Abstract
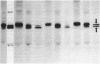
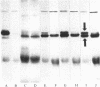
We are concerned with the mechanisms whereby hydrophilic proteins synthesized in the cytoplasm are translocated across one or two membranes into different cellular organelles. On the basis of a model of the translocation process to be described elsewhere, we propose an explanation of previous findings that the in vitro translocation across the endoplasmic reticulum of secretory proteins of higher eukaryotic cells appears to be obligatorily co-translational (i.e., occurs only while the polypeptide chain is being synthesized on the ribosome). We suggest that in vitro the intrachain disulfide bonds of the polypeptide rapidly form after it is released from the ribosome; the three-dimensional conformation of the chain is thereby stabilized and cannot undergo the unfolding that is required for post-translational translocation. In accord with this proposal, we show that the secretory preprotein human preprolactin, after translation and release from the ribosome, can indeed undergo translocation across endoplasmic reticulum membranes in vitro if the medium is sufficiently reducing. Those polypeptides that, in the absence of reducing agents, can be post-translationally translocated in vitro across bacterial, mitochondrial, and other types of membranes may generally lack intrachain disulfide bonds.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman M. J. Animal lipoproteins: chemistry, structure, and comparative aspects. J Lipid Res. 1980 Sep;21(7):789–853. [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Hunt J. S., Windham G. C. On the cysteine and cystine content of proteins. Differences between intracellular and extracellular proteins. J Mol Evol. 1977 Nov 25;10(2):155–160. doi: 10.1007/BF01751808. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Eichele G., Jansonius J. N. Three-dimensional structure of a pyridoxal-phosphate-dependent enzyme, mitochondrial aspartate aminotransferase. Proc Natl Acad Sci U S A. 1980 May;77(5):2559–2563. doi: 10.1073/pnas.77.5.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Garcia P. D., Walter P. In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell. 1986 May 9;45(3):397–406. doi: 10.1016/0092-8674(86)90325-9. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Durbin R. Is there a single pathway for the folding of a polypeptide chain? Proc Natl Acad Sci U S A. 1985 Jun;82(12):4028–4030. doi: 10.1073/pnas.82.12.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem. 1982;51:459–489. doi: 10.1146/annurev.bi.51.070182.002331. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Arpin M., Hannum C., Margoliash E., Sabatini D. D., Morimoto T. In vitro synthesis and posttranslational uptake of cytochrome c into isolated mitochondria: role of a specific addressing signal in the apocytochrome. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4368–4372. doi: 10.1073/pnas.78.7.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., McKean D. J. Synthesis of preprolactin and conversion to prolactin in intact cells and a cell-free system. J Biol Chem. 1978 Sep 25;253(18):6315–6318. [PubMed] [Google Scholar]
- Mueckler M., Lodish H. F. The human glucose transporter can insert posttranslationally into microsomes. Cell. 1986 Feb 28;44(4):629–637. doi: 10.1016/0092-8674(86)90272-2. [DOI] [PubMed] [Google Scholar]
- Olafson R. W., Jurásek L., Carpenter M. R., Smillie L. B. Amino acid sequence of Streptomyces griseus trypsin. Cyanogen bromide fragments and complete sequence. Biochemistry. 1975 Mar 25;14(6):1168–1177. doi: 10.1021/bi00677a011. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Nagabhushan N., Dzwiniel M., Smillie L. B., Whitaker D. R. Primary structure of alpha-lytic protease: a bacterial homologue of the pancreatic serine proteases. Nature. 1970 Oct 31;228(5270):438–442. doi: 10.1038/228438a0. [DOI] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Mahley R. W. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: reconstitution of the translocation and glycosylation of alpha-factor and invertase in a homologous cell-free system. Cell. 1986 Feb 28;44(4):619–628. doi: 10.1016/0092-8674(86)90271-0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M., Handwerger S., McLaurin W. D., Lanner M. Amino-acid sequence of human placental lactogen. Nat New Biol. 1971 Sep 8;233(36):59–61. doi: 10.1038/newbio233059a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Waters M. G., Blobel G. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol. 1986 May;102(5):1543–1550. doi: 10.1083/jcb.102.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]