Abstract
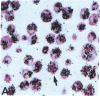
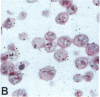
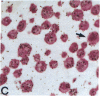
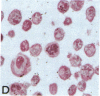
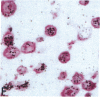
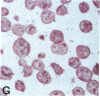
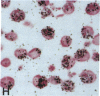
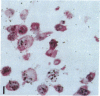
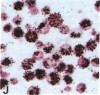
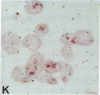
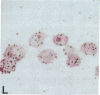
Human B lymphocytes latently infected with Epstein-Barr virus (EBV) synthesize two low molecular weight RNAs designated EBER 1 and 2. Using an in situ hybridization technique we have localized EBER 1 and 2 within the nucleus of single EBV-harboring B lymphocytes from established and recently transformed cell lines. As controls, the locations of the small nuclear RNA, U1, and the small cytoplasmic RNA, 7SL, were examined in HeLa and EBV-harboring cells. Because of possible functional similarities between EBERs and the adenovirus-associated (VA) RNAs, VAI was also localized; it appeared to be in the nucleus and cytoplasm, implying that VAI may have a different role than that of the nuclear-localized EBERs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Rymo L. Characterization of the major Epstein-Barr virus-specific RNA in Burkitt lymphoma-derived cells. J Virol. 1982 Feb;41(2):376–389. doi: 10.1128/jvi.41.2.376-389.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. A., Thimmappaya B. Construction and analysis of additional adenovirus substitution mutants confirm the complementation of VAI RNA function by two small RNAs encoded by Epstein-Barr virus. J Virol. 1985 Dec;56(3):750–756. doi: 10.1128/jvi.56.3.750-756.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. A., Thimmappaya B. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4789–4793. doi: 10.1073/pnas.80.15.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T., Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Borjeson J., Chessin L. N. Studies on human lymphocytes in vitro. IV. Comparative fine structural features of the established Burkitt lymphoma cell lines AL-1, EB-2 and phytomitogen-transformed lymphocytes. J Immunol. 1967 Aug;99(2):340–346. [PubMed] [Google Scholar]
- Godard C., Jones K. W. Detection of AKR MuLV-specific RNA in AKR mouse cells by in situ hybridization. Nucleic Acids Res. 1979 Jun 25;6(8):2849–2861. doi: 10.1093/nar/6.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Gurney T., Jr, Eliceiri G. L. Intracellular distribution of low molecular weight RNA species in HeLa cells. J Cell Biol. 1980 Nov;87(2 Pt 1):398–403. doi: 10.1083/jcb.87.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamrich M., Mahon K. A., Gavis E. R., Gall J. G. Histone RNA in amphibian oocytes visualized by in situ hybridization to methacrylate-embedded tissue sections. EMBO J. 1984 Sep;3(9):1939–1943. doi: 10.1002/j.1460-2075.1984.tb02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P., Arrand J. R. In vitro transcription of two Epstein-Barr virus specified small RNA molecules. Nucleic Acids Res. 1982 Jun 11;10(11):3407–3425. doi: 10.1093/nar/10.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R. Specific nucleotide sequences in HeLa cell inverted repeated DNA: enrichment for sequences found in double-stranded regions of heterogeneous nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):591–601. doi: 10.1016/0022-2836(77)90104-8. [DOI] [PubMed] [Google Scholar]
- Kallin B., Dillner J., Ernberg I., Ehlin-Henriksson B., Rosén A., Henle W., Henle G., Klein G. Four virally determined nuclear antigens are expressed in Epstein-Barr virus-transformed cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1499–1503. doi: 10.1073/pnas.83.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., De Robertis E. M., Cortese R. Order and intracellular location of the events involved in the maturation of a spliced tRNA. Nature. 1980 Mar 13;284(5752):143–148. doi: 10.1038/284143a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnigan H., Moyer R. W. Intracellular location of rabbit poxvirus nucleic acid within infected cells as determined by in situ hybridization. J Virol. 1985 Sep;55(3):634–643. doi: 10.1128/jvi.55.3.634-643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley R. P., Mariano T. M., Siekierka J., Mathews M. B. A mechanism for the control of protein synthesis by adenovirus VA RNAI. Cell. 1986 Feb 14;44(3):391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Jelinek W. Determination of nucleotide sequences from double-stranded regions of HeLa cell nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):571–589. doi: 10.1016/0022-2836(77)90103-6. [DOI] [PubMed] [Google Scholar]
- Rosa M. D., Gottlieb E., Lerner M. R., Steitz J. A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf W. E. Techniques for the autoradiography of diffusible compounds. Methods Cell Biol. 1976;13:171–193. doi: 10.1016/s0091-679x(08)61802-6. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Ullu E., Weiner A. M. Upstream sequences modulate the internal promoter of the human 7SL RNA gene. 1985 Nov 28-Dec 4Nature. 318(6044):371–374. doi: 10.1038/318371a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Weigel R., Fischer D. K., Heston L., Miller G. Constitutive expression of Epstein-Barr virus-encoded RNAs and nuclear antigen during latency and after induction of Epstein-Barr virus replication. J Virol. 1985 Jan;53(1):254–259. doi: 10.1128/jvi.53.1.254-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]