constructs for both seeding densities. In addition, a lower seeding density was more conducive to matrix synthesis (on a per cell basis) in agarose constructs.
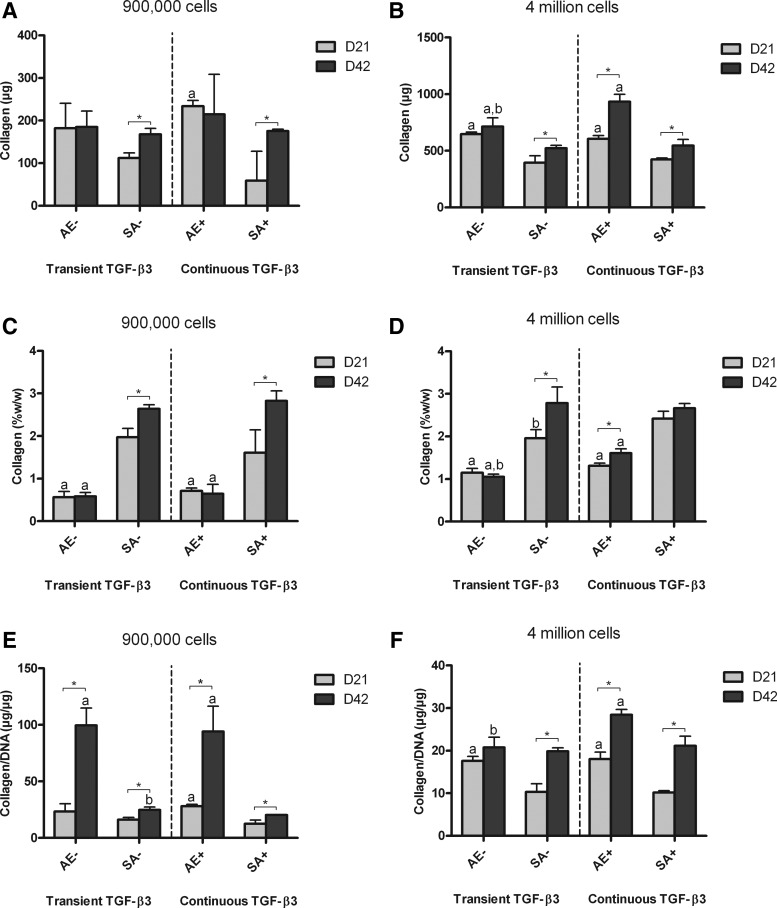
Similar trends were observed in collagen accumulated per construct (Fig. 3), with continuously supplemented agarose hydrogels accumulating significantly more collagen than other constructs by day 42 (Fig. 3B). When normalized to wet weight, however, the SA constructs accumulated more collagen than corresponding AE constructs, for both low and high seeding densities (Fig. 3C, D). When normalized to DNA content (Fig. 3E, F), collagen accumulation was observed as following similar trends to sGAG/DNA. Collagen synthesis (Collagen/DNA) did not appear to be dramatically affected by transient TGF-β3 supplementation for either SA or AE.
FIG. 3.
Collagen content of agarose (AE) and SA constructs for both transient (AE-, SA-) and continuous (AE+, SA+) TGF-β3 supplementation. (A) collagen content for low seeding density constructs (μg); (B) collagen content for high seeding density constructs (μg); (C) collagen content normalized to wet weight for low seeding density constructs (%w/w); (D) collagen content normalized to wet weight for high seeding density constructs (%w/w); (E) collagen content normalized to DNA content for low seeding density constructs (μg/μg); (F) collagen content normalized to DNA content for high seeding density constructs (μg/μg). ap<0.05 versus SA with same culturing conditions at same time point; bp<0.05 versus continuous TGF-β3 with same scaffold at same time point. *Denotes significant difference with p<0.05.
The temporal development of grafts engineered using hydrogel encapsulation and SA was also different. At high seeding densities, sGAG and collagen accumulation in AE constructs continued to increase from days 21 to 42 in continuously supplemented conditions (Figs. 2F and 3D). In contrast, ECM accumulation in SA constructs appeared to peak by day 21 (Figs. 2F and 3D), with smaller changes over the subsequent 21 days of culture. There were comparable levels of sGAG and greater levels of collagen accumulation (measured as %w/w) in day 21 SA grafts compared with day 42 agarose constucts (Figs. 2E, F and 3C, D).
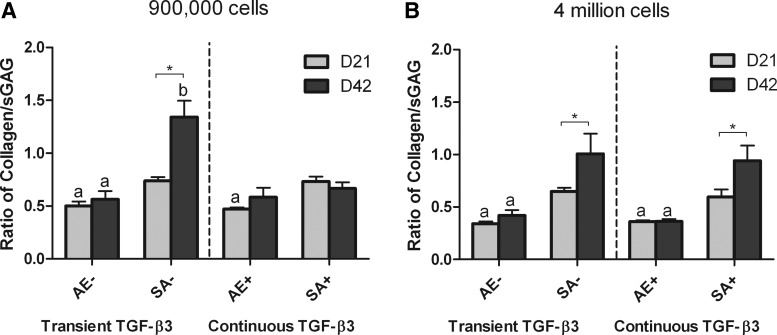
In normal articular cartilage, the tissue contains approximately three times more collagen than sGAG as a percentage of wet weight.41 To enable the comparison between the relative compositions of our engineered tissue with normal articular cartilage, we normalized collagen accumulation within all constructs to corresponding sGAG accumulation (Fig. 4). At higher seeding densities (Fig. 4B), SA constructs at day 42 displayed a ratio of ∼1, which was significantly greater than that of agarose constructs (less than 0.5). This would suggest that SA using a sufficient number of chondrocytes leads to the development of a tissue with a more cartilage-like composition compared with hydrogel encapsulation.
FIG. 4.
Ratio of collagen to sGAG content of agarose (AE) and SA constructs for both transient (AE−, SA−) and continuous (AE+, SA+) TGF-β3 supplementation at days 21 and 42. (A) low seeding density; (B) high seeding density. ap<0.05 versus SA with same culturing conditions at same time point; bp<0.05 versus continuous TGF-β3 with same scaffold at same time point. *Denotes significant difference with p<0.05.
The spatial accumulation of matrix components in tissues engineered using SA and hydrogel encapsulation
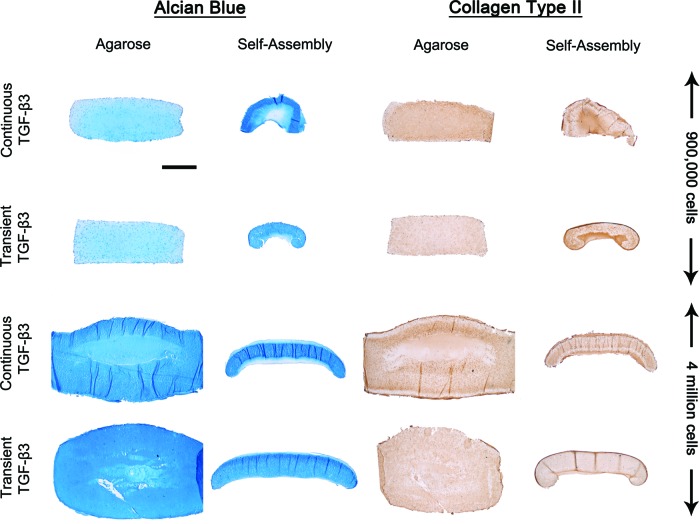
All constructs stained positively for sGAG and collagen type II (Fig. 5), with evidence of increased staining of collagen type II in constructs continuously supplemented with TGF-β3. SA at a higher seeding density resulted in the development of a more uniform tissue, with contraction and distortion of SA constructs witnessed at low seeding densities. High seeding density SA constructs exhibited a peripheral region with weak sGAG staining and strong collagen type II staining. At higher magnification, it was observed that the structure and organization of SA constructs mimics certain aspects of native articular cartilage (Fig. 6). Clustering of chondrocytes was observed in the deeper zones of the tissue. The superficial regions of the tissue stained intensely for type II collagen. Transiently supplemented SA constructs appeared more homogeneous than continuously supplemented constructs.
FIG. 5.
Alcian Blue staining for sGAG production, and type II collagen immunohistochemistry staining of agarose and SA constructs for both transient and continuous TGF-β3 supplementation at day 42. Scale bar=1 mm. Color images available online at www.liebertpub.com/tec
FIG. 6.
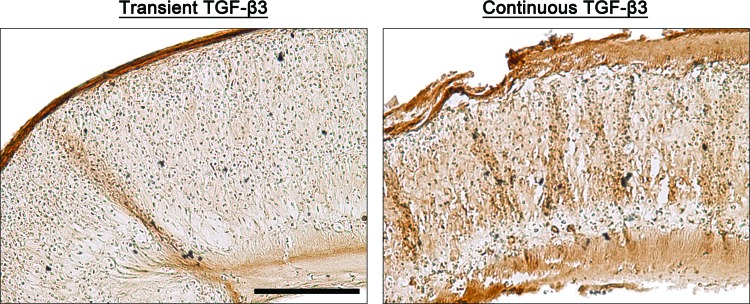
High magnification images of type II collagen immunohistochemistry staining of SA constructs for both transient and continuous TGF-β3 supplementation at day 42 (high seeding density constructs). Scale bar=250 μm. Color images available online at www.liebertpub.com/tec
We decided to only investigate high seeding density constructs from this point forward in the experiment, as it was clear from our analysis that the low seeding density generated an inadequate SA construct.
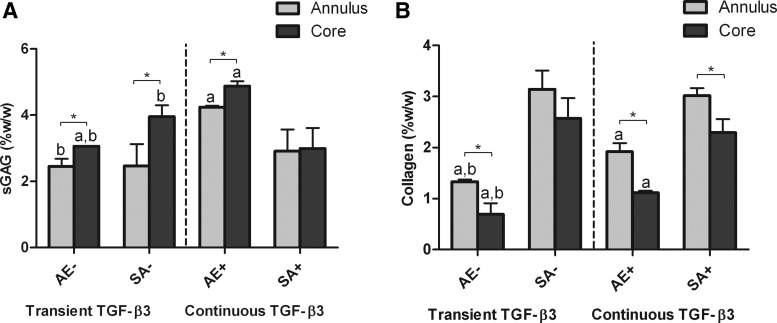
Prevention of core degradation is an important challenge when scaling up engineered grafts. To gain an appreciation of spatial variations in matrix synthesis within constructs engineered using AE and SA, we next compared sGAG and collagen accumulation in the core and annular regions of these constructs (Fig. 7). In each region of the constructs, similar trends were seen between groups in terms of respective sGAG and collagen accumulation. Continuously supplemented AE constructs accumulated significantly more sGAG than other constructs in both the core (4.87±0.14 %w/w) and annulus (4.24±0.04 %w/w). AE constructs accumulated significantly more sGAG in their core compared with their annuli, as did transiently supplemented SA constructs. In contrast to this, a more homogeneous sGAG distribution was observed in continuously supplemented SA constructs, with no significant difference found between core and annulus. As noted earlier, SA constructs accumulated significantly more collagen (measured as %w/w) than their corresponding AE constructs. Greater collagen accumulation was observed in the annular regions of all groups compared with their respective cores (although this was not significant in transiently supplemented SA constructs).
FIG. 7.
Biochemical composition of core and annular regions of agarose (AE) and SA constructs for both transient (AE−, SA−) and continuous (AE+, SA+) TGF-β3 supplementation at day 42. (A) sGAG content normalized to wet weight for high seeding density constructs (%w/w); (B) collagen content normalized to wet weight for high seeding density constructs (%w/w). ap<0.05 versus SA with same culturing conditions at same time point; bp<0.05 versus continuous TGF-β3 with same scaffold at same time point. *Denotes significant difference with p<0.05.
Total matrix synthesis is greater in hydrogels than SA
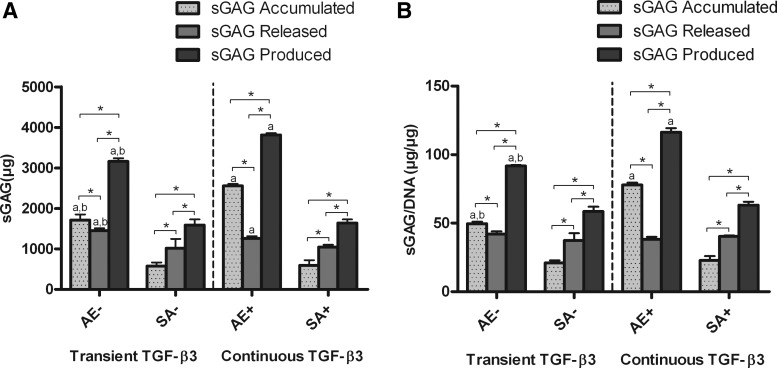
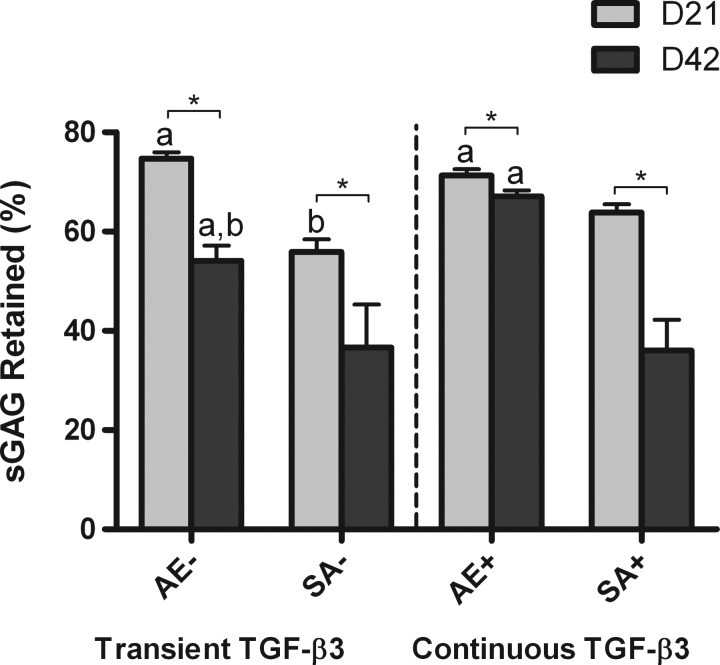
We next wished to determine whether the greater levels of sGAG accumulation within the agarose hydrogels (measured in μg) were due to greater total sGAG synthesis or enhanced retention of sGAG within the construct (Figs. 8 and 9). Agarose constructs were found to accumulate significantly more sGAG than corresponding SA constructs, but they also released more sGAG to the culture media, with the highest levels of sGAG release observed in transiently supplemented agarose constructs (1447.56±61.88 μg) (Fig. 8A). Continuously supplemented agarose constructs synthesized the greatest overall levels of sGAG (3809.72±48.58 μg). The total amount of sGAG synthesis in these AE constructs was approximately double that of SA constructs. To ascertain whether this increased level of sGAG production was due to changes in cell number, we normalized our results to DNA content (Fig. 8B). We found almost identical trends, indicating that the greater sGAG production within AE constructs was mainly due to an enhanced matrix synthesizing capacity of the encapsulated cells. Agarose hydrogels were also more efficient at retaining sGAG within the construct (Fig. 9), with continuously supplemented constructs retaining ∼67% of synthesized sGAG (day 42 samples). All constructs were found to retain a lower % of sGAG at day 42 compared to day 21.
FIG. 8.
Total sGAG accumulated, released, and produced for agarose (AE) and SA constructs for both transient (AE−, SA−) and continuous (AE+, SA+) TGF-β3 supplementation over 42 days. (A) sGAG for high seeding density constructs (μg); (B) sGAG normalized to DNA content for high seeding density constructs (μg/μg). ap<0.05 versus SA with same culturing conditions at same time point; bp<0.05 versus continuous TGF-β3 with same scaffold at same time point. *Denotes significant difference with p<0.05.
FIG. 9.
Percentage of sGAG retained in agarose (AE) and SA constructs for both transient (AE−, SA−) and continuous (AE+, SA+) TGF-β3 supplementation. ap<0.05 versus SA with same culturing conditions at same time point; bp<0.05 versus continuous TGF-β3 with same scaffold at same time point. *Denotes significant difference with p<0.05.
Discussion
The objective of this study was to directly compare the SA method to AE for engineering cartilaginous grafts using passaged chondrocytes. Two seeding densities were chosen, as it is known that a minimum number of cells are required to form stable SA constructs.31 We found that at a low seeding density of 900,000 cells (46,000 cells/mm2), generating a uniform 5 mm diameter SA construct proved difficult. Previous studies have shown that the minimum number of cells needed to generate a uniform 5 mm diameter chondrocyte SA construct is 2 million cells (102,000 cells/mm2).31 At the high seeding density (4 million cells total or 204,000 cells/mm2), we observed that sGAG and collagen synthesis was greater using AE compared with SA. When normalized to wet weight, however, SA constructs accumulated significantly greater levels of collagen compared with agarose gels. Consequently, SA led to the formation of an engineered cartilaginous tissue with a ratio of collagen to sGAG that is more comparable to native articular cartilage. A further benefit of SA is that such grafts can be generated within a relatively short time frame (∼3 weeks), with comparable sGAG levels and higher collagen levels to AE constructs. Shortened culture times are important for clinical translation of tissue engineered products.
An inherent advantage of using agarose hydrogels is the ability to easily control the height and width of the engineered tissue. However depending on the cell seeding density, the construct can experience bulging at the top and bottom surfaces, as was observed in the high seeding density AE constructs. SA constructs reached a maximum thickness of ∼1.4 mm. This is similar to previous studies18 where a SA thickness of ∼1 mm was reached. Articular cartilage thickness is on average 2.4±0.5 mm in human medial femoral condyles,42,43 demonstrating that further optimization is required if SA constructs are to be used to treat full thickness cartilage defects.
sGAG accumulation was greater in agarose constructs compared with SA. To assess whether this was simply due to greater retention of sGAG within hydrogels, or due to overall higher levels of sGAG synthesis, we evaluated sGAG release to the media. Both the amount of sGAG released and total sGAG retained was higher in the agarose hydrogels, clearly demonstrating that total sGAG synthesis was higher in this system. To determine whether this greater sGAG synthesis was due to greater cell proliferation in the hydrogel environment, we normalized our sGAG data to DNA content. Even by this measure, sGAG synthesis was higher in agarose hydrogels, indicating that altered ECM synthesis and not simply greater proliferation in the hydrogel environment was responsible for this different level of sGAG accumulation. This may be considered an advantage to using agarose, with more sGAG synthesis on a per cell basis, and also a greater percentage of sGAG retained within the constructs. This occurs despite higher levels of collagen accumulation (%w/w) in the SA constructs, which presumably play an important role in proteoglycan retention in engineered tissues, highlighting the benefit of agarose for maintaining synthesized matrix components. The higher levels of cartilage-specific ECM synthesis in the AE constructs may be due to the agarose promoting a more spherical chondrocyte morphology, which previously has been shown to support the re-establishment of a chondrogenic phenotype in passaged chondrocytes.3 It could also be due to the physical separation of cells within the agarose hydrogel; whereas in SA constructs, significant cell-to-cell contact occurs.
While chondrocytes appear more synthetically active in hydrogels, the composition of the engineered tissue (as a % of wet weight) as well as the relative amounts of collagen to proteoglycans are more similar to native articular cartilage in the SA constructs. It has been well documented that achieving native levels of collagen accumulation is more challenging than reaching native levels of proteoglycan accumulation in tissue-engineered cartilage.44–47 Indeed, rapid GAG synthesis has been hypothesized to be an impediment to collagen synthesis in chondrocyte seeded agarose hydrogels, with recent studies demonstrating that inducing enzymatic GAG loss during the early phase of culture can increase the ultimate collagen concentration and tensile properties of the engineered tissue.48 The local environment within SA constructs would appear to suppress sGAG synthesis while maintaining collagen synthesis at levels approaching that found in the agarose hydrogels. Therefore, in spite of the fact that both sGAG synthesis and retention were lower in SA constructs compared with AE constructs, it would appear that the SA process generates a tissue with a composition more akin to that of native articular cartilage.
By spatially analyzing the biochemical composition of the engineered tissues, we observed greater collagen accumulation in the annulus of constructs compared with their corresponding cores (Fig. 7). This could be due to gradients in nutrients and other regulatory molecules developing within the constructs. It should be noted that sGAG levels were comparable between the core and annuli of continuously supplemented SA constructs. This would suggest that collagen synthesis may be more sensitive to nutrient availability than sGAG synthesis.
It was noted that collagen type II staining was more intense in superficial regions of SA constructs, which is similar to native articular cartilage where staining is generally highest in the superficial tangential zone. Clustering of chondrocytes was also observed in the deeper zones of the SA tissues. Previous studies have demonstrated that organization of cartilaginous tissues generated by SA of MSCs mimic certain aspects of the native articular cartilage architecture. Specifically, these tissues stained intensely for collagen type II, and weakly for proteoglycans, in the superficial region of the engineered tissue.19 It may be that tension developing at the surface of SA constructs is contributing to the higher level of collagen type II production in the superficial region of the developing tissue. Greater nutrient/growth factor availability in this region of the engineered tissue could also play a role.
Since transient TGF-β3 stimulation has been shown to enhance chondrogenesis in bovine chondrocyte seeded agarose hydrogels,17 we compared the effect of such media supplementation conditions on the development of cartilaginous grafts engineered using both the SA and AE approaches. We found no clear benefit to transient TGF-β3 supplementation for either SA or AE, apart from the fact that the fiscal cost of this approach is lower than continuous growth factor supplementation. The discrepancy between our findings and that of previous studies17 may possibly be due to our use of expanded chondrocytes or species differences.
The lack of mechanical property data is a limitation of this study. The nonuniform shape of the SA tissue (and indeed agarose constructs at high seeding densities, see Fig. 5) led to varying and possibly unreliable mechanical testing results in pilot studies, and such tests were not undertaken as a part of the main study. The fact that chondrocytes were obtained from the femoral condyles of a 4 month-old pig might also be considered a limitation of the study. At this age, such animals have not reached skeletal maturity. Chondrocytes from such tissue would probably be more adept at producing cartilage-specific ECM than chondrocytes obtained from an older donor, as seen in bovine49,50 and human donors.51 As with many tissue engineering studies, cells were expanded and differentiated in high glucose (25 mM) culture medium. One possible implication of this could be hyperglycaemic conditions, leading to the copious production of hyaluronic acid (HA).52 Since rapid GAG synthesis has been hypothesized to be an impediment to collagen production,48 this additionally produced HA could inhibit collagen production of our tissue engineered constructs. Future studies will explore the influence of altered glucose conditions on tissue engineered cartilage.
In conclusion, a higher seeding density was required to develop robust cartilaginous grafts using a SA approach. If achieving such high numbers of chondrocytes is clinically feasible, the SA approach has many attractive attributes, including the generation of grafts with a high collagen content, and the development of a tissue with an architecture and a ratio of collagen to sGAG content more closely resembling native articular cartilage. The SA process also generated tissues with such high levels of ECM within a relatively short time frame. Coupled with the inherent advantages of a scaffold-free approach, the results of this study provide strong support for the future use of the SA approach for engineering functional cartilaginous grafts for clinical applications.
Acknowledgments
Funding was provided by the Irish Research Council for Science, Engineering and Technology (G30403), the SFI President of Ireland Young Researcher Award (08/Y15/B1336), and a European Research Council Starter Grant (StemRepair–Project number: 258463).
Disclosure Statement
No competing financial interests exist.
References
- 1.Erickson I.E., Huang A.H., Chung C., Li R.T., Burdick J.A., and Mauck R.L.Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A 15,1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang A.H., Stein A., Tuan R.S., and Mauck R.L.Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A 15,3461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benya P.D., and Shaffer J.D.Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30,215, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Sun D., Aydelotte M.B., and Maldonado B.Clonal analysis of the population of chondrocytes from the Swarm rat chondrosarcoma in agarose culture. J Orthop Res 4,427, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Buschmann M.D., Gluzband Y.A., Grodzinsky A.J., Kimura J.H., and Hunziker E.B.Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res 10,745, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Mauck R.L., Soltz M.A., Wang C.C., Wong D.D., Chao P.H., Valhmu W.B., Hung C.T., and Ateshian G.A.Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122,252, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Mauck R.L., Seyhan S.L., Ateshian G.A., and Hung C.T.Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng 30,1046, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Mauck R.L., Wang C.C.B., Oswald E.S., Ateshian G.A., and Hung C.T.The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage 11,879, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Buckley C.T., Thorpe S.D., and Kelly D.J.Engineering of large cartilaginous tissues through the use of microchanneled hydrogels and rotational culture. Tissue Eng Part A 15,3213, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Buckley C.T., Vinardell T., Thorpe S.D., Haugh M.G., Jones E., McGonagle D., and Kelly D.J.Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J Biomech 43,920, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Vinardell T., Buckley C.T., Thorpe S.D., and Kelly D.J.Composition-function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J Tissue Eng Regen Med 5,673, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Meyer E.G., Buckley C.T., Steward A.J., and Kelly D.J.The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J Mech Behav Biomed Mater 4,1257, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Buckley C.T., Meyer E.G., and Kelly D.J.The influence of construct scale on the composition and functional properties of cartilaginous tissues engineered using bone marrow-derived mesenchymal stem cells. Tissue Eng Part A 18,382, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Buckley C.T., Downey R., Mulhall K.J., and Kelly D.J.The role of environmental factors in regulating the development of cartilaginous grafts engineered using osteoarthritic human infrapatellar fat pad-derived stem cells. Tissue Eng Part A 18,1531, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinardell T., Sheehy E.J., Buckley C.T., and Kelly D.J.A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A 18,1161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauck R.L., Yuan X., and Tuan R.S.Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14,179, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Byers B.A., Mauck R.L., Chiang I.E., and Tuan R.S.Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A 14,1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J.C., and Athanasiou K.A.A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12,969, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Elder S.H., Cooley A.J., Borazjani A., Sowell B.L., To H., and Tran S.C.Production of hyaline-like cartilage by bone marrow mesenchymal stem cells in a self-assembly model. Tissue Eng Part A 15,3025, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Reginato A.M., Iozzo R.V., and Jimenez S.A.Formation of nodular structures resembling mature articular cartilage in long-term primary cultures of human fetal epiphyseal chondrocytes on a hydrogel substrate. Arthritis Rheum 37,1338, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Estrada L.E., Dodge G.R., Richardson D.W., Farole A., and Jimenez S.A.Characterization of a biomaterial with cartilage-like properties expressing type X collagen generated in vitro using neonatal porcine articular and growth plate chondrocytes. Osteoarthritis Cartilage 9,169, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Novotny J.E., Turka C.M., Jeong C., Wheaton A.J., Li C., Presedo A., Richardson D.W., Reddy R., and Dodge G.R.Biomechanical and magnetic resonance characteristics of a cartilage-like equivalent generated in a suspension culture. Tissue Eng 12,2755, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kim M., Kraft J.J., Volk A.C., Pugarelli J., Pleshko N., and Dodge G.R.Characterization of a cartilage-like engineered biomass using a self-aggregating suspension culture model: Molecular composition using FT-IRIS. J Orthop Res 29,1881, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraft J.J., Jeong C., Novotny J.E., Seacrist T., Chan G., Domzalski M., Turka C.M., Richardson D.W., and Dodge G.R.Effects of hydrostatic loading on a self-aggregating, suspension culture-derived cartilage tissue analog. Cartilage 2,254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H., Grynpas M., and Kandel R.A.Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials 18,1425, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Naumann A., Dennis J.E., Aigner J., Coticchia J., Arnold J., Berghaus A., Kastenbauer E.R., and Caplan A.I.Tissue engineering of autologous cartilage grafts in three-dimensional in vitro macroaggregate culture system. Tissue Eng 10,1695, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hoben G.M., Hu J.C., James R.A., and Athanasiou K.A.Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng 13,939, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Elder B.D., and Athanasiou K.A.Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res 26,238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elder B.D., and Athanasiou K.A.Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE 3,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ofek G., Revell C.M., Hu J.C., Allison D.D., Grande-Allen K.J., and Athanasiou K.A.Matrix development in self-assembly of articular cartilage. PLoS One 3,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revell C.M., Reynolds C.E., and Athanasiou K.A.Effects of initial cell seeding in self assembly of articular cartilage. Ann Biomed Eng 36,1441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elder B.D., and Athanasiou K.A.Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A 15,1151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elder B.D., and Athanasiou K.A.Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage 17,114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murdoch A.D., Grady L.M., Ablett M.P., Katopodi T., Meadows R.S., and Hardingham T.E.Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage. Stem Cells 25,2786, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Ando W., Tateishi K., Hart D.A., Katakai D., Tanaka Y., Nakata K., Hashimoto J., Fujie H., Shino K., Yoshikawa H., and Nakamura N.Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials 28,5462, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Ando W., Tateishi K., Katakai D., Hart D.A., Higuchi C., Nakata K., Hashimoto J., Fujie H., Shino K., Yoshikawa H., and Nakamura N.In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: Biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A 14,2041, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Zhang L., Su P., Xu C., Yang J., Yu W., and Huang D.Chondrogenic differentiation of human mesenchymal stem cells: A comparison between micromass and pellet culture systems. Biotechnol Lett 32,1339, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.J., Sah R.L., Doong J.Y., and Grodzinsky A.J.Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174,168, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Kafienah W., and Sims T.J.Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol Biol 238,217, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Ignat'eva N.Y., Danilov N.A., Averkiev S.V., Obrezkova M.V., Lunin V.V., and Sobol E.N.Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem 62,51, 2007 [Google Scholar]
- 41.Mow V.C., Ratcliffe A., and Robin Poole A.Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 13,67, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Hunziker E.B., Quinn T.M., and Hauselmann H.J.Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage 10,564, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Klein T.J., Malda J., Sah R.L., and Hutmacher D.W.Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev 15,143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freed L.E., Hollander A.P., Martin I., Barry J.R., Langer R., and Vunjak-Novakovic G.Chondrogenesis in a cell-polymer-bioreactor system. Exp Cell Res 240,58, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Waldman S.D., Spiteri C.G., Grynpas M.D., Pilliar R.M., and Kandel R.A.Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res 21,590, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Hung C.T., Mauck R.L., Wang C.C.B., Lima E.G., and Ateshian G.A.A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng 32,35, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Gemmiti C.V., and Guldberg R.E.Fluid flow increases type II collagen deposition and tensile mechanical properties in bioreactor-grown tissue-engineered cartilage. Tissue Eng 12,469, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Bian L., Crivello K.M., Ng K.W., Xu D., Williams D.Y., Ateshian G.A., and Hung C.T.Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A 15,2065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran-Khanh N., Hoemann C.D., McKee M.D., Henderson J.E., and Buschmann M.D.Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res 23,1354, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Erickson I.E., Van Veen S.C., Sengupta S., Kestle S.R., and Mauck R.L.Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Relat Res 469,2744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbero A., Grogan S., Schäfer D., Heberer M., Mainil-Varlet P., and Martin I.Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage 12,476, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Wang A., De La Motte C., Lauer M., and Hascall V.Hyaluronan matrices in pathobiological processes. FEBS J 278,1412, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]