Abstract
Objective: The purpose of this study was to test the efficacy of clomipramine and fluoxetine, controlled by placebo, and compare their action in children and adolescents with anxiety disorders.
Method: Thirty subjects (ages 7–17 years), who were diagnosed with generalized anxiety disorder and/or separation anxiety disorder and/or social phobia, were submitted to a 12 week double-blind, randomized, placebo-controlled trial of clomipramine and fluoxetine. The instruments included: the Schedule for Affective Disorders and Schizophrenia, the Multidimensional Anxiety Scale for Children, the Children's Depression Inventory, the Clinical Global Impressions, and the Children's Global Assessment Scale.
Results: All groups (clomipramine [n=9], fluoxetine [n=10], placebo [n=11]) showed a significant improvement after 12 weeks of treatment. There were significant differences between the fluoxetine and placebo groups in some ratings of anxiety severity and impairment. No significant differences were observed between clomipramine and placebo groups or between fluoxetine and clomipramine groups.
Conclusions: Treatment with placebo showed an unusual high response rate. Clomipramine showed similar efficacy compared with fluoxetine, although it was not superior to placebo.
Introduction
Anxiety disorders (AD) are the most prevalent psychiatric condition in childhood and adolescence, with 6–20% of this age group affected (Costello et al. 2005). The identification and treatment of these conditions may prevent negative repercussions during childhood and, possibly, in adulthood. The association of cognitive-behavioral therapy (CBT) with pharmacotherapy is considered the treatment of choice for young people with AD (Walkup et al. 2008). It has been suggested that the selective serotonin reuptake inhibitors (SSRIs) are effective and safe for the acute treatment of AD, including generalized anxiety disorder (GAD), separation anxiety disorder (SAD), and/or social phobia (SP) in children and adolescents (Seidel and Walkup 2006; Rynn et al. 2011). Differently from the SSRIs, clomipramine, a non-selective serotonin reuptake inhibitor, has barely been studied in patients with these anxiety conditions. The only (double-blind) treatment trial, with children with school refusal and neurotic disorder, failed to demonstrate any significant short-term effects of clomipramine. It is noteworthy, however, that subjects in this study had heterogeneous diagnoses and were submitted to small dose regimens (40–75 mg/day) (Berney et al. 1981). In contrast with the scarcity of studies with clomipramine in GAD, SAD, and SP, children and adolescents with another AD, obsessive-compulsive disorder (OCD), have long been successfully treated with this compound (Leonard et al. 1989). A metanalitic study has demonstrated the superiority of clomipramine over all SSRIs in the treatment of pediatric OCD, for which the SSRIs have been extensively studied and used (Geller et al. 2003).
The objective of this study was to test the efficacy of clomipramine, compared with fluoxetine and placebo, in children and adolescents with AD. It is hypothesized that clomipramine may be an effective alternative treatment to SSRIs for the acute treatment of GAD, SAD, and SP in children and adolescents.
Methods
The study included 30 children and adolescents (14 boys) between 7 and 17 years of age, with one or more diagnoses of AD (GAD, SAD, and/or SP). Subjects were excluded if they presented at the initial evaluation the following conditions: Comorbid diagnosis of major depressive episode; attention-deficit/hyperactivity disorder (ADHD) as a primary disorder; previous or current diagnosis of other psychiatric disorders or any organic brain disease; suicidal ideation; current treatment for anxiety or use of medication that affects the central nervous system; or pregnancy. Informed consent, signed by a legal guardian, was obtained. The study was approved by the Ethics Committee of the University of São Paulo Medical School Hospital.
The subjects were randomly divided into three groups (clomipramine, fluoxetine, and placebo), for a 12 week treatment. The medications were administered in flexible doses (adjusted according to clinical response, as evaluated by a child and adolescent psychiatrist). The doses of fluoxetine could vary from 10 mg to 40 mg/day for children and to 60 mg/day for adolescents; doses of clomipramine, from 25 mg/day to 5mg/kg/day or 150 mg/day for children and to 225 mg/day for adolescents. Placebo was administered in capsules, identical to those used for the active medications. Capsules were administered in the same quantity during the 12 weeks of treatment. Children and adolescents received two and three capsules, respectively. Their content was adjusted as the doses changed. A research assistant was responsible for the administration of medications. He was not blind regarding the treatments, and did not participate in evaluations of any of the subjects. After each clinical appointment, the psychiatrists instructed the research assistant whether the dose should be maintained or changed. In cases of a clinical worsening (at any time of treatment), lack of therapeutic response after 6 weeks of treatment, or intolerable side effects, subjects were withdrawn from the trial and continued to receive treatment at our service. Patients were followed weekly for the first month and then every 2 weeks until the end of the 12 weeks of treatment. Apart from the assessments that included the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (at baseline and week 12), which lasted longer (between 45 and 90 minutes), both clinical appointments and the independent evaluator's assessments lasted ∼30 minutes. The clinical appointments were conducted by three experienced child and adolescent psychiatrists. All were aware of the research protocol, which established that the clinical appointments should only focus on the medical treatments, including: The evaluation of anxiety symptoms, of laboratory workup, and of side effects; and that no psychotherapeutic intervention should be delivered (particularly CBT's expositions/response preventions). During the clinical appointments, the psychiatrist provided only basic information about anxiety disorders to all patients and parents/legal guardians. Assessments included the following instruments: K-SADS infant version (Ambrosini 2000), Multidimensional Anxiety Scale for Children (MASC) (March et al. 1997), Children's Depression Inventory (CDI) (Kovacs 1983), the National Institute for Mental Health (NIMH) Clinical Global Impressions (CGI) (Guy 1976), and Children's Global Assessment Scale (CGAS) (Shaffer et al. 1983).
Statistical analysis
Initially, a descriptive analysis of the variables was performed. An analysis of variance (ANOVA) was performed to compare the three groups in the variables (MASC, CGI, C-GAS, CDI) at week 0 and at week 12 using the Scheffé test (when ANOVA was significant). The paired Student t test was applied to compare the measurements at weeks 0 and 12 in each group, separately. For the evaluation of response and remission to treatment, the Fisher test was used. The effect of treatment was evaluated by regressive analysis using the “generalized estimating equations” (GEE) method, with robust standard errors estimation. The model included the treatment groups, time of evaluation and the interaction among them, considering the placebo group as reference for the treatment, and time as a continuous variable. The autoregressive correlation (AR-1) was the internal correlation assumed in the analyses of the MASC (total and subscales) and the CGI variables. For the C-GAS analysis, equal correlations within each subject measurements were assumed (exchangeable correlation), as with AR-1 correlation structure, the model did not fit. The GEE analyses were also conducted considering fluoxetine as reference to contrast with clomipramine. Graphic methods were used to show outcome changes over time (weeks). All analyses were done based on intention to treat. The level of significance was set at α=0.05. The analyses were performed using software S-PLUS v. 6.2 for Windows (http://lib.stat.cmu.edu/).
Results
Thirty subjects were included, 9 in the group treated with clomipramine, 10 in the group treated with fluoxetine, and 11 in the control group (placebo). There were three dropouts, two patients from the fluoxetine group (one for worsening of the clinical condition and one for noncompliance); one patient from the clomipramine group (because of side effects). Statistical analysis of the demographic characteristics of the three treatment groups was not performed because of the small sample size. All three groups showed similar average age: 11.4 in the placebo group, 11.2 in the clomipramine group, and 11.6 in the fluoxetine group (at baseline). The percentages of males were 54.5% (n=6) in the placebo group, 50% (n=5) in the fluoxetine group, and 33.3% (n=3) in the clomipramine group. Concerning the socioeconomic status, subjects were equally divided in the fluoxetine group (upper/middle class: 50%/n=5; lower class: 50%/n=5), and in the placebo group (upper/middle class: 45.4%/n=5; lower class: 45.4%/n=5; missing data: 1 subject). In the clomipramine group, 14.3% (n=1) were from the upper/middle class, and 58.7% (n=6) were from the lower class (missing data: 2 subjects).
In the initial evaluation, all groups showed similarities in relation to the level of anxiety in all subscales of the MASC (Table 1) and in the global evaluations – CGI mean scores (placebo: 4.82, SD: 0.4; clomipramine: 5, SD: 0.7; fluoxetine: 4.9, SD: 0.56) and C-GAS mean scores (placebo: 58, SD: 6.81; clomipramine: 54, SD: 9.81; fluoxetine: 53.3, SD: 6.78). None of the groups showed high levels of depressive symptoms, as verified by the CDI mean scores (placebo: 9.18, SD: 5.03; clomipramine: 10, SD 6.38; fluoxetine: 9.2, SD: 6.44).
Table 1.
Description of the Evolution of the Anxiety Symptoms in the Three Groups, by Means of the Multidimensional Anxiety Scale for Children (MASC) Scores – Total and Subscales in Weeks 0 and 12
| MASC scores | Clomipramine | Fluoxetine | Placebo |
|---|---|---|---|
| MASC total score - mean (SD) | |||
| Week 0 |
58.7 (13.14) |
56.5 (10.67) |
57.09 (15.32) |
| Week 12 |
46.2 (16.55) |
33.5 (5.52) |
47.3 (14.79) |
| Physical Symptoms Subscale - mean (SD) | |||
| Week 0 |
57.5 (10.38) |
56.7 (10.14) |
52.7 (9.44) |
| Week 12 |
45.5 (12.3) |
38.8 (4.22) |
42.3 (9) |
| Harm Avoidance Subscale - mean (SD) | |||
| Week 0 |
42.7 (9.44) |
44.9 (9.84) |
45.9 (11.7) |
| Week 12 |
38 (11.83) |
35.6 (11.69) |
37.6 (12.02) |
| Social Anxiety Subscale - mean (SD) | |||
| Week 0 |
56.4 (14.17) |
60.3 (13.2) |
58 (16.14) |
| Week 12 |
51 (15.54) |
37.7 (3.45)* |
54.1 (13.86)* |
| Separation Anxiety Subscale - mean (SD) | |||
| Week 0 |
66.6 (12.01) |
68 (13.54) |
65.9 (14.98) |
| Week 12 |
54.1 (16.96) |
44.8 (8.5) |
60.2 (14.21) |
| Anxiety Disorder Index Subscale (ADI) - mean (SD) | |||
| Week 0 |
54.6 (14.42) |
50.4 (10.03) |
51.5 (14.48) |
| Week 12 | 43.3 (13.0) | 29.6 (3.54)** | 44.3 (12.93)** |
p=0.039; **p=0.037.
Effects of treatment
The three groups showed significant improvements, as observed in the comparisons of CGI (scale of severity) and C-GAS between weeks 0 and 12: CGI (placebo, p<0.001/clomipramine, p=0.001/fluoxetine, p<0.001); C-GAS (placebo, p=0.011/clomipramine, p=0.001/fluoxetine, p<0.001). There was no significant difference between weeks 0 and 12 in the CDI, in any of the three groups (placebo, p=0.079/clomipramine, p=0.143/fluoxetine, p=0.082).
The response rates to the treatment (CGI=1 or 2) were 87.5% (n=7) in the clomipramine group, 100% (n=8) in the fluoxetine group, and 77.7% (n=7) in the placebo group. The rates of remission (CGI=1) were 75% (n=6) in the clomipramine group, 100% (n=8) in the fluoxetine group, and 44.4% (n=4) in the placebo group, with a significant difference between the fluoxetine and the placebo groups in the average of remission (p=0.029). In the comparisons among all groups, performed in the 12th week, a significant difference was observed favoring the fluoxetine group, when compared with the placebo group, in the “social anxiety” (p=0.039) and “index of anxiety” (p=0.037) subscales of the MASC, and in the C-GAS (p=0.023). Significant differences between the clomipramine and the placebo groups or between the fluoxetine and the clomipramine groups were not observed in any variables studied (Table 1).
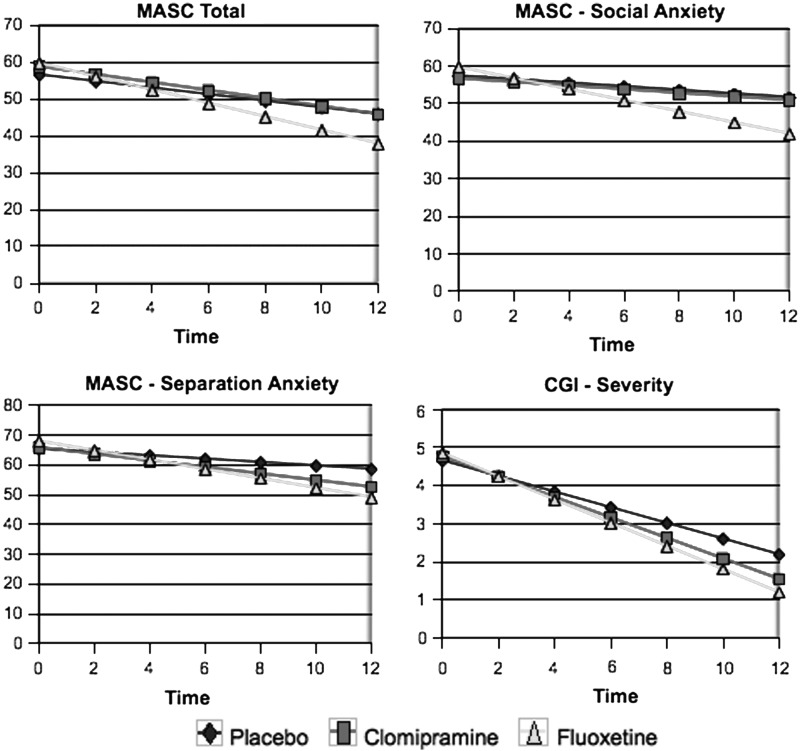
In the regressive analysis, a significant effect was observed for time in all variables. Regarding the effect of the treatment groups, considering the placebo group as a reference, no significant difference was found. However, there was a significant interference of the fluoxetine group in the action of time observed in the variables: “Total score” (p=0.029), “social anxiety” (p=0.007), and “separation anxiety” (p=0.008) subscales of the MASC; and the “CGI – severity scale” (p=0.003). In the C-GAS, the analysis showed a significant effect of the fluoxetine group (placebo as a reference), independent of the time factor (p=0.023), and a significant effect of group and time interaction (p<0.001). A significant effect was also observed for this variable in the clomipramine group in the action of time (p=0.016). There was no significant difference between the fluoxetine and the clomipramine groups, considering fluoxetine as a reference. The data in Figure 1 show the results of the regressive analysis for the “MASC total score,” “social anxiety,” and “separation anxiety” subscales, and for the CGI – severity scale.
FIG. 1.
Comparison through regressive analysis among the clomipramine, fluoxetine, and placebo groups for the variables “total score,” “social anxiety,” and “separation anxiety” subscales of the Multidimensional Anxiety Scale for Children (MASC); and the Clinical Global Impressions (CGI) – severity scale.
Dosages
The average daily doses in week 12 were 118.75 mg for clomipramine, and 35 mg for fluoxetine.
Side effects
The side effects described later in this article were not statistically analyzed, because of the small number of subjects. They represent the ones observed more often in each group. In the clomipramine group, 33.3% of the subjects (n=3) reported a feeling of confusion more often than did subjects in the fluoxetine and placebo groups. In the fluoxetine group, sedation (66.7%: n=6), malaise (44.4%: n=4), abdominal discomfort (40%: n=4), excessive salivation (33.3%: n=3), tachycardia (33.3%: n=3), and excessive sweating (30%: n=3) were more frequently observed than in the other groups. In the placebo group, there were more frequent reports of sleep disturbances (30%: n=3) and agitation (40%: n=4) than in the other groups.
Discussion
To our knowledge, this is the first study to compare clomipramine and fluoxetine, antidepressants of distinct classes, with placebo for the treatment of GAD, SAD, and/or SP in children and adolescents.
All three groups showed significant improvement of symptoms after treatment. Interestingly, the placebo group showed an unusual high rate of response (77.7%), higher than in other studies with anxious children and adolescents (rates in clinical trials range from 10 to 47% [Gittelman-Klein and Klein 1971; Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2001; Rynn et al. 2001; Birmaher et al. 2003; Beidel et al. 2007; Wagner et al. 2004; Walkup et al. 2008]). The analysis of the data showed similar treatment response in both the clomipramine and fluoxetine groups, as no significant differences between them were observed. However, differently from the initial hypothesis, no significant differences were observed between the clomipramine and placebo groups. In some evaluation measures, the fluoxetine group significantly differed from the placebo group.
In adults, an analysis of the United States Food and Drug Administration (FDA) Summary Basis of Approval reports for 11 antidepressants for major depressive disorder (MDD) found that the magnitude of placebo response was the single most powerful variable associated with the outcome of an antidepressant trial (Khan et al. 2003). For studies with placebo response rates<30%, the occurrence of a statistically significant effect favoring drug was close to 3 in 4. In contrast, when placebo response rate was >30%, only 1 in 5 antidepressants showed significant separation. A review of controlled trials of psychotropic drugs for children and adolescents with internalizing disorders found that, as in adults, the magnitude of placebo response was the most powerful predictor of the outcome of a trial, rather than the response of the active treatment itself (Cohen, et al. 2010). This makes sense, considering the wide variation in the percentage of responders to active compounds in most studies with children and adolescents with internalizing disorders: 36–71% (MDD), 21–65% (OCD), and 56–91% (AD) (Bridge et al. 2007). Therefore, the ‘‘success’’ of a trial is more dependent on the placebo response. Indeed, the absence of difference between clomipramine and placebo in the present study was, probably, because of the high placebo response, which, ultimately, influenced the outcome of this trial.
Many aspects could have influenced the high placebo response. First, the frequent assessments of the subjects, despite psychotherapeutic interventions have been avoided during all clinical appointments. Also, participation in a clinical trial, itself, may have a therapeutic benefit, through educational support about the disorder, frequent and structured meetings with professionals, and the expectation of an effective treatment that a clinical trial may bring. The quality of the physician–patient relationship may influence the placebo effect as well. Professionals who show confidence in treatment and caring for patients seem most likely to influence a placebo effect (Sandler 2005). In a meta-analysis of 24 clinical trials, in which active treatment was CBT for treating anxiety disorders (GAD, SAD, SP, simple phobia, and panic disorder) in children and adolescents, In-Albon and Schneider (2007) reported data from two studies that included psychoeducation on anxiety as a control group condition. This procedure showed equal efficacy when compared with active treatment. These results could suggest that improvement in symptoms of AD in children and adolescents can be achieved purely with psychoeducation on anxiety. Therefore, the information about AD provided in our clinical trial (with no encouragement or instructions to exposure/response prevention to feared situations) may have contributed to the high placebo response.
Furthermore, the present study included subjects diagnosed primarily with one or more anxiety disorders without clinically significant comorbidities, which may also have influenced the placebo response, as the rate of improvement caused by the placebo effect varies in different psychiatric diagnoses. In outpatients treated for depression or anxiety, antidepressants and anxiolytics fail to demonstrate superiority over placebo 50% of the time, whereas 30–50% of patients improve with placebo (Khan et al. 2002). Khan and colleagues (2005) evaluated the placebo response in different psychiatric disorders (psychosis, OCD, GAD, major depression, post-traumatic stress disorder, and panic disorder). Heterogeneity was observed in the placebo response, as well as in the drug response among the various disorders. The difference in response between drug and placebo groups was greater in OCD and psychosis. The authors suggested that the placebo response might be related to the subjective distress of patients, and those who have greater insight about their illness (as in depressive and anxiety disorders) would have a greater chance of response to nonspecific treatments.
By contrasting the sample of the present study with samples of children and adolescents with anxiety disorders from other trials, the presence of possible cultural and socioeconomic differences could have also played a role in the higher placebo response observed. A review by Cohen and colleagues (2010) found a strong negative correlation between the percentage of Caucasian patients and the magnitude of the placebo response, that is, the more Caucasian patients, the lower the placebo response. The authors hypothesized that this association could be related to socioeconomic variables, such as low socioeconomic status and early life adversities, which are risk factors for internalizing disorders. In addition, they observed that the expectations of medication treatment may be different across cultural /ethnic/racial groups, leading to differences in response to placebo. In our study, ∼50% of the sample was from the lower socioeconomic class, contrasting with other trials, in which most of subjects were from the middle class (Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2001; Birmaher 2003; Beidel 2007; Walkup 2008).
Excluding trials on pediatric OCD, the studies with tricyclic antidepressants for AD in children and adolescents, most of them with imipramine, have shown controversial results (Gittelman-Klein and Klein 1971; Klein et al. 1992; Bernstein et al. 2000). The only placebo-controlled clinical trial to evaluate clomipramine in children and adolescents with school refusal also showed similarity between its clinical response and the one observed with placebo (Berney et al. 1981). In this study, subjects may have taken subdoses, as only low doses of clomipramine (40–75 mg/day) were used. Moreover, the trial included subjects with rather comprehensive diagnoses: Neurotic disorders with school refusal, which included subjects with anxiety, sensitivity, obsessive-compulsive phenomena, phobias, somatic symptoms, hypochondriasis, or hysterical symptoms. In contrast, in the present study, doses of clomipramine used were higher (mean dose: 118.75 mg/range: 25–175 mg) and well tolerated. Additionally the inclusion criteria were stricter, with only subjects with specific anxiety disorders (according to American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [DSM-IV] diagnostic criteria [American Psychiatric Association 1994]). Despite these methodological differences, the clomipramine group did not differ from the placebo group.
The regressive analysis has not shown the fluoxetine group to have a statistically better effect than the placebo group for most of measurements. A group effect was observed by modifying the effect of time (in some of the primary measures of evaluation), which suggests that the presence of the medication influenced an improved response in those individuals. Although these results are in line with the findings of the literature that shows the efficacy of the SSRI for the treatment of AD in children and adolescents (Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2001; Rynn et al. 2001; Birmaher et al. 2003; Wagner et al. 2004; Walkup et al. 2008), they are less robust. Again, the high placebo response found in our trial has probably influenced these results.
Limitations
The main limitation of this study is related to the restricted number of participants, which prevents generalization of the results. The difficulty of enrolling subjects within the strict inclusion criteria adopted was the main reason for the small sample size of this study. Nonetheless, among the subjects who met DSM-IV diagnostic criteria for one or more AD and were eligible for the study, some subjects were not able to follow the protocol, for various reasons: Some subjects' parents did not allow them to take medications; it was impossible for some subjects (or parents) to attend weekly appointments; loss of contact with some other subjects; and, finally, four of these individuals showed significant improvement after being considered eligible for the trial during the initial evaluation process (before the beginning of treatment), and, therefore, they no longer met inclusion criteria to be randomized to the study. If, on the one hand, the fact of excluding psychiatric and neurological comorbidities has been an advantage of studying the effects of particular drugs on ADs, on the other hand, it has become more difficult to obtain a more robust sample, thus limiting the extension of the findings to populations of children and adolescents with ADs.
Acknowledgments
We thank the colleagues who contributed to the accomplishment of this study.
Disclosures
No competing financial interests exist.
References
- Ambrosini PJ: Historical development and present status of the Schedule for Affective Disorders and Schizophrenia for School-Age (K-SADS). J Am Acad Child Adolesc Psychiatry 39:49–58, 2000 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S: SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry 46:1622–1632, 2007 [DOI] [PubMed] [Google Scholar]
- Berney T, Kolvin I, Bhate SR, Garside RF, Jeans J, Kay B, Scarth L: School phobias: A therapeutic trial with clomipramine and short-term outcome. Br J Psychiatry 133:110–118, 1981 [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Borchardt CM, Perwien AR, Crosby RD, Kushner MG, Thuras PD, Last CG: Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry 39:276–283, 2000 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, Bridge J, Heo J, Brent DA: Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry 42:415–423, 2003 [DOI] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA: Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA 297:1683–1696, 2007 [DOI] [PubMed] [Google Scholar]
- Cohen D, Consoli A, Bodeau N, Purper–Ouakil D, Deniau E, Guile JM, Donnelly C: Predictors of placebo response in randomized controlled trials of psychotropic drugs for children and adolescents with internalizing disorders. J Child Adolesc Psychopharmacol 20:39–47, 2010 [DOI] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, Angold A: The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatric Clin N Am 14:631–648, 2005 [DOI] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Stewart SE, Mullin B, Martin A, Spencer T, Faraone SV: Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder Am J Psychiatry 160:1919–1928, 2003 [DOI] [PubMed] [Google Scholar]
- Gittelman–Klein R, Klein D: Controlled imipramine treatment of school phobia. Arch Gen Psychiatry 25:204–207, 1971 [PMC free article] [PubMed] [Google Scholar]
- Guy W: ECDEU Assessment Manual for Psychopharmacology. 2nd ed. Washington DC: US Government Printing Office; 1976 [Google Scholar]
- In-Albon T, Schneider S: Psychotherapy of childhood anxiety disorders: A meta-analysis. Psychother Psychosom 76:15–24, 2007 [DOI] [PubMed] [Google Scholar]
- Khan A, Detke M, Khan SR, Mallinckrodt C: Placebo response and antidepressant clinical trial outcome. J Nerv Ment Dis 191:211–218, 2003 [DOI] [PubMed] [Google Scholar]
- Khan A, Khan S, Brown WA: Are placebo controls necessary to test new antidepressants and anxiolytics? Int J Neuropsychopharmacol 5:193–197, 2002 [DOI] [PubMed] [Google Scholar]
- Khan A, Kolts RL, Rapaport MH, Krishnan KR, Brodhead AE, Browns WA: Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med 35:743–749, 2005 [DOI] [PubMed] [Google Scholar]
- Klein RG, Koplewicz HS, Kanner A: Imipramine treatment of children with separation anxiety disorder. J Am Acad Child Adolesc Psychiatry 31:21–28, 1992 [DOI] [PubMed] [Google Scholar]
- Kovacs M.The Children's Depression Inventory: A self-rated depression scale for school age youngsters. Pittsburgh: University of Pittsburgh, School of Medicine; 1983 [Google Scholar]
- Leonard HL, Swedo SE, Rapoport JL, Koby EV, Lenane MC, Cheslow DL, Hamburger SD: Treatment of obsessive-compulsive disorder with clomipramine and desipramine in children and adolescents. A double-blind crossover comparison. Arch Gen Psychiatry 46:1088–1092, 1989 [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK: The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36:554–565, 1997 [DOI] [PubMed] [Google Scholar]
- Research Unit on Pediatric Psychopharmacology Anxiety Study Group: Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 344:1279–1285, 2001 [DOI] [PubMed] [Google Scholar]
- Rynn M, Puliafico A, Heleniak C, Rikhi P, Ghalib K, Vidair H: Advances in pharmacotherapy for pediatric anxiety disorders. Depress Anxiety 28:76–87, 2011 [DOI] [PubMed] [Google Scholar]
- Rynn MA, Siqueland L, Rickels K: Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry 158:2008–2014, 2001 [DOI] [PubMed] [Google Scholar]
- Sandler A: Placebo effects in developmental disabilities: Implications for research and practice. Ment Retard Dev Disabil Res Rev 11:164–170, 2005 [DOI] [PubMed] [Google Scholar]
- Seidel L, Walkup JT: Selective serotonin reuptake inhibitor use in the treatment of the pediatric non-obsessive-compulsive disorder anxiety disorders. J Child Adolesc Psychopharmacol 16:171–179, 2006 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S: A Children'S Global Assessment Scale (CGAS). Arch Gen Psychiatry 40:1228–31, 1983 [DOI] [PubMed] [Google Scholar]
- Wagner KD, Berard R, Stein MB, Wetherhold E, Carpenter DJ, Perera P, Gee M, Davy K, Machin A: A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry 61:1153–1162, 2004 [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]