Abstract
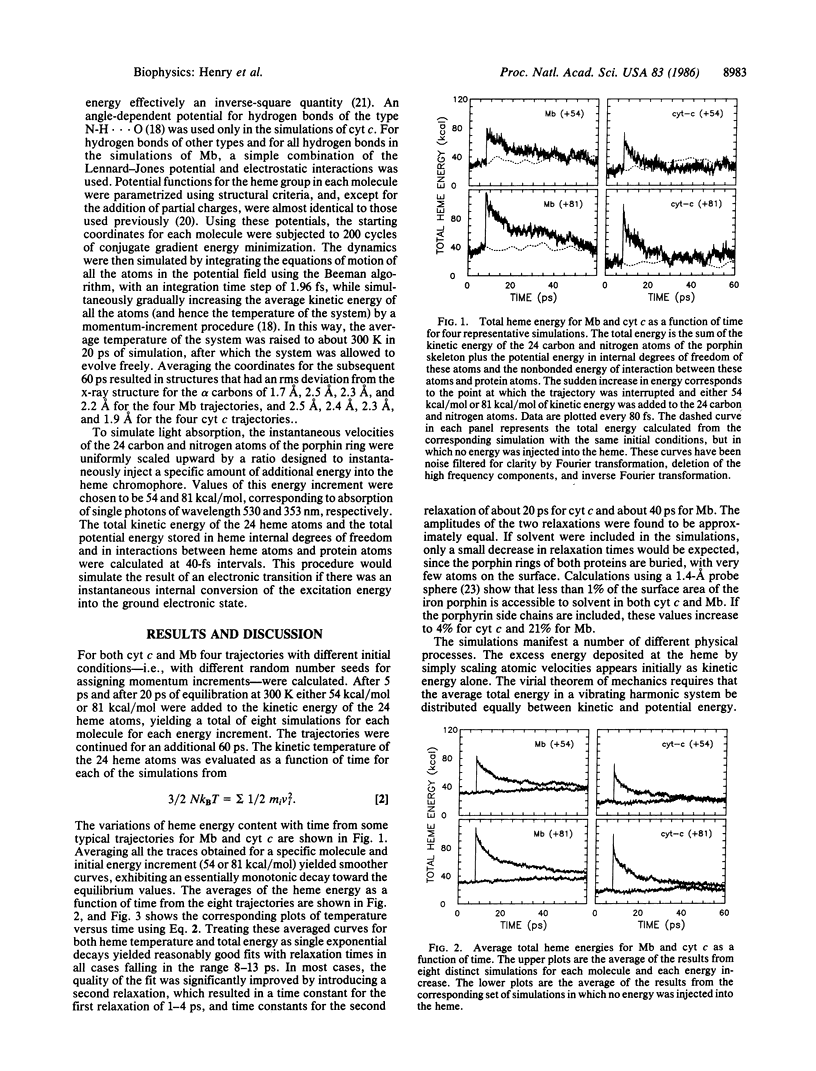
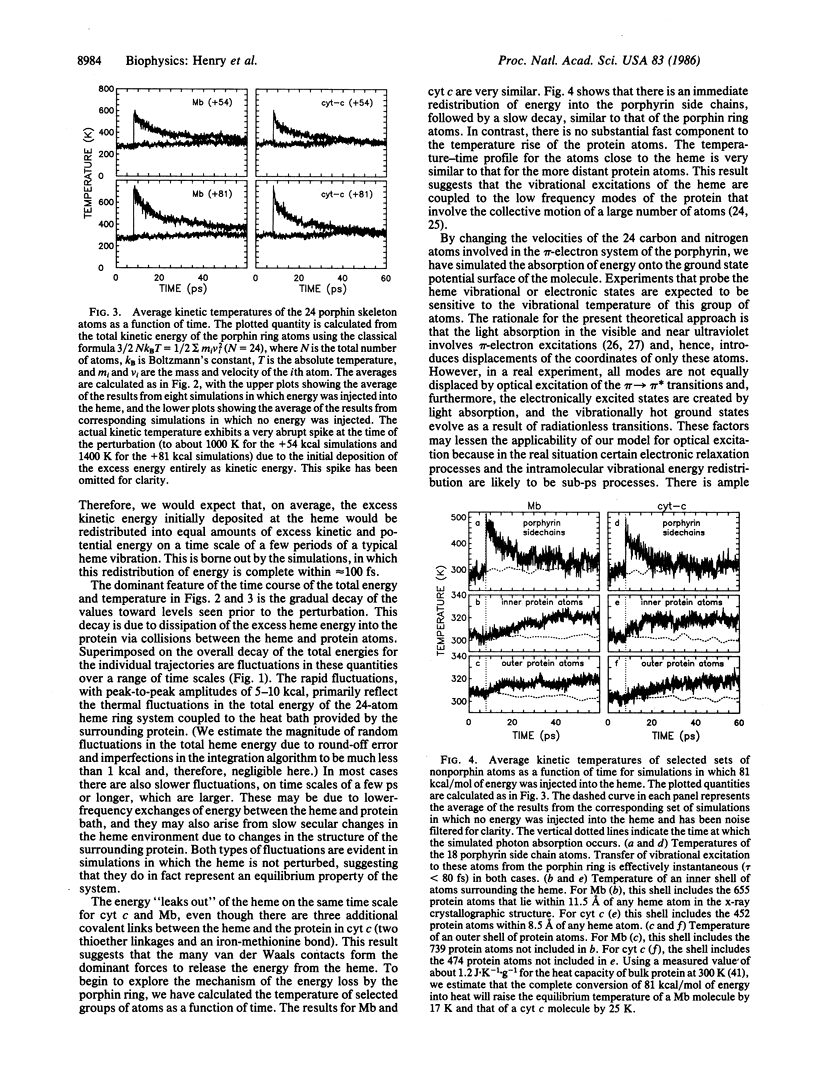
In transient optical experiments the absorbed photon raises the vibrational temperature of the chromophore. In heme proteins at room temperature conversion of a 530-nm photon into vibrational energy is estimated to raise the temperature of the heme by 500-700 K. Cooling of the heme is expected to occur mainly by interacting with the surrounding protein. We report molecular dynamics simulations for myoglobin and cytochrome c in vacuo that predict that this cooling occurs on the ps time scale. The decay of the vibrational temperature is nonexponential with about 50% loss occurring in 1-4 ps and with the remainder in 20-40 ps. These results predict the presence of nonequilibrium vibrational populations that would introduce ambiguity into the interpretation of transient ps absorption and Raman spectra and influence the kinetics of sub-ns geminate recombination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks B., Karplus M. Harmonic dynamics of proteins: normal modes and fluctuations in bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6571–6575. doi: 10.1073/pnas.80.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff D. A., Hochstrasser R. M., Steele A. W. Geminate recombination of O2 and hemoglobin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5606–5610. doi: 10.1073/pnas.77.10.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius P. A., Hochstrasser R. M., Steele A. W. Ultrafast relaxation in picosecond photolysis of nitrosylhemoglobin. J Mol Biol. 1983 Jan 5;163(1):119–128. doi: 10.1016/0022-2836(83)90032-3. [DOI] [PubMed] [Google Scholar]
- Cornelius P. A., Steele A. W., Chernoff D. A., Hochstrasser R. M. Different dissociation pathways and observation of an excited deoxy state in picosecond photolysis of oxy- and carboxymyoglobin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7526–7529. doi: 10.1073/pnas.78.12.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S., Spiro T. G., Johnson C. K., Dalickas G. A., Hochstrasser R. M. Picosecond resonance Raman evidence for unrelaxed heme in the (carbonmonoxy)myoglobin photoproduct. Biochemistry. 1985 Sep 24;24(20):5295–5297. doi: 10.1021/bi00341a003. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Scott T. W., Fisanick G. J., Simon S. R., Findsen E. W., Ondrias M. R., Macdonald V. W. Localized control of ligand binding in hemoglobin: effect of tertiary structure on picosecond geminate recombination. Science. 1985 Jul 12;229(4709):187–190. doi: 10.1126/science.4012316. [DOI] [PubMed] [Google Scholar]
- Greene B. I., Hochstrasser R. M., Weisman R. B., Eaton W. A. Spectroscopic studies of oxy- and carbonmonoxyhemoglobin after pulsed optical excitation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5255–5259. doi: 10.1073/pnas.75.11.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Levitt M., Eaton W. A. Molecular dynamics simulation of photodissociation of carbon monoxide from hemoglobin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2034–2038. doi: 10.1073/pnas.82.7.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. Dynamics of proteins: elements and function. Annu Rev Biochem. 1983;52:263–300. doi: 10.1146/annurev.bi.52.070183.001403. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Levitt M., Meirovitch H. Integrating the equations of motion. J Mol Biol. 1983 Aug 15;168(3):617–620. doi: 10.1016/s0022-2836(83)80305-2. [DOI] [PubMed] [Google Scholar]
- Levitt M., Sander C., Stern P. S. Protein normal-mode dynamics: trypsin inhibitor, crambin, ribonuclease and lysozyme. J Mol Biol. 1985 Feb 5;181(3):423–447. doi: 10.1016/0022-2836(85)90230-x. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. H., Rand S. D., Rentzepis P. M. Mechanisms for excited state relaxation and dissociation of oxymyoglobin and carboxymyoglobin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2292–2296. doi: 10.1073/pnas.78.4.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurkuusk J. Specific heat measurements on lysozyme, chymotrypsinogen, and ovalbumin in aqueous solution and in solid state. Acta Chem Scand B. 1974;28(4):409–417. doi: 10.3891/acta.chem.scand.28b-0409. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]


