Abstract
Objective: The use of early response/nonresponse (ER/ENR) to antipsychotics as a predictor for ultimate response/nonresponse (UR/UNR) may help decrease inefficacious treatment continuation. However, data have been limited to adults, and ER/ENR has only been determined using time-consuming psychopathology rating scales. In the current study, we assessed if early improvement on the Clinical Global Impressions-Improvement (CGI-I) scale predicted UR/UNR in psychiatrically ill youth started on antipsychotic treatment.
Methods: Seventy-nine youth aged 6–19 years, with schizophrenia spectrum disorders, treated naturalistically with aripiprazole, olanzapine, quetiapine, risperidone, or ziprasidone and evaluated monthly, were divided into ER/ENR groups at week 4, using at least “minimally improved” on the CGI-I scale. Prediction using week 4 ER/ENR status for UR (CGI-I=at least “much improved”), effectiveness and adverse effect outcomes at 8–12 weeks were assessed.
Results: At 4 weeks, 45.6% of subjects were ER and 54.4% were ENR without differences regarding baseline demographic, illness, and treatment variables, except for higher age (p=0.034) and maximum risperidone dose (p=0.0043) in ENR. ER/ENR status at 4 weeks predicted UR/UNR at week 12 significantly (p<0.0001): Sensitivity=68.9%, specificity=85.3%, positive predictive value=86.1%, negative predictive value=67.4%. At weeks 4, 8, and 12, ER patients improved significantly more on the CGI-I, CGI-Severity, and Children's Global Assessment of Functioning scales, and more ER patients reached UR compared with ENR patients (83.3% vs. 34.9%, all p<0.0001). ENR patients had more extrapyramidal side effects (EPS) at weeks 4, 8, and 12 (p=0.0019–0.0079). UR was independently associated with ER (odds ratio [OR]=18.09; 95% confidence interval [CI]=4.71–91.68, p<0.0001) and psychosis not otherwise specified (NOS) (OR=4.82 [CI: 1.31–21.41], p=0.017) (r2=0.273, p<0.0001).
Conclusions: Older age and EPS were associated with ENR; ENR and schizophrenia were associated with UNR in naturalistically treated youth with schizophrenia spectrum disorders. Early CGI-I-based treatment decisions require further consideration and study.
Introduction
The time course of response to antipsychotic (AP) medications in schizophrenia spectrum disorders has attracted considerable interest (Agid et al. 2003; Correll et al. 2003; Leucht et al. 2007; Kinon et al. 2008; Leucht et al. 2008; Kinon et al. 2010). This is in part because no reliable response predictors/biomarkers exist that can guide clinicians as to when to give up on a medication trial and initiate another one (Kane et al. 2003; Kane and Correll 2010; Correll et al. 2011a). In this context, a clinical marker of AP response/nonresponse has gained significant traction. The focus has been on presence/absence of early improvement in the first few weeks after initiation of the AP treatment.
The hypothesis of early-onset action of APs proposes no notable delay in onset of action, and a gradual improvement toward a plateau (Agid et al. 2003). The early-onset hypothesis of AP action is opposed to the previously widely held delayed-onset of action hypothesis, according to which there is a delay of 2–3 weeks from the initiation of AP treatment to the onset of specific therapeutic effects.
Previously, it has been suggested that the early effects of APs are the result of changes in nonspecific symptoms, such as relief from anxiety and agitation, rather than a change in core psychotic symptoms. However, studies (Agid et al. 2003, Kapur et al. 2005) have shown that antipsychotic treatment leads to early and robust improvement in psychotic symptoms that is not just secondary to nonspecific changes, as the improvement is seen on psychosis-specific items on the Positive and Negative Syndrome Scale (PANSS, Kay et al. 1988) and Brief Psychiatric Rating Scale (BPRS, Overall 1974). A pooled analysis of double-blind studies including 7450 patients has shown greater improvement in the first 2 treatment weeks than in the subsequent 2 weeks, measured as reductions in total scores on the BPRS and PANSS (Agid et al. 2003). These findings have been replicated and extended to 1 year of AP treatment during which also the greatest degree of symptomatic improvement occurred in the first 2–4 weeks (Leucht et al. 2005). In one study (Kapur et al. 2005), a significant AP effect was shown to occur as early as within 24 hours of medication initiation.
More importantly, early-onset AP benefits have shown to be a stable predictor of clinical response in adults with schizophrenia (Agid et al. 2003; Kinon et al. 2010). Other studies have identified early AP nonresponse regarding total symptom scores as a robust predictor of subsequent nonresponse (Correll et al. 2003; Kinon et al. 2008; Leucht et al 2008), and early nonresponse (ENR) has been associated with less reduction in positive and negative symptoms (Lambert et al. 2007). In most studies, early response/nonresponse (ER/ENR) has been defined as ≥20/<20% reduction on the PANSS or BPRS. In addition to predicting greater symptom reduction at study endpoint and overall treatment response as measured by at least 20% or 40% reduction in the PANSS or BPRS total score, there have also been significant differences between early responders and early nonresponders in other domains not directly related to PANSS or BPRS scores. These have included more favorable outcomes in early responders regarding higher rates of remission (Schennach-Wolff et al. 2010), longer time to all-cause treatment discontinuation (Liu-Seifert et al. 2005), lower overall and non-medication related treatment cost (Ascher-Svanum et al. 2008a), and higher levels of social and physical functioning as measured by the SF 36® (Ascher-Svanum et al. 2008a).
Although several studies have investigated the early onset of action hypothesis, there is no clear consensus on the definition and the predictive value of ER of AP drugs (Schennach-Wolff et al. 2010). This is because studies have included different patient populations regarding duration of illness and used different response and remission criteria (Correll et al. 2011b). Moreover, ER patterns, which are measured relative to baseline, also depend upon baseline illness severity (Lambert et al. 2009).
These methodological differences may have affected the results. For example, the time to predictive response may need to be much longer than 2 weeks in first-episode patients. As studies in first-episode schizophrenia spectrum disorder patients have found predictive improvement after as long as 6 weeks (Derks et al. 2010), 8 weeks (Emsley et al. 2006), or 10 weeks (Gallego et al. 2011), there may be a mixture of patient groups with early and with more delayed response patterns early on in the illness course.
Moreover, the demonstration of improved outcomes in early responders versus early nonresponders is generally based on group means. To provide a more fine-grained appraisal, recent studies have focused on trajectories of response. For example, Levine et al. (2010) identified five treatment response trajectories characterized by varying levels of symptomatic improvement. In this study, group members with better treatment response trajectories had the shortest duration of illness and the lowest dropout rates.
Moreover, to date, it has been unclear how these findings can be translated into clinical practice, as clinicians generally do not have the time to conduct a full PANSS or BPRS rating as part of their care. Conversely, the Clinical Global Impressions-Improvement scale (CGI-I, Guy 1976) is a simple, seven point Likert scale that can easily be utilized in busy clinical settings (Berk et al. 2008). It has the advantages of being simple and not time-consuming, and having clinical face validity. In addition, the CGI-I scores have been correlated to PANSS and BPRS total score reductions in equipercentile ranking studies by Leucht and colleagues (Leucht et al. 2006). In these analyses, a 20% reduction in the PANSS or BPRS scale was equivalent to a rating of “minimally improved” on the CGI-I, whereas a rating of “much improved” on the CGI-I corresponded to a reduction of 40–50% in the PANSS or BPRS total score.
However, to date, no study has attempted to assess if one can predict ultimate response (UR) reliably by presence/absence of ER defined by a rating of at least minimal improvement on the CGI-I. Post-hoc analyses in studies in which CGI as well as PANSS or BPRS assessments were obtained cannot be used to test this hypothesis, as ratings on the more detailed psychopathology scale very likely affect CGI ratings. Furthermore, to our knowledge, the hypothesis of early onset of AP effects has not been tested in children and adolescents with schizophrenia spectrum disorders, and most analyses included patients from randomized controlled studies, which may reduce the generalizability of the findings.
To fill this gap in the literature, we analyzed data from an ongoing study of children and adolescents who are treated naturalistically with AP medications based on clinical need, aiming to assess if 1) presence/absence of at least minimal improvement on the CGI-I at week 4 is a significant predictor of response/nonresponse at week 12, 2) the ER paradigm extends to patients with psychotic disorder not otherwise specified (NOS) in addition to schizophrenia, and 3) ER predicted by CGI-I at week 4 is associated with better outcomes in other efficacy and tolerability domains.
Methods
Study setting and design
Data were collected as part of the Second-Generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY) study (Correll et al. 2009), an ongoing inception cohort study of AP use in youth. From December 2001 to September 2007, patients were recruited from pediatric inpatient and outpatient services of The Zucker Hillside Hospital, Queens, New York. Legal guardians/participants ages 18–19 years of age signed informed consent, minors ages 9–17 years signed informed assent, and minors <9 years old were exempt from signing assent for this North Shore-Long Island Jewish Health System Institutional Review Board-approved study.
Subjects
Inclusion criteria for the SATIETY study (Correll et al. 2009) were: Age 4–19 years; psychosis, mood, or aggressive spectrum disorder prompting clinician's choice of AP initiation; and consent/baseline assessments obtained within ≤7 days of new AP initiation. Exclusion criteria were: Treatment with >1 AP; active/past eating disorder; biochemical evidence of thyroid dysfunction; acute medical disorders; pregnancy/breastfeeding; wards of the state (as research consent by a public agency representative within 1 week was unlikely); and anticipated leaving of the catchment area within <4 weeks.
Data for this report were restricted to youth with 1) American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV; American Psychiatric Association 1994) schizophrenia spectrum disorders (i.e., schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, and psychotic disorder not otherwise specified [PsyNOS]); 2) at least a 4 week and either 8 or 12 week CGI-I, (Guy 1976) assessment (in order to determine early as well as UR/UNR); 3) non-clozapine AP treatment; and 4) confirmed AP adherence (i.e., taking ≥70% of the prescribed medication based on interview, not interrupting AP treatment against medical advice for >5 half-lives, and measurable AP blood levels).
Treatment
Patients received clinician's choice of AP treatment. Informed consent/assent was obtained after the AP choice was made by the treating clinician. Dosing, co-medications and treatment changes were based on clinical necessity. Although this study focused on second-generation APs (SGAs), patients starting any AP were screened.
Outcomes
Primary outcome for the current study were the sensitivity and specificity of ER/ENR defined as a CGI-I (Guy 1976) score of at least “minimally improved” (corresponding to at least a 20% reduction in the PANSS or BPRS total score, [Leucht et al. 2006]) for predicting UR/UNR, defined as a CGI-I score of at least “much improved” (corresponding to at least a 40–50% reduction in the PANSS or BPRS total score [Leucht et al. 2006]) at last-observation-carried-forward (LOCF) study endpoint (i.e., week 8 or 12). Secondary outcomes included the predictive value of ER/ENR for UR/UNR in patient subgroups (i.e., schizophrenia, schizoaffective disorder, schizophreniform disorder vs. PsyNOS; AP-naïve vs. non-naïve patients), as well as changes in illness severity (CGI-I, CGI-Severity [CGI-S; Guy 1976]), functional status Children's Global Assessment Scale (CGAS; Shaffer et al. 1983), treatment response (i.e., CGI-I: much or very much improved), all-cause and specific-cause discontinuation, duration of inpatient stay (for inpatients only), as well as adverse effects (i.e., incidence of akathisia, Parkinsonian side effects, absolute and relative body weight and sex- and age-adjusted body mass index change, as well as the incidence of weight gain ≥7%).
Assessments
At baseline, psychiatric diagnoses, based on DSM-IV criteria, race/ethnicity, socioeconomic status, and past treatment history were assessed by chart review, discussion with treatment providers, and clinical interview of the patient/caregiver. Structured research interviews were not conducted. Socioeconomic status was categorized according to Hollingshead (1975), ranging from 1 (highest) to 5 (lowest). Postpubertal status (Tanner stage 3–5) was determined at baseline through inspection and interview of the patient and/or caregiver.
Psychopathology and adverse effect ratings in this open, naturalistic study were mostly conducted by author C.U.C. Three additional raters were trained during the course of the study by C.U.C. with regular recalibration, but without formal inter-rater reliability testing. At baseline and monthly, CGI-S was used to rate illness severity on a scale of 1 (normal, not at all ill) to 7 (extremely ill). At monthly postbaseline assessments, the CGI-I was used to rate illness improvement on a scale from 1 (very much improved) to 7 (very much worse). At baseline and monthly, the CGAS was used to assess symptomatic as well as psychosocial functioning. CGAS is a numeric scale that ranges from 0 to 100, the highest scores being the highest functioning. Quality of Life was measured with the Sheehan Disability Scale (Sheehan 1983), but the scale was introduced midstream into the study. Because of limited data in the current study sample, data were not analyzed.
For the purposes of this study, we selected a priori extrapyramidal side effects (EPS), akathisia, and weight change as potential correlates of early or ultimate response status, as these are easily assessed in clinical care, are related to the degree of dopaminergic blockade (EPS, akathisia) and have relevance for subjective well-being, compliance and physical health. At baseline and monthly, subjects were assessed after ≥8 hours of overnight fasting for height, body weight, and fasting laboratory values (metabolic data were not analyzed for this study). Height was measured three times, using the stadiometer Seca 214. Body weight was measured using the Tanita Body Composition Analyzer TBF-310. As described before (Correll et al. 2009), patients were weighed clothed, with emptied pockets and without shoes or socks, using the following subtraction schedule: Persons >5 feet wearing long trousers and long-sleeve shirt/sweatshirt: −3 lbs; if dressed with short pants or short-sleeve/light shirt: −2.5 lbs; if dressed with short pants and short-sleeve/light shirt: −2 lbs; if just wearing underwear: −1.5 lbs. For persons measuring <5, but >4 feet, an additional 0.5 lbs were subtracted from the formula. For persons <4 feet, an additional 1 lb was subtracted. Sex- and age-adjusted BMI z-scores were calculated using a web-based calculator (http://www.kidsnutrition.org/bodycomp/bmiz2.html).
Presence of EPS was assessed with the Simpson–Angus Scale (SAS; Simpson and Angus 1970) and defined as at least one item being rated mild or higher (excluding tremor and glabellar tap, as these can be affected by other non-EPS related conditions [e.g., autism, mental retardation] or medications [e.g., lithium]). Akathisia was defined as a score of at least mild (score of 2) on the global clinical assessment (range 0–5) on Barnes Akathisia Scale (BARS; Barnes 1989).
AP treatment adherence was assessed through patient and caregiver interview as well as monthly AP blood levels. AP plasma levels were measured with liquid chromatography at Cooper Laboratory (Nathan Kline Institute, Orangeburg, NY).
Statistical analyses
Patients with two or more postbaseline assessments (4 weeks to establish ER status and at least one additional assessment at 8 and/or 12 weeks to assess outcome) comprised the modified intent-to-treat sample. Consistent with prior studies in this area, LOCF analyses were utilized throughout. As mentioned, ER was defined as a rating of at least “minimally improved” (i.e., ≤3) on the CGI-I at 4 weeks. Subsequently, ENR was defined as a rating of “no change” or worse (i.e., ≥4) on the CGI-I at 4 weeks. UR was defined as a rating of “much improved” or “very much improved” (i.e., 1 or 2) on the CGI-I at 8–12 weeks, and UNR was defined as a rating of “minimally improved” or worse (i.e., CGI-I≥3) at 8–12 weeks.
For the primary outcome, we calculated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for patients with ER/ENR regarding UR, using χ2 tests for the overall 2×2 outcome table. Sensitivity was defined as the proportion of ultimate responders at 12 weeks who were identified as early responders at 4 weeks (“true positive”). Specificity was defined as the proportion of early nonresponders at 12 weeks who were identified as early nonresponders at 4 weeks (“true negative”). The PPV was defined as the proportion of early responders at week 4 who were ultimate responders at week 12, and the NPV was defined as the proportion of nonresponders at week 4 who were ultimate nonresponders at week 12.
As up until now, the ER paradigm has been assessed only in patients with schizophrenia, we performed exploratory subgroup analyses for patients with a diagnosis of schizophrenia/schizoaffective/schizophreniform disorder or of psychosis NOS/brief psychotic disorder, and in AP-naïve patients or AP non-naïve patients.
Further, we compared baseline values across ER and ENR groups, using χ2 or Fisher's exact tests for categorical variables and t test for continuous variables. As EPS and AP doses were larger in the ENR groups, we also conducted an exploratory logistic regression analysis, investigating the effect of EPS on UR status, covarying the analysis for mean AP chlorpromazine equivalent doses. Finally, we conducted a backward elimination logistic regression analysis of UR, entering ENR status, EPS during the 12 week treatment period, and all baseline variables that were statistically significantly different between ER and ENR patients (i.e., age), as well as diagnostic subgroup (i.e., schizophrenia/schizoaffective/schizophreniform disorder or PsyNOS/brief psychotic disorder) and AP-naïve status in the initial model. For categorical variables remaining significant in this model predicting UR, we further calculated the number-needed-to-treat by dividing 1 by the difference between the two UR rates.
All data were analyzed with JMP 5.0.1, 1989–2003 (SAS Institute, Inc, Cary, NC). All tests were two sided, and α was set at <0.05.
Results
Patient population
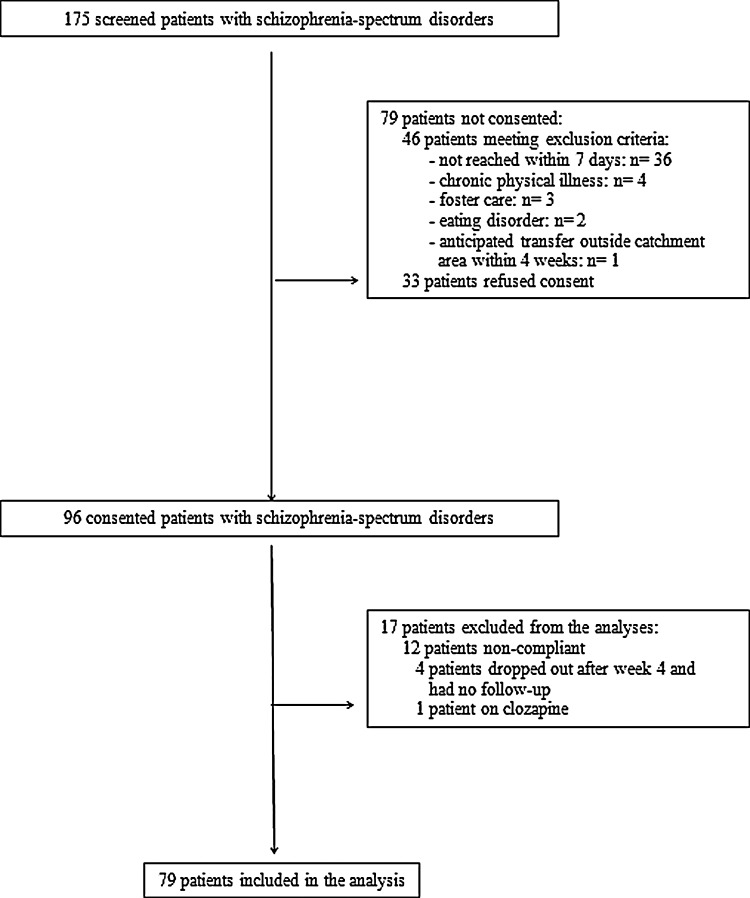
Of 175 screened patients with a schizophrenia spectrum diagnosis, 79 patients were not consented into the study because they were either ineligible (n=46) or refused consent (n=33) (Fig. 1). Therefore, 96 (54.9%) patients with schizophrenia spectrum disorders were consented into the SATIETY study. Of these, 79 (82.3%) were included in the final analyses and 17 were excluded because of partial or full nonadherence based on the interview, or unmeasurable AP blood levels after baseline (n=12), dropping out after week 4 (n=4), or treatment with clozapine (n=1) (Fig. 1). The 96 (n=79+n=17) refusing/ineligible/excluded patients were not significantly different from the 79 included patients except for the following differences between included and excluded patients: 1) More schizophrenia and schizophreniform disorder diagnoses (32.9% vs. 8.6% p=0.00095), less pervasive developmental disorder (PDD) NOS among the excluded patients (84.0% vs. 63.3%, p=0.00095), and more AP-naïve patients in the included group (77.2% vs. 58.0%, p=0.034).
FIG. 1.
Patient flow.
Baseline characteristics
Patients were on average 15.2±2.8 years old (range: 6.6–19.8), predominantly male (58.2%), postpubertal (86.1%), and had a wide ethnic distribution (Table 1). Most of the patients had a primary diagnosis of PsyNOS (63.3%) or schizophrenia (29.1%). The most common comorbid diagnoses were attention-deficit/hyperactivity disorder (ADHD) (12.7%), lifetime substance use disorder (12.7%), and anxiety disorders (11.4%). Altogether, 33 patients (60%) had a psychiatric family history and 14 (25.4%) had a first- or second-degree relative with schizophrenia.
Table 1.
Baseline Demographic and Illness Characteristics
| Demographic and illness characteristics | Total (n=79) | Early responder (n=36) | Early nonresponder (n=43) | p value |
|---|---|---|---|---|
| Age, years, mean±SD |
15.2±2.8 |
14.5±3.3 |
15.8±2.4 |
0.034 |
| Postpubertal status, n (%) |
68 (86.1) |
28 (77.8) |
40 (93.0) |
0.10 |
| Sex male, n (%) |
46 (58.2) |
22 (61.1) |
24 (55.8) |
0.64 |
| Race, n (%) |
|
|
|
0.91 |
| Caucasian |
29 (36.7) |
13 (36.1) |
16 (37.2) |
|
| Black/African-American |
27 (34.2) |
14 (38.9) |
13 (30.2) |
|
| Hispanic |
11 (13.9) |
5 (13.9) |
6 (14.0) |
|
| Asian |
6 (7.6) |
2 (5.6) |
4 (9.3) |
|
| Other |
6 (7.6) |
2 (5.6) |
4 (9.3) |
|
| Inpatients, n (%) |
62 (78.5) |
30 (83.3) |
32 (74.4) |
0.34 |
| Number of past admissions, mean±SD |
0.2±0.6 |
0.1±0.5 |
0.3±0.6 |
0.27 |
| Socioeconomic status, mean±SD |
2.6±0.9 |
2.7±0.9 |
2.6±1.0 |
0.82 |
| Weight, kg, mean±SD |
57.6±16.2 |
57.3±18.3 |
57.8±14.2 |
0.89 |
| BMI %-tile, mean±SD |
54.5±30.9 |
58.3±32.4 |
52.3±29.6 |
0.32 |
| Weight status, n (%) |
|
|
|
0.31 |
| Underweight |
6 (7.6) |
3 (8.3) |
3 (7.0) |
|
| Normal weight |
56 (70.9) |
22 (61.1) |
34 (79.1) |
|
| Overweight |
6 (7.6) |
4 (11.1) |
2 (4.7) |
|
| Obese |
11 (13.9) |
7 (19.4) |
4 (9.3) |
|
| Primary diagnosis, n (%) |
|
|
|
0.92 |
| Psychotic disorder not otherwise specified |
50 (63.3) |
22 (61.1) |
28 (65.1) |
|
| Schizophrenia |
23 (29.1) |
11(30.6) |
12 (27.9) |
|
| Schizophreniform disorder |
3 (3.8) |
1 (2.8) |
2 (4.7) |
|
| Schizoaffective disorder |
2 (2.5) |
1 (2.8) |
1 (2.3) |
|
| Brief psychotic disorder |
1 (1.3) |
1 (2.8) |
0 (0) |
|
| CGI-S, mean±SD |
5.6±0.8 |
5.7±0.9 |
5.6±0.8 |
0.66 |
| CGAS, mean±SD |
34.0±8.8 |
33.6±8.1 |
34.2±9.3 |
0.78 |
| Psychiatric family history, n (%) | ||||
| Any psychiatric disorder |
33 (60.0) |
11 (50.0) |
22 (66.7) |
0.22 |
| Schizophrenia family history | ||||
| First degree relative |
2 (3.6) |
2 (9.1) |
0 (0) |
0.16 |
| Second degree relative |
12 (21.8) |
4 (18.2) |
8 (24.2) |
0.74 |
| Comorbidity, n (%) | ||||
| Attention-deficit/hyperactivity disorder |
10 (12.7) |
7 (19.4) |
3 (7.0) |
0.17 |
| Lifetime substance use disorder |
10 (12.7) |
5 (13.9) |
5 (11.6) |
1.00 |
| Anxiety disorder |
9 (11.4) |
5 (13.9) |
4 (9.3) |
0.73 |
| Disruptive behavior disordersa |
6 (7.6) |
4 (11.1) |
2 (4.7) |
0.40 |
| Obsessive compulsive disorder |
5 (6.3) |
2 (5.6) |
3 (7.0) |
1.00 |
| Pervasive developmental disorder |
3 (3.8) |
1 (2.8) |
2 (4.7) |
1.00 |
| Depressive spectrum disordersb |
3 (3.8) |
2 (5.6) |
1 (2.3) |
0.59 |
| Mental retardation | 4 (5.1) | 1 (2.8) | 3 (7.0) | 0.62 |
Disruptive behavior disorders=oppositional defiant disorder, conduct disorder, intermittent explosive disorder, impulse control disorder not otherwise specified.
Depressive spectrum disorders=major depressive disorder, depressive disorder not otherwise specified, adjustment disorder with depressed mood.
CGAS, Children's Global Assessment of Functioning Scale; CGI-S, Clinical Global Impressions-Severity.
Bolded p-values indicate p<0.05.
There were no significant differences between ENR and ER patients in gender, race, socioeconomic status, body weight, number of past admissions, or inpatient status, except for older age in ENR patients (15.8±2.4 vs. 14.5±3.3 years, p=0.034). Similarly, patients in both groups had equivalent levels of psychopathology and global functioning at baseline, with an average CGI-S score of 5.6±0.8 (i.e., between “markedly ill” and “severely ill”) and a mean CGAS of 34.0±8.8 (i.e., “major impairment in several areas and unable to function in one area”) (Table 1).
Treatment characteristics
Most patients (77.2%) were AP-naïve (i.e., ≤7 days of lifetime treatment) at baseline (Table 2). The most common AP prescribed was risperidone (45.6%), followed by olanzapine (20.3%), aripiprazole (17.7%), quetiapine (11.4%), and ziprasidone (5.1%).
Table 2.
Treatment Characteristics
| Treatment characteristics | ±Total (n=79) | Early responder (n=36) | Early nonresponder (n=43) | p value |
|---|---|---|---|---|
| Antipsychotic- naïve, n (%) |
61 (77.2) |
27 (75.0) |
34 (79.1) |
0.67 |
| Number of antipsychotic trials (mean±SD) |
0.5±0.9 |
0.6±1.1 |
0.4±0.7 |
0.44 |
| Antipsychotic treatment | ||||
| Risperidone, n (%) |
36 (45.6) |
18 (50.0) |
18 (41.9) |
0.47 |
| Starting dose (mg±SD) |
0.8±0.5 |
0.7±0.4 |
0.9±0.6 |
0.16 |
| Days until max dose (mean±SD) |
24.6±23.8 |
20.5±24.3 |
28.8±23.4 |
0.32 |
| Maximum daily dose (mg±SD) |
3.1±1.7 |
2.2±1.6 |
4.0±1.9 |
0.0043 |
| Olanzapine, n (%) |
16 (20.3) |
7 (19.4) |
9 (20.9) |
0.87 |
| Starting dose (mg±SD) |
3.0±1.1 |
3.6±1.3 |
2.6±1.0 |
0.13 |
| Days until max dose (mean±SD) |
26.5±27.8 |
29.0±28.3 |
24.4±27.2 |
0.75 |
| Maximum daily dose (mg±SD) |
12.6±7.4 |
14.3±7.9 |
11.3±7.0 |
0.43 |
| Aripiprazole, n (%) |
14 (17.7) |
4 (11.1) |
10 (23.3) |
0.24 |
| Starting dose (mg±SD) |
5.2±4.5 |
3.1±1.3 |
6.1±5.2 |
0.29 |
| Days until max dose (mean±SD) |
31.2±26.9 |
24.0±19.0 |
34.1±29.0 |
0.54 |
| Maximum daily dose (mg±SD) |
17.0±9.0 |
14.4±7.7 |
18.0±9.4 |
0.51 |
| Quetiapine, n (%) |
9 (11.4) |
5 (13.9) |
4 (9.3) |
0.73 |
| Starting dose (mg±SD) |
45.8±35.3 |
45.0±32.6 |
46.9±38.7 |
0.94 |
| Days until max dose (mean±SD) |
22.0±19.0 |
17.8±22.9 |
27.3±11.8 |
0.48 |
| Maximum daily dose (mg±SD) |
369.4±270.2 |
310.0±214.0 |
443.8±330.6 |
0.49 |
| Ziprasidone, n (%) |
4 (5.1) |
2 (5.6) |
2 (4.7) |
1.00 |
| Starting dose (mg±SD) |
25.0±10.0 |
20.0±0 |
30.0±14.1 |
0.42 |
| Days until max dose (mean±SD) |
20.8±10.7 |
23.5±13.4 |
18.0±7.1 |
0.66 |
| Maximum daily dose (mg±SD) |
120.0±44.7 |
100.0±56.6 |
140.0±28.3 |
0.47 |
| Pooled CPZ equivalent doses (mg±SD) | ||||
| Mean pooled maximum CPZ equivalent dose |
228.0±184.7 |
198.2±173.2 |
253.5±194.0 |
0.19 |
| Mean CPZ equivalent dose at week 4 |
182.6±149.0 |
159.6±110.1 |
201.4±174.3 |
0.22 |
| Mean CPZ equivalent dose at week 8 |
205.9±171.3 |
180.5±153.5 |
228.2±185.4 |
0.23 |
| Mean CPZ equivalent dose at week 12 |
205.9±189.6 |
188.0±182.1 |
220.1±195.2 |
0.46 |
| Co-medication | ||||
| Total number of co-medications |
0.9±0.9 |
0.9±0.7 |
0.9±1.1 |
0.95 |
| None, n (%) |
27 (34.6) |
10 (27.8) |
17 (40.5) |
0.24 |
| Antidepressants, n (%) |
20 (25.6) |
9 (25.0) |
11 (26.2) |
0.91 |
| Mood stabilizers, n (%) |
11 (14.1) |
5 (13.9) |
6 (14.3) |
1.00 |
| Stimulants, n (%) |
3 (3.9) |
2 (5.6) |
1 (2.4) |
0.59 |
| Anticholinergics, n (%) |
13 (16.7) |
5 (13.9) |
8 (19.1) |
0.76 |
| Anxiolytics-sedatives, n (%) |
14 (18.0) |
7 (19.4) |
7 (16.7) |
0.75 |
| α-agonists, n (%) |
3 (3.9) |
2 (5.6) |
1 (2.4) |
0.59 |
| Other co-medications | 4 (5.1) | 3 (8.3) | 1 (2.4) | 0.33 |
CPZ, chlorpromazine.
Bolded p-values indicate p<0.05.
There were no differences in treatment characteristics between the ER and ENR groups, except for the maximum daily dose of risperidone (2.2±1.6 vs. 4.0±1.9 mg, p=0.0043), risperidone dose at 4 and 8 weeks, and risperidone level at 8 weeks (data not shown). However, across all APs together, there were no significant differences in maximum daily chlorpromazine equivalent doses or in mean chlorpromazine equivalent doses at week 4, 8, and 12 (Table 2).
A minority of 27 patients (34.6%) received no co-medications during the 12 week study. The co-medications primarily used were antidepressants (25.6%), anxiolytics/sedatives (18%), and anticholinergics (16.7%). There was no difference in the use of co-medications between the ER and ENR groups.
Response trajectory
At week 4, 36 patients (45.6 %) were ER and 43 (54.4 %) were ENR, using a CGI-I of ≤3 threshold. At week 12, 45 patients (57.0%) were UR and 34 (43%) were UNR, using a threshold of CGI-I ≤2.
Predictive value of ER/ENR
The predictive value analysis (Table 3) of early ER/ENR predicting UR/UNR showed a specificity of 85.3%, an NPV of 67.4%, a sensitivity of 68.9%, and a PPV of 86.1% (χ2 p<0.0001).
Table 3.
Predictive Value Analysis of Early Response at Week 4 for Ultimate Response at Weeks 8–12 (LOCF)
| Sample | Sensitivity | Specificity | Positive predictive value | Negative predictive value | p value of 2×2 table |
|---|---|---|---|---|---|
| Total sample (n=79) |
68.9% |
85.3% |
86.1% |
67.4% |
<0.0001 |
|
Subgroup analysis by diagnostic groups | |||||
| Schizophrenia/Schizoaffective disorder (n=28) |
83.3% |
81.3% |
76.9% |
86.7% |
0.0016 |
| Psychosis NOS/ Brief psychotic disorder (n=51) |
63.6% |
88.9% |
91.3% |
57.1% |
0.0004 |
|
Subgroup analysis by treatment history | |||||
| Antipsychotic-naïve (n=61) |
67.6% |
91.7% |
92.6% |
64.7% |
<0.0001 |
| Antipsychotic-non-naïve (n=18) | 75.0% | 70.0% | 66.7% | 77.8% | 0.15 |
LOCF=last-observation-carried-forward; NOS, not otherwise specified.
Bolded p-values indicate p<0.05.
The exploratory subgroup analyses in patients with PsyNOS/brief psychosis or schizophrenia/schizoaffective disease showed a higher sensitivity and, especially, NPV for patients with schizophrenia than for PsyNOS (sensitivity: 83.3% vs. 63.6%, NPV: 86.7% vs. 57.1%). By contrast, specificity and, especially, PPV were higher in PsyNOS patients (specificity: 88.9% vs. 81.3%, PPV: 91.3% vs 76.9%). Similarly, in the AP-naïve subgroup, specificity and PPV were higher than in the patients with prior AP exposure history (specificity: 91.7% vs. 70.0%, PPV: 92.6% vs. 66.7%) whereas sensitivity and NPV were only slightly higher in AP-exposed patients (sensitivity: 75.0% vs. 67.6%, NPV: 77.8% vs. 64.7%).
Efficacy, effectiveness and tolerability outcomes
Compared with ENR patients, ER patients had significantly better scores at every visit in CGI-I, CGI-S, and CGAS (all p<0.0001) (Table 4). At week 12, significantly more ER patients than ENR patients reached UR status (83.3% vs. 34.9%, p<0.0001, number-needed-to-treat: 3).
Table 4.
Treatment Outcomes
| Outcomes | Total (n=79) | Early responder (n=36) | Early nonresponder (n=43) | p value |
|---|---|---|---|---|
| All-cause discontinuation, n (%) |
19 (24.4) |
5 (13.9) |
14 (33.3) |
0.064 |
| Discontinuation, reasons,an (%) | ||||
| Inefficacy |
8 (10.4) |
1 (2.8) |
7 (17.1) |
0.061 |
| Intolerability |
9 (11.7) |
3 (8.3) |
6 (14.6) |
0.49 |
| Loss of contact |
4 (5.2) |
2 (5.6) |
2 (4.9) |
1.00 |
| Number of inpatient days (mean±SD) |
25.6±21.7 |
20.7±19.5 |
30.1±23.5 |
0.09 |
| Response (CGI-I</=2), n (%) |
45 (57.0) |
30 (83.3) |
15 (34.9) |
<0.0001 |
| CGI-I, mean±SD | ||||
| 4 weeks |
2.6±0.5 |
1.7±0.5 |
3.4±0.5 |
<0.0001 |
| 8 weeks |
2.4±0.9 |
1.8±1.0 |
3.0±0.7 |
<0.0001 |
| 12 weeks |
2.1±0.8 |
1.5±0.7 |
2.7±0.9 |
<0.0001 |
| CGI-S change, mean±SD | ||||
| 4 weeks |
−1.2±0.8 |
−1.9±1.0 |
−0.6±0.7 |
<0.0001 |
| 8 weeks |
−1.4±1.0 |
−2.0±1.2 |
−0.8±0.8 |
<0.0001 |
| 12 weeks |
−1.7±1.2 |
−2.4±1.4 |
−1.2±1.1 |
<0.0001 |
| CGAS change, mean±SD | ||||
| 4 weeks |
11.9±7.3 |
18.1±8.3 |
6.7±6.4 |
<0.0001 |
| 8 weeks |
15.2±9.4 |
20.6±9.7 |
10.6±9.1 |
<0.0001 |
| 12 weeks |
18.0±11.0 |
23.5±11.2 |
13.0±10.8 |
<0.0001 |
| EPS, n (%) | ||||
| 4 weeks |
15 (21.4) |
2 (6.1) |
13 (35.1) |
0.0035 |
| 8 weeks |
14 (19.7) |
2 (6.1) |
12 (31.6) |
0.0079 |
| 12 weeks |
14 (19.4) |
1 (3.0) |
13 (33.3) |
0.0019 |
| Anytime at 4, 8 or 12 weeks |
22 (30.6) |
4 (12.2) |
18 (46.2) |
0.0018 |
| Akathisia, n (%) | ||||
| 4 weeks |
5 (6.6) |
2 (5.7) |
3 (7.3) |
1.00 |
| 8 weeks |
5 (6.5) |
1 (2.9) |
4 (9.5) |
0.37 |
| 12 weeks |
4 (5.2) |
1 (2.9) |
3 (7.1) |
0.62 |
| Anytime at 4, 8 or 12 weeks |
6 (7.8) |
2 (5.7) |
4 (9.5) |
0.53 |
| Weight change, kg, mean±SD | ||||
| 4 weeks |
3.1±2.6 |
3.0±2.1 |
3.3±3.0 |
0.64 |
| 8 weeks |
4.8±3.7 |
4.9±3.0 |
4.8±4.2 |
0.92 |
| 12 weeks |
5.7±4.3 |
5.5±3.5 |
5.9±4.9 |
0.65 |
| Weight % change, mean±SD, 12 weeks |
11.0±8.8 |
10.4±7.0 |
11.6±10.2 |
0.56 |
| Weight gain ≥7%, n (%), 12 weeks |
51 (64.5) |
24 (66.7) |
27 (62.8) |
0.72 |
| BMI change, kg/m3, mean±SD | ||||
| 4 weeks |
1.1±0.9 |
1.1±0.8 |
1.1±1.1 |
0.91 |
| 8 weeks |
1.7±1.3 |
1.7±1.1 |
1.6±1.4 |
0.75 |
| 12 weeks |
1.9±1.5 |
1.9±1.3 |
2.0±1.7 |
0.73 |
| BMI z-score change, mean±SD | ||||
| 4 weeks |
0.4±0.4 |
0.4±0.4 |
0.4±0.4 |
0.96 |
| 8 weeks |
0.7±1.0 |
0.8±0.9 |
0.6±1.0 |
0.23 |
| 12 weeks | 0.6±0.6 | 0.6±0.5 | 0.6±0.7 | 0.83 |
Total may be >100% of all-cause discontinuation because of discontinuation for more than one reason.
BMI, body mass index; CGAS, Children's Global Assessment of Functioning Scale; CGI-I, Clinical Global Impressions- Improvement; CGI-S, Clinical Global Impressions-Severity; EPS, Extrapyramidal Side Effects.
Bolded p-values indicate p<0.05.
The total rate of all cause discontinuation was 24.4%, and there was a trend for ENR patients to discontinue treatment more often than ER patients (33.3% vs.13.9%, p=0.064). This was primarily the result of more ENR patients discontinuing because of inefficacy (p=0.061) (Table 4).
Side effects
Altogether, 30.8% of patients had at least mild EPS at least at one postbaseline assessment. A significantly higher proportion of ENR patients had EPS at weeks 4, 8, and 12 compared with ER patients (p=0.0019–0.0079) (Table 4). Occurrence of any EPS during the 12 week study was significantly associated with ENR status, both in univariate analyses (46.2% vs. 12.2%, p=0.0018), as well as after controlling for AP-naive status and chlorpromazine equivalent AP doses (p=0.0029).
Only a few patients (7.8%) developed akathisia during the study, and there was no significant group difference in the incidence of akathisia between the ER and ENR groups. At week 12, patients had gained an average of 5.7±4.3 kg and 51 (64.5%) had gained at least 7% of their baseline body weight, but ER and ENR patients also did not differ regarding these outcomes.
Logistic regression model of ultimate response
Entering age, diagnostic subgroup, AP-naïve status at baseline, and ER status as well as EPS during the study period into the model, the following two variables emerged as independent predictors of UR (r2=0.273, p<0.0001): ER status at week 4 (odds ratio [OR]: 18.09, 95% confidence interval [CI]: 4.71–91.68, p<0.0001), and PsyNOS/brief psychotic disorder (OR: 4.82, 95% CI: 1.31–21.41, p=0.017).
Discussion
In this first study that utilized the clinically scalable CGI-I assessment as the sole tool to categorize patients into ER and ENR groups, ER or ENR at week 4 accurately predicted subsequent response or nonresponse at week 12 in youth with schizophrenia spectrum disorders who were primarily AP naïve and treated naturalistically with SGAs. Moreover, ER patients had significantly greater improvements in CGI-S and CGAS scores than ENR patients. Furthermore, ENR status was predicted by higher age and EPS ratings, whereas UNR status was predicted by ENR and a diagnosis of schizophrenia.
Studies in adults with chronic schizophrenia have repeatedly shown that ER or ENR to APs at week 1 or 2, generally defined as at least a 20% reduction in PANSS or BPRS total score, is a robust predictor of later response or nonresponse (Correll et al. 2003; Leucht et al. 2007; Ascher-Svanum et al. 2008a; Kinon et al. 2008; Leucht et al. 2008; Kinon et al. 2010; Hatta et al. 2011). Moreover, these studies have also shown that early AP treatment response is associated with significantly better outcomes, indicated by greater improvements on scales and dimensions not directly related to PANSS or BPRS scores that were used to group patients into ER and ENR subjects (Correll et al. 2003; Leucht et al. 2007; Ascher-Svanum et al. 2008a; Kinon et al. 2008; Leucht et al. 2008; Kinon et al. 2010; Hatta et al. 2011). However, to date, all studies that have investigated the predictive validity of early clinical response for later response included adult patients in randomized controlled or open-label trials, and used changes on the PANSS or BPRS as measures of ER/ENR and of UR.
This study extends the prior research in several ways. Most importantly, we examined pediatric patients in a naturalistic treatment setting, and we used the CGI-I rating for the assessment of ER and UR, both features that increase the generalizability and clinical applicability of the findings. Moreover, we included a majority of patients with PsyNOS, a diagnostic group that has not been examined with the ER paradigm.
We found that a CGI-I of less than “minimally improved” at week 4 was a significant predictor of UNR status (defined as a CGI-I>2) as well as of significantly less improvement on the CGI-S and CGAS at weeks 8 and 12. Specificity level for the prediction of UNR was high (85.3%), indicating a high probability that nonresponders at week 12 were correctly identified at week 4. High specificity and high NPV are needed to avoid unnecessarily changing treatment in patients who would have responded. PPV was also high (86.1%), whereas sensitivity (68.9%) and NPV (67.4%) were moderate. For patients with schizophrenia and for AP-non-naïve patients the NPV (86.7% and 77.8%, respectively) was higher than for the entire cohort of patients that included a larger proportion of patients with PsyNOS. These results indicate that a diagnosis of schizophrenia or a prior exposure to APs seem to improve the predictive value of ENR to antidopaminergic medications in this patient group. This finding is in line with analyses in adult first-episode schizophrenia samples in whom the 2 week predictive value of ENR was generally not as good as seen in chronically ill patients (Emsley et al. 2006; Gallego et al. 2011; Stauffer et al. 2011). On the other hand, we found that the PPV of ER is very high among patients with PsyNOS/brief psychosis.
For example, one study of predominantly adults (16–40 years of age) with first-episode schizophrenia (Stauffer et al. 2011) examined early response to AP treatment, defined as a 26.2% improvement on the PANSS total score at week 2, as a predictor of response at week 12 (defined as ≥50% reduction of the PANSS total score). These thresholds for ER and UR are comparable to the ones used in our study, according to an equipercentile ranking that linked ratings of levels of illness severity and improvement measured on the CGI-S and CGI-I to ratings on the PANSS or BPRS total score (Leucht et al. 2006). In that study, a 20% reduction in total BPRS or PANSS score was comparable to “minimally improved” on the CGI-I, and a 40–50% reduction in total BPRS or PANSS score was comparable to rating of at least “much improved” on the CGI-I.
Two weeks was chosen as the early response assessment time point in the study by Stauffer et al. (2011), as in many other studies, on the basis of previous investigations, in which the bulk and/or significantly greater improvement in symptoms occurred during weeks 1 and 2 of treatment compared with weeks 3 and 4 (Agid et al. 2003; Leucht et al. 2005). Stauffer and colleagues found 43.1% of patients to be early responders, which is almost the same as the 45.6% early responders in our study, although early response in our study was measured at 4 and not 2 weeks. However, in the adult first-episode sample, using a ≥50% reduction on the PANSS total score for UR, only 39% of patients had UR at week 12 compared with 57% in our study in which a CGI-I of “much or very much improved” defined UR. However, the study by Stauffer et al. (2011) had only 21.6%/30.5% AP-naïve patients in the ER/ENR groups, respectively, compared with 75.0%/79.1% in our study. The two to three times higher proportion of AP-naïve patients might be part of the reason why in our sample more patients achieved UR status. Moreover, participants in our study were pediatric patients, compared with patients 16–40 years of age in the study by Stauffer et al (2011). This finding of more ultimate responders in our study suggests that PsyNOS and AP-naïveté-enriched samples or early-onset psychosis patients may have a more favorable response than adult first-episode schizophrenia patients, a finding also reported in two independent, large Australian samples (Schimmelmann et al. 2007; Amminger et al. 2011). Nevertheless, the sensitivity analysis of ER at 2 weeks to predict UR at 12 weeks in the adult first-episode sample yielded an NPV of 80% and specificity of 74%, which is comparable to the NPV of 86.7% and the specificity of 81.3% in our patient subgroup with schizophrenia and schizoaffective disorder, and to the NPV of 77.8% and specificity of 70% in our AP-non-naïve subgroup. Interestingly, in a subgroup analysis of patients ≤21 and >21 years of age, Stauffer et al. (2011) found no difference in the predictive characteristics of ER/ENR, supporting that the ER paradigm can be extended to younger age groups. Extension of the ER paradigm to adolescents was also recently confirmed by a post-hoc analysis of a randomized, placebo-controlled study, showing that ER at week 3 was most predictive (more so than at week 2) of UR and remission at week 6 in adolescents 13–17 years of age with non-first-episode schizophrenia who had been randomized to aripiprazole (Correll et al. 2013).
In our study, ER/ENR assessments were only available at week 4 after baseline and not before, whereas most prior studies focused on week 2 (or week 1). However, some studies of first-episode patients point to the importance of a later time point to determine ER/ENR status for this patient group. In an analysis of the European First Episode Study (EUFEST), the rate and predictors of remission within 12 months (according to Andreasen's remission criteria of a score of mild or less on eight predefined PANSS items, Andreasen et al. 2005) was assessed (Derks et al. 2010). All variables that were significantly associated with remission (PANSS, CGI) were included as predictors in the analyses. The authors found that the prediction of remission significantly improved by the inclusion of 4 and 6 week assessments instead of choosing only week 2, and that the CGI-S score at 4 weeks significantly improved the prediction of remission even when the PANSS rating was excluded. In another first-episode study, time to clinical response (defined as ≥20% improvement in PANNS total score) was determined (Emsley et al. 2006), and the investigators found that time to AP response varied widely and that 22.5% of patients did not respond until after 4 weeks. These results are in line with a 16 week study by Gallego et al. (2011) in which many patients responded between weeks 8 and 16, and in which percentage reduction in symptom severity score at week 4, but not at week 2 or week 8, was associated with UR status at week 16. Therefore, results from these studies indicate that a 4 week time point might be reasonable to determine ER status in first-episode or recent-onset patients.
In some early prediction studies, the same threshold for ER as for UR was used (Ascher-Svanum et al. 2008a). In our study, we chose a greater improvement threshold to determine UR than to determine ER. In two studies by Kinon et al. (2008, 2010) the greatest total predictive accuracy of early response (defined as ≥20% improvement on the PANSS total score) existed for UR using a threshold of ≥40% or ≥50% improvement in the PANSS total score, with lesser accuracy for UR definitions, using ≥20% or ≥30% improvement in PANSS total score, or Andreasen's remission criteria. These results suggest that a higher threshold for later response than for early response might be more appropriate, in addition to being also clinically more meaningful (Correll et al. 2011b).
One study of adults with chronic schizophrenia used the CGI-I to determine ER (Hatta et al. 2011), using the same ER criteria as we did (i.e., at least “minimally improved” on the CGI-I) but at week 2, in order to predict UR at week 4 (defined as ≥50% improvement of PANSS total score). Therefore, their threshold for later response was comparable to ours, but employed at an earlier time point. Hatta and colleagues (2011) found ER to risperidone in primarily AP-naïve patients to predict later nonresponse. Importantly, however, different from our study, the CGI ratings were not made independently of PANSS ratings. Therefore, raters' CGI sores were likely influenced by the much more time-consuming and detailed PANSS assessments. The finding of utility of CGI ratings is consistent with results by Leucht and Engel (2006) who found the CGI to be as sensitive as the BPRS in detecting efficacy differences among AP drugs; however, again, CGI and BPRS ratings were not made independent of each other. In this regard, our study adds to these results, suggesting that the clinically very feasible global CGI assessments, performed without co-rating of a detailed psychopathology scale, have acceptable and clinically useful predictive value to determine UR status early in the treatment course of a new AP trial.
Notably, we found that significantly more patients in the ENR group than in the ER group had EPS at week 4, 8, and 12, and that EPS at any time postbaseline point was associated with ENR, a finding that was independent of AP-naive status and chlorpromazine equivalent AP doses. Although in one study of chronically ill schizophrenia patients (Kinon et al. 2010), EPS was not related to ENR or UNR, EPS was related to UNR in an earlier study using first-generation APs (Kinon et al. 1993), and EPS was related to both ENR and UNR in a study of first-episode patients (Stauffer et al. 2011). In the latter study, both the ER and the ENR group had a significant worsening of Parkinsonian symptoms early on. In the last 6 weeks, EPS improved in responders, but not in nonresponders, and significant between-group differences were seen at weeks 10 and 12. These converging findings indicate that dopamine dysfunction, uncovered by EPS in response to AP treatment, is a marker of both ENR and UNR, which may be most detectable in early phase illness and AP-naïve patients. Similarly, in a previous first-episode study, baseline EPS was associated with poorer treatment response (Chatterjee et al. 1995), and tardive dyskinesia has also been associated with less AP efficacy and poorer treatment outcomes (Asher-Svanum et al. 2008b). By contrast, akathisia, which may be more related to the noradrenergic transmitter system (Wilbur et al. 1988), was not related to ENR or UNR in our or prior studies. Finally, neither we nor Stauffer and colleagues (2011) detected a significant relationship between body weight gain and either ER or UR status.
Limitations
The results from this study need to be interpreted within its limitations. These include the relatively low number of patients, large age range, mixture of schizophrenia and PsyNOS patients, lack of formal diagnostic interviews and inter-rater reliability testing of the CGI-I assessments, naturalistic treatment with co-medications being permitted, the absence of CGI-I ratings before week 4, and absence of PANSS or BPRS ratings to compare the performance of the CGI with more detailed PANSS/BPRS ratings. On the other hand, absent PANSS/BPRS ratings avoided the problem of criterion conflation, in that the singular performance of the pragmatic CGI scale without incorporating rating scale-based information could be tested in a naturalistic treatment setting.
Conclusions
Older age and EPS were associated with ENR, and ENR and a schizophrenia diagnosis were associated with UNR at 12 weeks in naturalistically treated youth with schizophrenia spectrum disorders. The utility of CGI-I based early treatment decisions needs to be considered and studied further, in both youth and adults.
Clinical Significance
To our knowledge, this is the first study that demonstrated the validity of the early response paradigm in a naturalistically treated pediatric sample of mostly AP-naïve youth and those with PsyNOS. The study employed CGI ratings as a stand-alone efficacy measure that is easily scalable into clinical care. All of these features increase the generalizability and clinical relevance of these findings. To further extend these data, the utility of CGI ratings to determine ER and UR needs to be tested in general clinical settings. Moreover, studies using different raters who assess patients on the CGI and the PANSS or BPRS are needed, in order to compare the diagnostic accuracy of these two approaches. EPS ratings should be included, and the predictive value of the full Simpson–Angus scale versus a few screening items should be investigated in future studies also, in order to increase the feasibility of using early EPS assessments as an additional marker for UNR in schizophrenia spectrum patients.
Disclosures
Drs. Stentebjerg-Olesen, Jeppesen, Pagsberg, Kapoor, Chekuri, and Al-Jadiri have no conflicts of interest to report. Dr. Fink-Jensen has received grant support from Novo Nordisk A/S. Dr. Kishimoto has received consultant fees from Dainippon Sumitomo, Otsuka, and Pfizer, and speaker's honoraria from Banyu, Dainippon Sumitomo, Eli Lilly, Janssen, Novartis, Otsuka, and Pfizer. He has received grant support from the Byoutaitaisyakenkyukai Fellowship (Fellowship of Astellas Foundation of Research on Metabolic Disorders) and an Eli Lilly Fellowship for Clinical Psychopharmacology. Dr. Kane has been a consultant and/or advisor to or has received honoraria from Alkermes, Amgen, Bristol-Myers Squibb, Eli Lilly, Forrest, Genentech, Gerson Lehrman, Intracellular Therapeutics, Janssen, Johnson and Johnson, Lundbeck, Merck, Novartis, Otsuka, Roche, and Sunovion. He is a shareholder of MedAvante. Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, Alexza, Bristol-Myers Squibb, Cephalon, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Lundbeck, Medavante, Medscape, Merck, Ortho-McNeill/Janssen/J&J, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Takeda, Teva, and Vanda. He has received grant support from Bristol-Myers Squibb, Janssen/J&J, and Otsuka. Dr. Carbon has a family relationship with Dr. Correll.
References
- Agid O, Kapur S, Arenovich T, Zipursky RB: Delayed-onset hypothesis of antipsychotic action: A hypothesis tested and rejected. Arch Gen Psychiatry 60:1228–1235, 2003 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Amminger GP, Henry LP, Harrigan SM, Harris MG, Alvarez–Jimenez M, Herrman H, Jackson HJ, McGorry PD: Outcome in early-onset schizophrenia revisited: Findings from the Early Psychosis Prevention and Intervention Centre long-term follow-up study. Schizophr Res 131:112–119, 2011 [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Carpenter WT, Kane JM, Lasser RA, Marder SR, Weinberger DR: Remission in schizophrenia: Proposed criteria and crationale for consensus. Am J Psychiatry 162:441–449, 2005 [DOI] [PubMed] [Google Scholar]
- Ascher–Svanum H, Nyhuis AW, Faries DE, Kinon BJ, Baker RW, Shekhar A: Clinical, functional, and economic ramifications of early nonresponse to antipsychotics in the naturalistic treatment of schizophrenia. Schizophr Bull 34:1163–1171, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher–Svanum H, Zhu B, Faries D, Peng X, Kinon BJ, Tohen M. Tardive dyskinesia and the 3-year course of schizophrenia: results from a large, prospective, naturalistic study. J Clin Psychiatry 69:1580–1588, 2008b [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676, 1989 [DOI] [PubMed] [Google Scholar]
- Berk M, Ng F, Dodd S, Callaly T, Campbell S, Bernardo M, Trauer T: The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract 14:979–983, 2008 [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 152:1724–1729, 1995 [DOI] [PubMed] [Google Scholar]
- Correll CU, Cañas F, Larmo I, Levy P, Montes JM, Fagiolini A, Papageorgiou G, Rossi A, Sturlason R, Zink M: Individualizing antipsychotic treatment selection in schizophrenia: characteristics of empirically derived patient subgroups. Eur Psychiatry 26:3–16, 2011a [DOI] [PubMed] [Google Scholar]
- Correll CU, Kishimoto T, Nielsen J, Kane JM: Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther 33:B16–39, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Malhotra AK, Kaushik S, McMeniman M, Kane JM: Early prediction of antipsychotic response in schizophrenia. Am J Psychiatry 59:441–448, 2003 [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Zhao Q, Carson WH, Marcus R, McQuade R, Forbes A, Mankoski R: Validity of early antipsychotic response to aripiprazole in adolescents with schizophrenia and its predictive value for clinical outcomes. J Am Acad Child Adolesc Psychiatry 52:689–698, 2013 [DOI] [PubMed] [Google Scholar]
- Derks EM, Fleischhacker WW, Boter H, Peuskens J, Kahn RS: Antipsychotic drug treatment in first-episode psychosis. Should patients be switched to a different antipsychotic drug after 2, 4 or 6 weeks of nonresponse? J Clin Psychopharmacol 30:176–180, 2010 [DOI] [PubMed] [Google Scholar]
- Emsley R, Rabinowitz J, Medori R: Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry 163:743–745, 2006 [DOI] [PubMed] [Google Scholar]
- Gallego JA, Robinson DG, Sevy SM, Napolitano B, McCormack J, Lesser ML, Kane JM: Time to treatment response in first-episode schizophrenia: Should acute treatment trials last several months? J Clin Psychiatry 72:1691–1696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W: Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology, revised, Rockville, MD: National Institute of Mental Health; 1976 [Google Scholar]
- Hatta K, Otachi T, Sudo Y, Hayakawa T, Ashizawa Y, Takebayashi H, Hayashi N, Hamakawa H, Ito S, Nakase R, Usui C, Nakamura H, Hirata T, Sawa Y: Difference in early prediction of antipsychotic non-response between risperidone and olanzapine in the treatment of acute-phase schizophrenia. Schizophr Bull 128:127–135, 2011 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB: A four-factor index of social status. Unpublished working paper, Department of Sociology, Yale University, New Haven, CT, 1975 [Google Scholar]
- Kane JM, Leucht S, Carpenter D, Docherty JP, Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders: The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J Clin Psychiatry 64:5–19, 2003 [PubMed] [Google Scholar]
- Kane JM, Correll CU: Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry 71:1115–1124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Arenovich T, Agid I, Zipursky R, Lindborg S, Jones B: Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry 162:939–946, 2005 [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA: The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276, 1987 [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Chen L, Ascher–Svanum H, Stauffer VL, Kollack–Wlaker S, Sniadecki JL, Kane J: Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr Res 102:230–240, 2008 [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Chen L, Ascher–Svanum H, Stauffer VL, Kollack–Walker S, Zhou W, Kapur S, Kane JM: Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology 35:581–590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinon BJ, Kane JM, Johns C, Perovich R, Ismi M, Koreen A, Weiden P: Treatment of neuroleptic-resistant schizophrenic relapse. Psychopharmacol Bull 29:309–314, 1993 [PubMed] [Google Scholar]
- Lambert M, Naber D, Eich FX, Schacht M, Linden M, Schimmelmann BG: Remission of severely impaired subjective wellbeing in 727 patients with schizophrenia treated with amisulpride. Acta Psychiatr Scand115:106–13, 2007 [DOI] [PubMed] [Google Scholar]
- Lambert M, Schimmelmann BG, Naber D, Eich FX, Schulz H, Huber CG, Karow A: Early- and delayed antipsychotic response and prediction of outcome in 528 severely impaired patients with schizophrenia treated with amisulpride. Pharmacopsychiatry 42:277–283, 2009 [DOI] [PubMed] [Google Scholar]
- Leucht S, Busch R, Hamann J, Kissling W, Kane JM: Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 57:1543–1549, 2005 [DOI] [PubMed] [Google Scholar]
- Leucht S, Busch R, Kissling W, Kane J: Early prediction of antipsychotic nonresponse among patients with schizophrenia. J Clin Psychiatry 68:352–360, 2007 [DOI] [PubMed] [Google Scholar]
- Leucht S, Engel RR: The relative sensitivity of the Clinical Global Impressions Scale and the Brief Psychiatric Rating Scale in antipsychotic drug trials. Neuropsychopharmacology 31:406–412, 2006 [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR: Linking the PANSS, BPRS, and CGI: Clinical implications. Neuropsychopharmacology 31:2318–2325, 2006 [DOI] [PubMed] [Google Scholar]
- Leucht S, Shamsi SA, Busch R, Kissling W, Kane JM: Predicting antipsychotic drug response – replication and extension to six weeks in an international olanzapine study. Schizophr Res 101:312–319, 2008 [DOI] [PubMed] [Google Scholar]
- Levine SZ, Rabinowitz J, Case M, Ascher–Svanum H: Treatment response trajectories and their antecedents in recent-onset psychosis: A 2-year prospective study. J Clin Psychopharmacol 30:446–449, 2010 [DOI] [PubMed] [Google Scholar]
- Liu–Seifert H, Adams DH, Kinon BJ: Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugs. BMC Med 23:3–21, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE: The Brief Psychiatric Rating Scale. Psychological Measurements in Psychopharmacology. Mod Probl Pharmacopsychiatry 7:67–78, 1974 [PubMed] [Google Scholar]
- Schennach–Wolff R, Seemuller FH, Mayr A, Maier W, Klingberg S, Heuser I, Klosterkötter J, Gastpar M, Häfner H, Sauer H, Schneider F, Gaebel W, Jäger M, Möller HJ, Riedel M: An early improvement threshold to predict response and remission in first-episode schizophrenia. Br J Psychiatry 196:460–466, 2010 [DOI] [PubMed] [Google Scholar]
- Schimmelmann BG, Conus P, Cotton S, McGorry PD, Lambert M: Pre-treatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophr Res 95:1–8, 2007 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S: A childrens global assessment scale (CGAS). Arch Gen Psychiatry 40:1228–1231, 1983 [DOI] [PubMed] [Google Scholar]
- Sheehan DV. The Anxiety Disease. New York: Scribner; 1983 [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 212:11–19, 1970 [DOI] [PubMed] [Google Scholar]
- Stauffer VL, Case M, Kinon BJ, Conley R, Ascher–Svanum H, Kollack–Walker S, Kane J, McEvoy J, Lieberman J: Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res 187:42–48, 2011 [DOI] [PubMed] [Google Scholar]
- Wilbur R, Kulik FA, Kulik AV: Noradrenergic effects in tardive dyskinesia, akathisia and pseudoparkinsonism via the limbic system and basal ganglia. Prog Neuropsychopharmacol Biol Psychiatr 12:849–864, 1988 [DOI] [PubMed] [Google Scholar]