Abstract
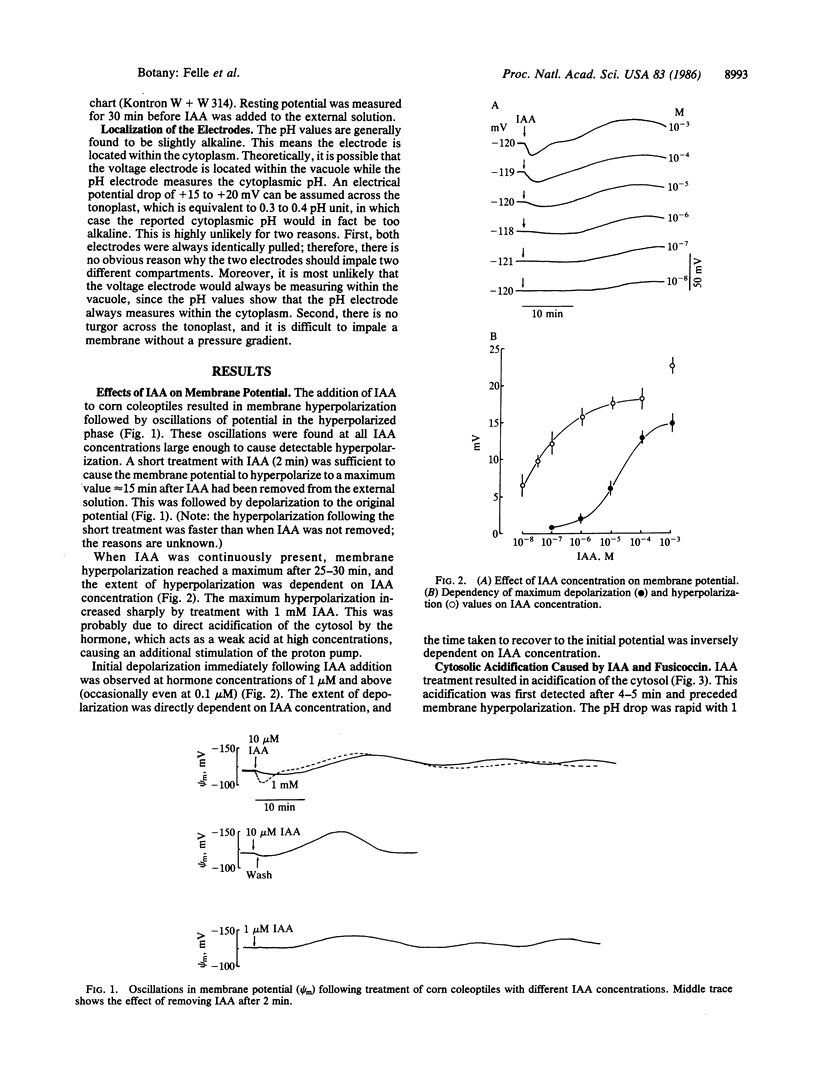
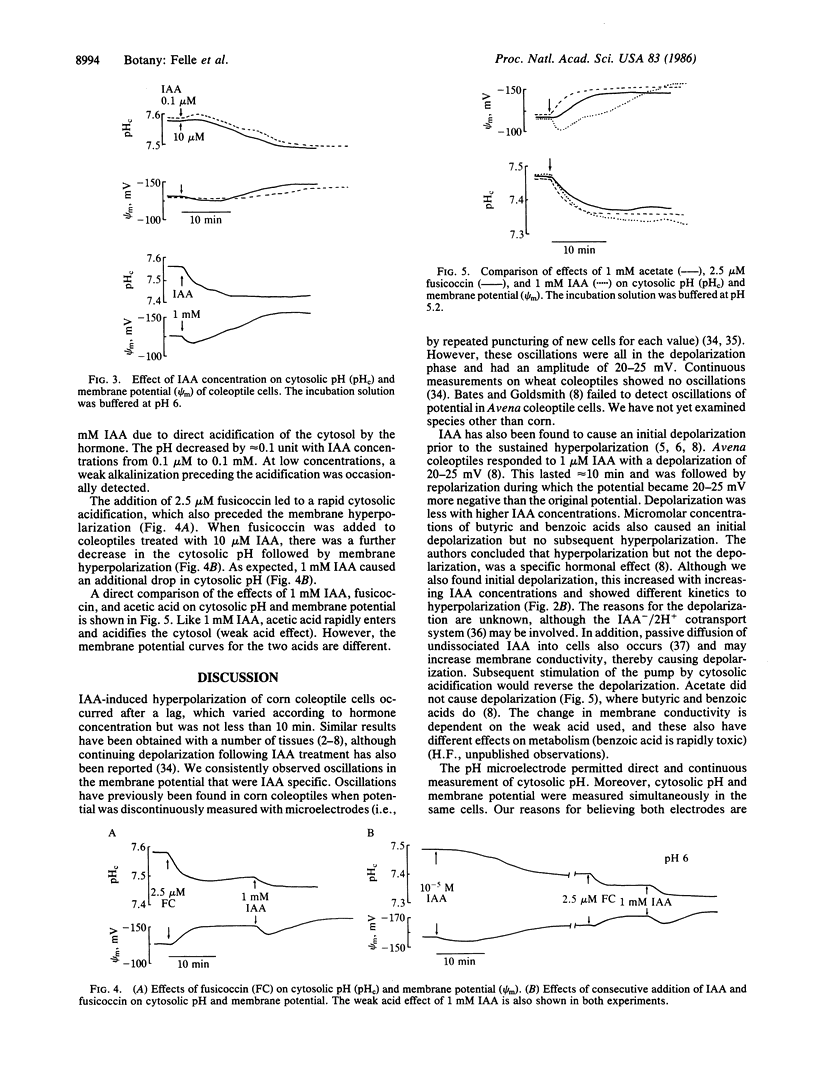
Microelectrodes were used to measure simultaneously the effects of indole-3-acetic acid (IAA) on membrane potential and cytosolic pH of corn coleoptile cells. IAA caused an initial depolarization followed by hyperpolarization, the latter displaying rhythmic oscillations. The extent of the changes in membrane potential was dependent on IAA concentration, and hyperpolarization, but not depolarization, could be detected with concentrations of IAA as low as 10 nM. Membrane hyperpolarization was preceded by a decrease in cytosolic pH. The decrease commenced ≈5 min after adding IAA and continued for 15-20 min before reaching a new steady state ≈0.1 pH unit lower than the original pH. The decrease in pH was readily detectable after treatment with 0.1 μM IAA. Fusicoccin and acetate, which, like IAA, induce elongation growth, caused a similar drop in cytosolic pH and subsequent membrane hyperpolarization, the decrease in pH commencing within seconds. The addition of fusicoccin to IAA-treated cells resulted in a further cytosolic acidification and membrane hyperpolarization. The two substances probably change cytosolic pH via different mechanisms. The results imply that one of the primary effects of auxins in coleoptiles is to lower cytosolic pH.
Keywords: auxins, elongation growth, membrane potential, pH microelectrodes
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertl A., Felle H., Bentrup F. W. Amine Transport in Riccia fluitans: Cytoplasmic and Vacuolar pH Recorded by a pH-Sensitive Microelectrode. Plant Physiol. 1984 Sep;76(1):75–78. doi: 10.1104/pp.76.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E., Prins H. B., Harper J. R., Higinbotham N. Rapid Hormone-induced Hyperpolarization of the Oat Coleoptile Transmembrane Potential. Plant Physiol. 1977 Mar;59(3):395–397. doi: 10.1104/pp.59.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced hydrogen ion excretion from Avena coleoptiles. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3092–3093. doi: 10.1073/pnas.70.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Effect of Indole-3-acetic Acid on Membrane Potentials of Oat Coleoptile Cells. Plant Physiol. 1970 Apr;45(4):527–528. doi: 10.1104/pp.45.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L. The action of auxin on plant cell elongation. CRC Crit Rev Plant Sci. 1985;2(4):317–365. doi: 10.1080/07352688509382200. [DOI] [PubMed] [Google Scholar]
- Felle H. A study of the current-voltage relationships of electrogenic active and passive membrane elements in Riccia fluitans. Biochim Biophys Acta. 1981 Aug 6;646(1):151–160. doi: 10.1016/0005-2736(81)90282-0. [DOI] [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. J. Rapid induction of selective transcription by auxins. Mol Cell Biol. 1985 Jun;5(6):1197–1203. doi: 10.1128/mcb.5.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U., Higinbotham N., Pallaghy C. K. Electrochemical evidence of specific action of indole acetic acid on membranes in Mnium leeaves. Z Naturforsch B. 1972 Oct;27(10):1239–1242. doi: 10.1515/znb-1972-1024. [DOI] [PubMed] [Google Scholar]
- Vesper M. J., Evans M. L. Nonhormonal induction of H efflux from plant tissues and its correlation with growth. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6366–6370. doi: 10.1073/pnas.76.12.6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Legocka J., Edelman L., Key J. L. An Analysis of Growth Regulator Interactions and Gene Expression during Auxin-Induced Cell Elongation Using Cloned Complementary DNAs to Auxin-Responsive Messenger RNAs. Plant Physiol. 1985 Apr;77(4):847–850. doi: 10.1104/pp.77.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]


