Abstract
A 3.7 Mb region of rat chromosome 13 (45.2–49.0 Mb) affects blood pressure (BP) in females only, indicating the presence of gender-specific BP loci in close proximity to the Renin locus. In the present study, we used a series of Dahl salt-sensitive/Mcwi (SS)-13 Brown Norway (BN) congenic rat strains to further resolve BP loci within this region. We identified 3 BP loci affecting female rats only, of which the 2 smaller loci (line9BP3 and line9BP4) were functionally characterized by sequence and expression analysis. Compared with SS, the presence of a 591 Kb region of BN chromosome 13 (line9BP3) significantly lowered BP by 21 mmHg on an 8% NaCl diet (153±7 vs 174±5 mmHg, P<0.001). Unexpectedly, the addition of 23 Kb of BN chromosome 13 (line9BP4) completely erased the female-specific BP protection on 8% NaCl diet, suggesting that BN hypertensive allele(s) reside in this region. The congenic interval of the protective line 9F strain contains 3 genes (Optc, Prelp, and Fmod) and the hypertensive line 9E contains 1 additional gene (Btg2). Sequence analysis of the 2 BP loci revealed a total of 282 intergenic variants, with no coding variants. Analysis of gene expression by RT-qPCR revealed strain- and gender-specific differences in Prelp, Fmod, and Btg2 expression, implicating these as novel candidate genes for female-specific hypertension.
Keywords: Hypertension, Genetics, Gender, Blood Pressure, Kidney
Introduction
Hypertension risk is defined by interaction of both environmental and genetic factors.1 Gender has historically been regarded as a covariate, but recent studies have begun analyzing male and female populations separately, and found that some genetic variants confer susceptibility to hypertension in a gender-specific manner.2–7 Such variants may define genetically and etiologically distinct subgroups of men and women with hypertension and have implications for rational selection of gender-specific treatments. However, reports on female cohorts are strikingly limited and greatly restricted by population sample size, emphasizing the need for additional strategies to identify gender-specific loci.
In a previous study,8 we used congenic mapping to identify gender-specific blood pressure (BP) loci on rat chromosome 13. This revealed a 3.7 Mb region of BN chromosome 13 (45.2–49.0 Mb) that reduced BP by 20 mmHg in females only, indicating the presence of female-specific BP allele(s).8 This SS-13BN congenic strain (referred to as line 9) contains 83 genes in the 3.7 Mb congenic interval (chr13:45.2–49.0 Mb), including the BP mediator Renin.8 In the present study, we refined the line 9 congenic interval to 3 female-specific BP loci and have functionally characterized the 2 smallest QTL (line9BP3 and line9BP4), which contained a total of 4 genes (Btg2, Prelp, Fmod, and Optc). Although no nonsynonymous coding variants were identified, we detected strain- and gender-specific differences in Btg2, Prelp, and Fmod expression. Collectively, we have identified specific candidate genes for BP that differentially impact salt-sensitive hypertension in females only.
Materials and Methods
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (MCW). Full descriptions of the 5 rat strains used in this study (Figure 1) are provided in the Expanded Materials and Methods.
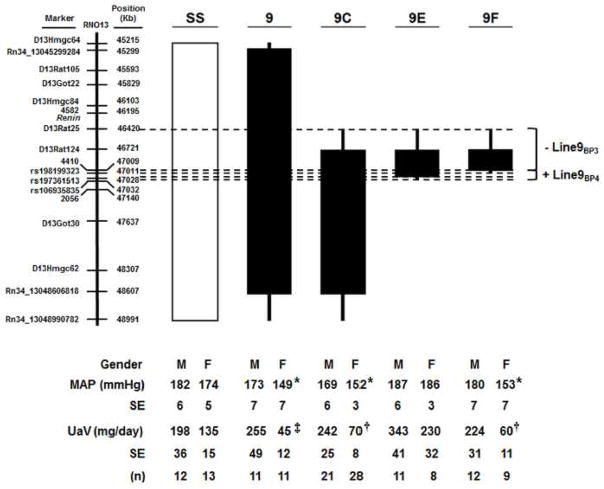
Figure 1.
Schematic representation of the SS-13BN congenic strains that were generated by introgressing segments of BN chromosome 13 (black) into the genetic background of the parental SS strain (white) by marker assisted breeding. Thin black bars represent chromosomal regions that could be BN or SS. Bottom: Mean arterial pressure (MAP) and urinary albumin excretion (UaV) of parental SS and SS-13BN congenics on 8% NaCl diet for 21 days. Values are means ± SEM from 8 to 28 animals per group. *P<0.05 vs. SS, †P<0.01 vs. SS, and ‡P<0.001 vs. line vs. SS, as determined by a 1-way ANOVA on Ranks followed by a Dunn’s post-hoc test (for MAP) or by a 1-way ANOVA followed by a Holm-Sidak post hoc test (for albuminuria). Data for male line 9C has been published previously.60
Blood Pressure Measurement
Mean arterial pressure (MAP) was measured by telemetry transmitter implantation with a catheter inserted into the abdominal aorta, as described previously.9
Measurement of Albumin Excretion
After 16 days of 8% NaCl diet, rats were acclimated in metabolic cages for 24 hours, followed by a 24-hour urine collection and measurement of albuminuria, as described previously.9
RT-qPCR
Total RNA from renal cortex and medulla of SS and SS-13BN congenic rats fed 0.4% NaCl (low salt) or 8% NaCl (high salt) diets for 7 days (n=4–6 per group) was synthesized to cDNA and transcript expression measured using an ABI HT7900 Real-Time machine (Applied BioSystems, Foster City, CA), as described previously.10 Primers are listed in Table S1.
Inflammation and Fibrosis RT-qPCR Array
Inflammatory and fibrotic gene expression was examined using a rat inflammatory cytokines and receptors RT2 Profiler PCR Array (PARN-120ZE-4, SABiosciences, Fredrick, MD), according to the manufacturer’s protocol.
Sequence Analysis
Genomic DNA sequence of BN (rn4 assembly) and SS/JrHsD/Mcwi were accessed from the RGD website and analyzed using, as described previously.9 Sequence analysis was performed using TargetScan,11 Variant Effect Predictor,12 Polyphen2,13 TRANSFAC, and MatInspector softwares.
Statistical analysis
Statistical analyses were performed using Sigma Plot 12.0 software. Data are presented as mean ± SEM. BP data were analyzed by 1-way ANOVA on Ranks followed by the Dunn’s post-hoc test. Albuminuria data were analyzed by 1-way ANOVA followed by the Holm-Sidak post-hoc test. Gene expression was analyzed by 1-way ANOVA followed by Tukey’s post-hoc test.
Expanded Materials and Methods are provided in the online-only Data Supplement.
Results
Blood Pressure
A series of SS-13BN congenic rat strains were phenotyped for MAP and urinary albumin excretion as depicted in Figure 1. After 21 days on 8% NaCl diet, the MAP of line 9 (173±7 mmHg, n=11), line 9C (169±6 mmHg, n=21), line 9E (187±6 mmHg, n=11), and line 9F (180±7 mmHg, n=12) male rats were not significantly different from SS (Figure 1). In contrast, after 21 days on 8% NaCl diet, the MAP of female rats from line 9 (149±7 mmHg, P<0.05, n=11), line 9C (152±3, P<0.05, n=28), and line 9F (153±7 mmHg, P<0.05, n=9) were significantly lower than line 9E (186±3 mmHg, n=8) and SS (174±5 mmHg, n=13) (Figure 1). The decreased MAP in line 9F females, but not line 9F males, indicates that female-specific BN protective allele(s) reside in the 591 Kb line 9F interval (chr13: 46,420,127–47,010,821 bp). Elevated BP in line 9E females suggests that BN hypertensive allele(s) are located within the 23 Kb region (chr13:47,008,948–47,031,810 bp) that differentiates line 9E from line 9F (Figure 1). However, because line 9E males were also hypertensive, it is not possible to establish whether the hypertensive BN allele(s) within the 23 Kb region are gender-specific based on BP alone. A protective locus also likely exists within the 2.0 Mb region (chr13:47.0–49.0 Mb) that lowered BP in line 9C.
Renal Damage
To assess renal damage, urine was collected from SS and SS-13BN congenic rats on 8% NaCl diet for 17 days and total urinary albumin excretion was quantified. Similar to BP, male albuminuria in lines 9 (255±49 mg), 9C (242±25 mg), 9E (343±41 mg) and 9F 224±31 mg) were not statistically different from male SS rats (198±36 mg) (Figure 1). Albuminuria in female lines 9 (45±12 mg, P<0.001), 9C (70±8 mg, P<0.01), and 9F (60±11 mg, P<0.01) was lower than SS (135±15 mg; Figure 1), suggesting that renal protection is secondary to significantly decreased BP in females of these congenic lines. In comparison, female line 9E rats had much higher albuminuria (230±32 mg, P=0.066) compared with SS (Figure 1), but was just below the threshold for statistical significance. Because line 9E and SS female rats are similarly hypertensive on 8% NaCl diet (186±3 vs. 174±5 mmHg), these data suggest that the BN allele(s) within the 23 Kb region (chr13:47,008,948–47,031,810 bp) may also modify renal damage independently of BP.
Sequence Analysis
Compared with the BN reference, a total of 5,143 variants were previously identified over the entire line 9 congenic interval.10 Further congenic mapping to narrow the protective 2.0 Mb BN interval (chr13:47.0–49.0 Mb) in line 9C to a more manageable size will be require before a more in depth analysis can be pursued. Therefore, below we have focused our analysis specifically on the smaller line9BP3 and line9BP4 quantitative trait loci (QTL) defined by the line 9E and 9F congenic strains.
Line9BP3
The line9BP3 QTL is a 591 Kb protective region of BN (chr13: 46,420,127–47,010,821 bp) that contains 3 genes (Optc, Prelp, and Fmod) and 264 total variants, with no coding variants and none predicted to interfere with 3′UTR. Of note, 10 variants were predicted to interfere with consensus transcription factor binding sites of the putative 5′ promoters of Optc, Prelp, and Fmod (Table S2).
Line9BP4
The line9BP4 QTL is a 23 Kb hypertensive region of BN (chr13:47,008,948–47,031,810 bp) that contains only Btg2 and 18 total variants, none of which are located in coding regions of Btg2 (Table S3). Of note, 9 variants were predicted to change consensus transcription binding sites and 2 variants were located in the 3′UTR, but were not predicted to interfere with any miRNA binding sites (Table S3).
Candidate Gene Expression
The BP and albuminuria (Figure 1) indicate that variant(s) modifying BP in a female-specific manner likely reside in the line9BP3 and line9BP4 QTLs. However, neither QTL had any predicted nonsynonymous variants within the protein coding regions that could account for BP changes. Instead, we hypothesized that noncoding variants could impact gene expression. To test this possibility, we performed RT-qPCR analysis of candidate gene expression (Optc, Prelp, Fmod, and Btg2) in kidneys of SS, line 9E, and line 9F congenic strains on low salt diet (0.4% NaCl) and after 7 days on high salt diet (8% NaCl). After 7 days of high salt diet, BP was not significantly different between strains (Figure S1), thus representing an early stage of the development of hypertension, at which point the underlying mechanisms can be assessed independently of BP changes.14
Line9BP3
The line9BP3 QTL significantly decreased BP in line 9F females, but not males (Figure 1). Line9BP3 contains 3 validated genes (Fmod, Prelp, and Optc) that do not have nonsynonymous changes. Optc is a class III small leucine-rich repeat protein (SLRP) that is largely restricted to the eye15 and has no reported role in BP or renal function. Based on this, and the relatively low Optc expression in the kidney that did not change in females, we have deprioritized Optc on the list of candidate genes. Compared with SS, both male and female line 9F rats on low (0.4% NaCl) and high (8% NaCl) salt diets had significantly decreased medullary expression of Prelp (male: −81 to −87% vs SS; P<0.001 and female: −83 to −89% vs SS; P<0.001) and Fmod (male: −97 to −99% vs SS; P<0.001 and female: −96 to −97% vs SS; P<0.001) (Table 1). Compared with SS, cortical expression in line 9F males and females also showed similar trends to the renal medulla, except for elevated Prelp expression in the renal cortex of line 9F females on both diets (Table 1).
Table.
Candidate Gene Expression in the Renal Medulla and Cortex of SS, Line 9F, and Line 9E Rats on Low-or High-Salt Diets
| Gene Groups | Medulla
|
Cortex
|
||
|---|---|---|---|---|
| LS | 7 Days HS | LS | 7 Days HS | |
| Prelp | ||||
| SS(male) | 1±0.1 | 0.7±0.04† | 1.4±0.5 | 0.6±0.1 |
| Line 9F (male) | 0.2±0.02* | 0.1±0.02* | 0.3±0.1* | 0.5±0.1 |
| SS (female) | 1±0.1 | 0.6±0.1† | 1.5±0.4 | 2.3±0.2 |
| Line 9F (female) | 0.2±0.02* | 0.1±0.01* | 1.6±0.2 | 1.9±0.2 |
| Fmod | ||||
| SS(male) | 1.0±0.1 | 0.8±0.1 | 1.1 ±0.2 | 1.5±0.3 |
| Line 9F (male) | 0.01±0.01* | 0.03±0.02* | 0.3±0.2* | 0.5±0.1* |
| SS (female) | 1.0±0.2 | 2.6±0.8*† | 1.3±0.3 | 1.3±03 |
| Line 9F (female) | 0.03±0.01* | 0.03±0.01* | 0.7±0.2 | 0.8±0.1 |
| Btg2 | ||||
| Line 9F (male) | 1.0±0.1 | 1.1±0.1 | 1.0±0.1 | 1.3±0.1 |
| Line 9F (male) | 1.3±0.2 | 1.0±0.3 | 1.2±0.2 | 1.1±0.2 |
| Line 9F (female) | 1.0±0.1 | 2.1±0.5 | 1.1±0.2 | 2.9±0.7† |
| Line 9E (female) | 1.3±0.3 | 2.1±0.5 | 1.9±0.2* | 2.6±0.2† |
Data are presented as mean fold-expression±SEM. Statistical significance was determined by 1-way ANOVA followed by Tukey post hoc test. HS indicates high salt; LS, Low salt, and SS, salt-sensitive.
Statistically significant between strains.
Statistically significant within strains.
Line9BP4
The line9BP4 QTL in line 9E females increased BP by 34mmHg compared with line 9F (Figure 1), indicating the presence of hypertensive BN allele(s). Only Btg2 resides in the line9BP4 congenic interval. In males, Btg2 expression was not significantly different in any of the groups tested (Table 1). In contrast, baseline Btg2 expression in renal cortex of line 9E females was 1.9±0.2-fold (P<0.05) higher than line 9F, suggesting that BN allele(s) in Line9BP4 increase baseline Btg2 expression independently of BP. After 7 days of 8% NaCl diet, Btg2 expression significantly increased 2.6±0.2- and 2.9±0.7-fold (P<0.05) in the cortex of both lines 9E and 9F, respectively (Table 1), indicating that Btg2 responds to salt challenge in females only. Similar trends were also observed in the renal medulla, but did not reach statistical significance.
Renin Expression
The renin-angiotensin system (RAS) is widely implicated in gender-specific BP.16–18 Moreover, BN allele(s) in a SS-13BN congenic that overlaps with lines 9E and 9F was reported to restore normal renin expression/function,10 prompting us to determine whether renin expression differed in lines 9E and 9F. After 7 days of 8% NaCl diet, renin expression was decreased 60–80% (P<0.05) by salt-challenge in all strains tested, but no differences between males or females of either strains were detected (Table S4). This indicates that gender-specific BP differences between line 9E and 9F rats are likely independent of Renin.
Tissue Remodeling Pathways
Dysfunction in the renal outer medulla has been suggested as a primary cause of salt-sensitive hypertension in the SS rat, leading to renal damage and fibrosis.19 Since Fmod, Prelp, and Btg2 have been implicated in tissue remodeling,20–25 we hypothesized that the differences in candidate gene expression would coincide with downstream changes in inflammatory and fibrosis pathways that mediated tissue remodeling. To test this possibility, we analyzed expression of 88 remodeling genes by RT-qPCR array in the renal medullas of SS, line 9E, and line 9F congenic strains on low salt diet (0.4% NaCl) and after 7 days on high salt diet (8% NaCl).
Line9BP3
Compared with SS, 38 out of 88 genes were uniquely downregulated (−1.5 to −61.5-fold) during salt-challenge in the protected line 9F females only (Table S5). The downregulated pathways included metalloproteases (6 out of 7), interleukins (6 out of 7), and members of the TGF-β pathway (12 out of 20) (Table S5), suggesting that multiple factors in tissue remodeling are actively suppressed in the protected line 9F females. Of the 38 genes downregulated in the line 9F females, 26 were also decreased (−1.5 to −13.3-fold) in the unprotected line 9F males during salt-challenge (Table S5).
Line9BP4
We also tested whether tissue remodeling pathways were upregulated in the hypertensive line 9E females compared with the protected line 9F. Surprisingly, 24 out of 88 genes were uniquely upregulated (1.5 to 5.6-fold) in line 9E females compared with line 9F females, of which only 1 was shared by the line 9E males (Table S5). Even more strikingly, 18 out of the 24 genes that were upregulated by salt-challenge in line 9E females were actively suppressed in the line 9F females (Table S5).
Discussion
Multiple human4, 7, 26 and rat27–36 studies have identified gender-specific BP loci; however, the majority of these have failed to translate to mechanistic discoveries. Previously, we identified a 3.7 Mb QTL on rat chromosome 13 (45.2–49.0 Mb) that attenuated salt-sensitive BP in female rats only.8 Here, we identified at least 3 QTL that mediated BP in females only (Figure 1). In the 2 smallest congenics (lines 9E and 9F), we reduced the total congenic intervals from 3.7 Mb to 614 Kb and identified 2 BP loci (line9BP3 and line9BP4), which contained a total of 3 differentially expressed candidate genes (Fmod, Prelp, and Btg2). Based on these findings, Fmod, Prelp, and Btg2 are novel BP candidate genes and potential therapeutic targets for female-specific genetic hypertension.
How does gender influence genetic hypertension?
Estrogen and the X chromosome are associated with lower BP,16–18 but it is largely unknown how these factors interact with genetic loci. Despite this, there is clear indication that some BP loci differ between males and females4, 7, 26–36 and it is highly likely that several mechanisms exist. One possibility is that promoter response elements (RE) for hormone receptors (e.g., estrogen or androgen receptors) or X-linked transcription factors are disrupted by genetic variants, which modifies gender-specific expression of a candidate gene that influences disease pathogenesis. For example, genetic variants frequently disrupt the estrogen RE in the promoter of the tumor suppressor gene, BRCA1.37 The resulting downregulation of BRCA1 protein then increases breast cancer risk.38
Similar to BRCA1,37 our data suggest that Btg2 expression is likely regulated by estrogen and/or X-linked factors, because changes in Btg2 expression were specific to females only (Table 1). This fits with previous evidence of BTG2 promoter regulation by estrogen receptor (ER) response elements.39–41 By sequence analysis, we also identified multiple conserved ER response elements in the rat Btg2 promoter and in close proximity to sequence variants; however, none were predicted to be disrupted by sequence variants. Several other transcription factors (TF) that were predicted to bind the Btg2 promoter (ERRα, Gata-1/-2/-3, and Myb) also interact with ER signaling,42–44 suggesting that estrogen could also indirectly regulate the Btg2 promoter by interacting with other transcriptional machinery. Because ER forms TF complexes (e.g., with ERRα,44 Gata-1/-2/-3,42 and Myb43), it is also possible that variation in binding sites of these TF in the Btg2 promoter (Table S3) could influence ER-mediated Btg2 expression in the kidney. We also detected a putative binding site for the gender-determining transcription factor Sry in the Btg2 promoter (Table S3). However, although Sry is widely implicated in male hypertension,18 it is Y-linked and therefore likely not accountable for the female-specific changes in Btg2 expression that we observed in line 9E (Table 1).
Gender might also influence response to the effects of a genetic locus (i.e., hypersensitivity in one gender versus the other). Our data suggest that only line 9F females are protected by low Prelp and Fmod expression (Figure 1), despite these genes being strongly downregulated in both genders (Table 1). This fits with evidence that multiple mouse knockouts (e.g., COX2,45 LDLR,46 and eNOS47) and the mRen2.Lewis transgenic rat model48 show sexual dimorphism in BP and hypertension risk. Although transgenic models do not constitute a “genetic locus” per se, one could argue that these transgenic models demonstrate dimorphic phenotypes based on gender and genotype interactions.
Candidate Genes
Line9BP3
Compared with SS, the BN-derived allele(s) in line9BP3 significantly decreased Fmod and Prelp expression in line 9F females (Table), which predisposed only the line 9F females to lower BP and albuminuria (Figure 1). Fmod and Prelp belong to the class II family of SLRPs, which has been previously implicated in renal damage and fibrosis.20 Fmod is expressed in the peritubular kidney,23 is elevated during renal fibrosis,49 and interacts directly with the profibrotic mediator, TGF-β.50 Prelp is also highly expressed in the kidney50 and in other tissues regulates the inflammatory mediator, NF-κB.51 Additionally, both Fmod and Prelp are also implicated in complement pathway activation,52 which has been associated with increased kidney damage.53 Collectively, these data support that the downregulation of remodeling pathways in line 9E (Table S5) are likely due to decreased expression of Fmod and Prelp (Table 1).
Line9BP4
Btg2 is the only gene residing in line9BP4 and is elevated in female line 9E rats prior to salt-challenge (Tab1e 1), suggesting that elevated Btg2 expression predisposes line 9E females to hypertension (Figure 1). Btg2 is highly expressed in the kidney proximal tubules,54 but has no previously reported role in BP or renal damage. Btg2 is a transcriptional co-regulator involved in cell-cycle,41, 55 apoptosis,55 inflammation,24 and fibrosis.56 In addition to regulation by ER,24 The Btg2 promoter is also regulated by NF-κB24 and p5357, 58 in response to multiple inflammatory stimuli, including TGF-β56 and TNF-α.24 Collectively, these data suggest that similar Btg2-dependent mechanism(s) might influence female-specific renal inflammation and fibrosis.
Line 9 is a compound BP QTL
The existence of both line9BP3 and line9BP4 demonstrate that line 9 is a compound BP QTL. Moreover, the hypertensive line9BP3 QTL (Btg2) offsets the BP lowering effects of the protective line9BP4 QTL (Fmod and Prelp) (Figure 1), indicating that these are interdependent BP loci. However, at present it is unclear whether line9BP3 and line9BP4 are merely subtractive, or have an epistatic relationship (i.e., phenotypic manifestation of one variant is dependent on the other).59 Based on the literature,24, 50, 51, 56 it is possible that Btg2, Fmod, and/or Prelp could indirectly interact through common pathways (e.g., TGF-β, NF-κB, and complement factor). However, epistatic interaction(s) between Btg2, Fmod, and Prelp have yet to be tested empirically.
In addition to line9BP3 and line9BP4, at least one other BP QTL resides within the original line 9 congenic interval,8 because the addition of flanking BN genome (chr13:47.0–49.0 Mb) lowered BP (−Δ22 mmHg) in the female line 9C rats compared with SS (Figure 1). This additional BP locus offset the hypertensive effects of line9BP4, indicating the existence of potentially 3 epistatic BP loci within a 2.6 Mb interval (chr13:46.4–49.0Mb). Our data also suggest that the third BP locus in line 9C (chr13:47.0–49.0 Mb) is unique to females only. We recently published that at least 2 other BP loci reside in the line 9 congenic interval using male rats only.60 One BN locus (chr13:47.2–49.0 Mb; referred to as line9BP2) was hypertensive and the other BN locus, containing Renin (chr13:46.1–47.2; referred to as line9BP1), lowered BP in male rats.60 At present, it appears that the male BP QTL identified previously60 and the female BP QTL identified here (Figure 1) are likely not shared. Although the protective line9BP1 (male) and line9BP3 (female) QTL overlapped, the line 9E males were not protective, suggesting that protective BP loci in this region are gender-specific. Likewise, the overlapping hypertensive line9BP2 (male) and protective line9BP4 (female) QTL had opposite phenotypes, indicating that BP loci in these QTL are very likely not shared. Further congenic mapping, ZFN-mutagenesis, and molecular analysis will be required to (1) narrow the protective BP allele(s) in line 9C (chr13:47.0–49.0 Mb) and (2) test whether epistasis or purely additive effects regulate overall hypertension risk for females in the line 9 congenic region.
Perspectives
The observed gender differences in BP regulation are longstanding,18 but the mechanism(s) specific to genetic hypertension in females are largely unresolved. Despite this, the majority of BP studies have focused primarily on male hypertension. Here, we identified a 614 Kb region on rat chromosome 13 that contains 2 female-specific BP loci. Within these loci we identified 3 differentially expressed candidate genes (Btg2, Fmod, and Prelp) that are specific to female BP and offer potential therapeutic targets for treating female hypertension. Future studies will be needed to test the functional roles of Btg2, Fmod, and Prelp in the kidney and potentially other tissues involved in the development of hypertension.
Supplementary Material
Novelty and Significance.
1) What Is New?
We identified 3 novel female-specific BP loci: the protective line9BP3 BP QTL (chr13:46,420,127–47,010,821 bp), the adjacent hypertensive line9BP4 QTL (chr13:47,008,948–47,031,810 bp), and another protective region (chr13:47.0–49.0) that has yet to be refined.
The BP lowering line9BP3 QTL contains 2 candidate genes (Fmod and Prelp) that were downregulated in both males and females, but attenuated BP in females only.
The BP elevating line9BP4 QTL contains 1 candidate gene (Btg2) that was upregulated in females only and increased BP in females only.
2) What Is Relevant?
Gender differences in BP are long recognized, but the mechanism(s) specific to genetic hypertension in females are largely unresolved.
Here we identified 2 novel BP loci (line9BP3 and line9BP4) that contain 3 differentially expressed candidate genes (Fmod, Prelp, and Btg2).
Although several others have identified larger BP QTL in females with many candidate genes, this is the first report of female-specific BP loci being reduced to 1 or 2 gene resolution.
3) Summary
We refined the line 9 congenic interval (chr13:45.2–49.0 Mb) to 2 female-specific BP QTL (591 Kb and 23 Kb) that contain a total of 3 differentially expressed candidate genes. We also discovered a third female-specific BP locus (chr13:47.0–49.0 Mb) that will require further congenic mapping to narrow the causative allele(s).
Acknowledgments
We thank Elisabeth Metzger, Elleanor Christenson, Rebecca Schilling, Allison Zappa, Anne Temple, Marc Casati, Nadia Barreto, Jaime Wendt-Andrae, and Michael Tschannen for their technical support.
Sources of Funding
M.J.F. is supported by a National Heart, Lung, and Blood Institute training grant (5T32HL007792). This study was supported by National Heart, Lung, and Blood Institute grant 5P01HL082798 to A.S.G. and C.M.Q.
Footnotes
Disclosures: No
References
- 1.Cowley AW., Jr The Genetic Dissection of Essential Hypertension. Nat Rev Genet. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Nejatizadeh A, Arif E, Akhtar S, Gupta M, Tyagi S, Goyal AK, Jain SK, Qadar Pasha MA. Multi-Locus Interactions of Vascular Homeostasis Genes in Essential Hypertension: A Gender-Based Study. Clinica chimica acta; international journal of clinical chemistry. 2009;405:87–93. doi: 10.1016/j.cca.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama T, Kuroi N, Sano M, Tabara Y, Katsuya T, Ogihara T, Makita Y, Hata A, Yamada M, Takahashi N, Hirawa N, Umemura S, Miki T, Soma M. Mutation of the Follicle-Stimulating Hormone Receptor Gene 5′-Untranslated Region Associated with Female Hypertension. Hypertension. 2006;48:512–518. doi: 10.1161/01.HYP.0000233877.84343.d7. [DOI] [PubMed] [Google Scholar]
- 4.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O’Connor DT. Population-Based Sample Reveals Gene-Gender Interactions in Blood Pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 5.Peter I, Shearman AM, Zucker DR, Schmid CH, Demissie S, Cupples LA, Larson MG, Vasan RS, D’Agostino RB, Karas RH, Mendelsohn ME, Housman DE, Levy D. Variation in Estrogen-Related Genes and Cross-Sectional and Longitudinal Blood Pressure in the Framingham Heart Study. J Hypertens. 2005;23:2193–2200. doi: 10.1097/01.hjh.0000188728.66183.92. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Zhu H, Dong Y, Treiber FA, Snieder H. Effects of Angiotensinogen and Angiotensin Ii Type I Receptor Genes on Blood Pressure and Left Ventricular Mass Trajectories in Multiethnic Youth. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2006;9:393–402. doi: 10.1375/183242706777591335. [DOI] [PubMed] [Google Scholar]
- 7.Seda O, Tremblay J, Gaudet D, Brunelle PL, Gurau A, Merlo E, Pilote L, Orlov SN, Boulva F, Petrovich M, Kotchen TA, Cowley AW, Jr, Hamet P. Systematic, Genome-Wide, Sex-Specific Linkage of Cardiovascular Traits in French Canadians. Hypertension. 2008;51:1156–1162. doi: 10.1161/HYPERTENSIONAHA.107.105247. [DOI] [PubMed] [Google Scholar]
- 8.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW., Jr Multiple Blood Pressure Loci on Rat Chromosome 13 Attenuate Development of Hypertension in the Dahl S Hypertensive Rat. Physiol Genomics. 2007;31:228–235. doi: 10.1152/physiolgenomics.00280.2006. [DOI] [PubMed] [Google Scholar]
- 9.Flister MJ, Prisco SZ, Sarkis AB, O’Meara CC, Hoffman M, Wendt-Andrae J, Moreno C, Lazar J, Jacob HJ. Identification of Hypertension Susceptibility Loci on Rat Chromosome 12. Hypertension. 2012;60:942–948. doi: 10.1161/HYPERTENSIONAHA.112.198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stodola TJ, de Resende MM, Sarkis AB, Didier DN, Jacob HJ, Huebner N, Hummel O, Saar K, Moreno C, Greene AS. Characterization of the Genomic Structure and Function of Regions Influencing Renin and Angiogenesis in the Ss Rat. Physiol Genomics. 2011;43:808–817. doi: 10.1152/physiolgenomics.00171.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman RC, Farh KK, Burge CB, Bartel DP. Most Mammalian Mrnas Are Conserved Targets of Micrornas. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the Consequences of Genomic Variants with the Ensembl Api and Snp Effect Predictor. Bioinformatics. 2010;26:2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramensky V, Bork P, Sunyaev S. Human Non-Synonymous Snps: Server and Survey. Nucleic acids research. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C, Stingo FC, Ahn KW, Liu P, Vannucci M, Laud PW, Skelton M, O’Connor P, Kurth T, Ryan RP, Moreno C, Tsaih SW, Patone G, Hummel O, Jacob HJ, Liang M, Cowley AW., Jr Increased Proliferative Cells in the Medullary Thick Ascending Limb of the Loop of Henle in the Dahl Salt-Sensitive Rat. Hypertension. 2013;61:208–215. doi: 10.1161/HYPERTENSIONAHA.112.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Goff MM, Bishop PN. Focus on Molecules: Opticin. Exp Eye Res. 2007;85:303–304. doi: 10.1016/j.exer.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan JC. Sex and the Renin-Angiotensin System: Inequality between the Sexes in Response to Ras Stimulation and Inhibition. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;294:R1220–1226. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- 17.Reckelhoff JF. Gender Differences in the Regulation of Blood Pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg K, Ji H. Sex Differences in Primary Hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW, Jr, Ferreri NR, Yeo NC, Liang M. Renal Medullary Micrornas in Dahl Salt-Sensitive Rats: Mir-29b Regulates Several Collagens and Related Genes. Hypertension. 2010;55:974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer L. Small Leucine-Rich Proteoglycans in Kidney Disease. J Am Soc Nephrol. 2011;22:1200–1207. doi: 10.1681/ASN.2010050570. [DOI] [PubMed] [Google Scholar]
- 21.Le Goff MM, Sutton MJ, Slevin M, Latif A, Humphries MJ, Bishop PN. Opticin Exerts Its Anti-Angiogenic Activity by Regulating Extracellular Matrix Adhesiveness. J Biol Chem. 2012;287:28027–28036. doi: 10.1074/jbc.M111.331157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeansson M, Granqvist AB, Nystrom JS, Haraldsson B. Functional and Molecular Alterations of the Glomerular Barrier in Long-Term Diabetes in Mice. Diabetologia. 2006;49:2200–2209. doi: 10.1007/s00125-006-0319-z. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer L, Raslik I, Grone HJ, Schonherr E, Macakova K, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small Proteoglycans in Human Diabetic Nephropathy: Discrepancy between Glomerular Expression and Protein Accumulation of Decorin, Biglycan, Lumican, and Fibromodulin. Faseb J. 2001;15:559–561. doi: 10.1096/fj.00-0493fje. [DOI] [PubMed] [Google Scholar]
- 24.Kawakubo H, Carey JL, Brachtel E, Gupta V, Green JE, Walden PD, Maheswaran S. Expression of the Nf-Kappab-Responsive Gene Btg2 Is Aberrantly Regulated in Breast Cancer. Oncogene. 2004;23:8310–8319. doi: 10.1038/sj.onc.1208008. [DOI] [PubMed] [Google Scholar]
- 25.Karve TM, Rosen EM. B-Cell Translocation Gene 2 (Btg2) Stimulates Cellular Antioxidant Defenses through the Antioxidant Transcription Factor Nfe2l2 in Human Mammary Epithelial Cells. J Biol Chem. 2012;287:31503–31514. doi: 10.1074/jbc.M112.367433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschini N, MacCluer JW, Goring HH, Cole SA, Rose KM, Almasy L, Diego V, Laston S, Lee ET, Howard BV, Best LG, Fabsitz RR, Roman MJ, North KE. A Quantitative Trait Loci-Specific Gene-by-Sex Interaction on Systolic Blood Pressure among American Indians: The Strong Heart Family Study. Hypertension. 2006;48:266–270. doi: 10.1161/01.HYP.0000231651.91523.7e. [DOI] [PubMed] [Google Scholar]
- 27.Ueno T, Tremblay J, Kunes J, Zicha J, Dobesova Z, Pausova Z, Deng AY, Sun Y, Jacob HJ, Hamet P. Gender-Specific Genetic Determinants of Blood Pressure and Organ Weight: Pharmacogenetic Approach. Physiological research/Academia Scientiarum Bohemoslovaca. 2003;52:689–700. [PubMed] [Google Scholar]
- 28.Moreno C, Dumas P, Kaldunski ML, Tonellato PJ, Greene AS, Roman RJ, Cheng Q, Wang Z, Jacob HJ, Cowley AW., Jr Genomic Map of Cardiovascular Phenotypes of Hypertension in Female Dahl S Rats. Physiol Genomics. 2003;15:243–257. doi: 10.1152/physiolgenomics.00105.2003. [DOI] [PubMed] [Google Scholar]
- 29.Herrera VL, Tsikoudakis A, Ponce LR, Matsubara Y, Ruiz-Opazo N. Sex-Specific Qtls and Interacting Loci Underlie Salt-Sensitive Hypertension and Target Organ Complications in Dahl S/Jrhs Hypertensive Rats. Physiol Genomics. 2006;26:172–179. doi: 10.1152/physiolgenomics.00285.2005. [DOI] [PubMed] [Google Scholar]
- 30.Cicila GT, Rapp JP, Wang JM, St Lezin E, Ng SC, Kurtz TW. Linkage of 11 Beta-Hydroxylase Mutations with Altered Steroid Biosynthesis and Blood Pressure in the Dahl Rat. Nat Genet. 1993;3:346–353. doi: 10.1038/ng0493-346. [DOI] [PubMed] [Google Scholar]
- 31.Cicila GT, Dukhanina OI, Kurtz TW, Walder R, Garrett MR, Dene H, Rapp JP. Blood Pressure and Survival of a Chromosome 7 Congenic Strain Bred from Dahl Rats. Mamm Genome. 1997;8:896–902. doi: 10.1007/s003359900607. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko Y, Herrera VL, Didishvili T, Ruiz-Opazo N. Sex-Specific Effects of Dual Et-1/Ang Ii Receptor (Dear) Variants in Dahl Salt-Sensitive/Resistant Hypertension Rat Model. Physiol Genomics. 2005;20:157–164. doi: 10.1152/physiolgenomics.00108.2004. [DOI] [PubMed] [Google Scholar]
- 33.Yagil C, Sapojnikov M, Kreutz R, Katni G, Lindpaintner K, Ganten D, Yagil Y. Salt Susceptibility Maps to Chromosomes 1 and 17 with Sex Specificity in the Sabra Rat Model of Hypertension. Hypertension. 1998;31:119–124. doi: 10.1161/01.hyp.31.1.119. [DOI] [PubMed] [Google Scholar]
- 34.Harris EL, Phelan EL, Thompson CM, Millar JA, Grigor MR. Heart Mass and Blood Pressure Have Separate Genetic Determinants in the New Zealand Genetically Hypertensive (Gh) Rat. J Hypertens. 1995;13:397–404. [PubMed] [Google Scholar]
- 35.Clark JS, Jeffs B, Davidson AO, Lee WK, Anderson NH, Bihoreau MT, Brosnan MJ, Devlin AM, Kelman AW, Lindpaintner K, Dominiczak AF. Quantitative Trait Loci in Genetically Hypertensive Rats. Possible Sex Specificity. Hypertension. 1996;28:898–906. doi: 10.1161/01.hyp.28.5.898. [DOI] [PubMed] [Google Scholar]
- 36.Carswell HV, Anderson NH, Clark JS, Graham D, Jeffs B, Dominiczak AF, Macrae IM. Genetic and Gender Influences on Sensitivity to Focal Cerebral Ischemia in the Stroke-Prone Spontaneously Hypertensive Rat. Hypertension. 1999;33:681–685. doi: 10.1161/01.hyp.33.2.681. [DOI] [PubMed] [Google Scholar]
- 37.Xu CF, Chambers JA, Solomon E. Complex Regulation of the Brca1 Gene. J Biol Chem. 1997;272:20994–20997. doi: 10.1074/jbc.272.34.20994. [DOI] [PubMed] [Google Scholar]
- 38.Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT. Decreased Expression of Brca1 Accelerates Growth and Is Often Present During Sporadic Breast Cancer Progression. Nat Genet. 1995;9:444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- 39.Karmakar S, Foster EA, Smith CL. Estradiol Downregulation of the Tumor Suppressor Gene Btg2 Requires Estrogen Receptor-Alpha and the Rea Corepressor. Int J Cancer. 2009;124:1841–1851. doi: 10.1002/ijc.24133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-Wide Analysis of Estrogen Receptor Binding Sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 41.Paruthiyil S, Cvoro A, Tagliaferri M, Cohen I, Shtivelman E, Leitman DC. Estrogen Receptor Beta Causes a G2 Cell Cycle Arrest by Inhibiting Cdk1 Activity through the Regulation of Cyclin B1, Gadd45a, and Btg2. Breast cancer research and treatment. 2011;129:777–784. doi: 10.1007/s10549-010-1273-5. [DOI] [PubMed] [Google Scholar]
- 42.Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M. Positive Cross-Regulatory Loop Ties Gata-3 to Estrogen Receptor Alpha Expression in Breast Cancer. Cancer research. 2007;67:6477–6483. doi: 10.1158/0008-5472.CAN-07-0746. [DOI] [PubMed] [Google Scholar]
- 43.Mitra P, Pereira LA, Drabsch Y, Ramsay RG, Gonda TJ. Estrogen Receptor-Alpha Recruits P-Tefb to Overcome Transcriptional Pausing in Intron 1 of the Myb Gene. Nucleic acids research. 2012;40:5988–6000. doi: 10.1093/nar/gks286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giguere V. To Err in the Estrogen Pathway. Trends in endocrinology and metabolism: TEM. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- 45.Yang T, Huang YG, Ye W, Hansen P, Schnermann JB, Briggs JP. Influence of Genetic Background and Gender on Hypertension and Renal Failure in Cox-2-Deficient Mice. American journal of physiology. Renal physiology. 2005;288:F1125–1132. doi: 10.1152/ajprenal.00219.2004. [DOI] [PubMed] [Google Scholar]
- 46.Trieu VN, Uckun FM. Male-Associated Hypertension in Ldl-R Deficient Mice. Biochemical and biophysical research communications. 1998;247:277–279. doi: 10.1006/bbrc.1998.8776. [DOI] [PubMed] [Google Scholar]
- 47.Miller AA, Hislop AA, Vallance PJ, Haworth SG. Deletion of the Enos Gene Has a Greater Impact on the Pulmonary Circulation of Male Than Female Mice. American journal of physiology. Lung cellular and molecular physiology. 2005;289:L299–306. doi: 10.1152/ajplung.00022.2005. [DOI] [PubMed] [Google Scholar]
- 48.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex Differences in Circulating and Renal Angiotensins of Hypertensive Mren(2). Lewis but Not Normotensive Lewis Rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalamajski S, Oldberg A. The Role of Small Leucine-Rich Proteoglycans in Collagen Fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the Small Interstitial Proteoglycans Biglycan, Decorin and Fibromodulin with Transforming Growth Factor Beta. Biochem J. 1994;302 ( Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rucci N, Rufo A, Alamanou M, Capulli M, Del Fattore A, Ahrman E, Capece D, Iansante V, Zazzeroni F, Alesse E, Heinegard D, Teti A. The Glycosaminoglycan-Binding Domain of Prelp Acts as a Cell Type-Specific Nf-Kappab Inhibitor That Impairs Osteoclastogenesis. J Cell Biol. 2009;187:669–683. doi: 10.1083/jcb.200906014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Happonen KE, Sjoberg AP, Morgelin M, Heinegard D, Blom AM. Complement Inhibitor C4b-Binding Protein Interacts Directly with Small Glycoproteins of the Extracellular Matrix. J Immunol. 2009;182:1518–1525. doi: 10.4049/jimmunol.182.3.1518. [DOI] [PubMed] [Google Scholar]
- 53.Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, Dechend R, Gratze P, Luft FC, Muller DN. Complement Activation in Angiotensin Ii-Induced Organ Damage. Circulation research. 2005;97:716–724. doi: 10.1161/01.RES.0000182677.89816.38. [DOI] [PubMed] [Google Scholar]
- 54.Melamed J, Kernizan S, Walden PD. Expression of B-Cell Translocation Gene 2 Protein in Normal Human Tissues. Tissue & cell. 2002;34:28–32. doi: 10.1054/tice.2001.0220. [DOI] [PubMed] [Google Scholar]
- 55.Li F, Liu J, Park ES, Jo M, Curry TE., Jr The B Cell Translocation Gene (Btg) Family in the Rat Ovary: Hormonal Induction, Regulation, and Impact on Cell Cycle Kinetics. Endocrinology. 2009;150:3894–3902. doi: 10.1210/en.2008-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park S, Lee YJ, Lee HJ, Seki T, Hong KH, Park J, Beppu H, Lim IK, Yoon JW, Li E, Kim SJ, Oh SP. B-Cell Translocation Gene 2 (Btg2) Regulates Vertebral Patterning by Modulating Bone Morphogenetic Protein/Smad Signaling. Molecular and cellular biology. 2004;24:10256–10262. doi: 10.1128/MCB.24.23.10256-10262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rouault JP, Falette N, Guehenneux F, Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P, Pain B, Shaw P, Berger R, Samarut J, Magaud JP, Ozturk M, Samarut C, Puisieux A. Identification of Btg2, an Antiproliferative P53-Dependent Component of the DNA Damage Cellular Response Pathway. Nat Genet. 1996;14:482–486. doi: 10.1038/ng1296-482. [DOI] [PubMed] [Google Scholar]
- 58.Duriez C, Falette N, Audoynaud C, Moyret-Lalle C, Bensaad K, Courtois S, Wang Q, Soussi T, Puisieux A. The Human Btg2/Tis21/Pc3 Gene: Genomic Structure, Transcriptional Regulation and Evaluation as a Candidate Tumor Suppressor Gene. Gene. 2002;282:207–214. doi: 10.1016/s0378-1119(01)00825-3. [DOI] [PubMed] [Google Scholar]
- 59.Rapp JP, Joe B. Use of Contiguous Congenic Strains in Analyzing Compound Qtls. Physiol Genomics. 2012;44:117–120. doi: 10.1152/physiolgenomics.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flister MJ, Hoffman MJ, Reddy P, Jacob HJ, Moreno C. Congenic Mapping and Sequence Analysis of the Renin Locus. Hypertension. 2013;61:850–856. doi: 10.1161/HYPERTENSIONAHA.111.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.