Abstract
It is widely accepted that chronic inflammation plays an active role in cancer. Inflammatory immunocytes and related cytokines in the tumor microenvironment are supposed to be a “double-edged sword” in colorectal cancer (CRC) initiation and progression. Interleukin-17 (IL-17), a pleiotropic proinflammatory cytokine, can promote cancer-elicited inflammation and prevent cancer cells from immune surveillance. Despite controversy, IL-17 is generally considered to be a promoter in CRC progression. In this review, we devote to summarize the current progress regarding the role of IL-17 in tumor initiation and progression, as well as the prognostic value in CRC.
1. Introduction
Human colorectal cancer (CRC) is an important contributor to cancer mortality and morbidity in developed countries [1]. It is generally accepted that most solid tumors including CRC are linked to chronic inflammation [2]. Due to various inflammatory cells and cytokines in tumor microenvironment, these tumors have been referred to as ‘‘wounds that do not heal” [3]. Accumulating evidence has shown that the functional disturbance of the immune system is directly associated with tumor stages and clinical prognosis of patients [4]. However, the interaction between immune cells, inflammatory cytokines, and cancer evolution is still largely unknown. Plentiful studies have demonstrated that innate and adaptive immunity play a critical role in the initiation and progression of CRC [5]. Recently, several inflammatory cytokines have been shown to promote CRC progression [6].
IL-17 (IL-17A), initially termed as cytotoxic T-lymphocyte-associated antigen (CTLA)-8, is the founding member of IL-17 cytokine family consisting of six homologous proteins (from IL-17A to IL-17F) [7, 8]. A large body of evidence suggests that IL-17 is an essential proinflammatory cytokine due to inducing a mass of cytokines and chemokines secretion by distinct cell types, such as mesenchymal cells and myeloid cells, which recruit monocytes and neutrophils into the site of inflammation [9]. Moreover, IL-17 promotes the expression of antimicrobial peptides from epithelial cells and facilitates host defense against infections [10, 11]. This evidence indicates that IL-17 is an important inflammatory cytokine which links innate and adaptive immunity. Besides, IL-17 has been demonstrated to play an active role in allergy, autoimmune diseases, allograft transplantation, and cancer [12–15]. Recently, several studies have shown that IL-17 has either a protumor or antitumor role in different cancer models [16]. But in CRC, the majority of studies consider that IL-17 acts as a promoter in tumor initiation and progression. Particularly, the ablation of IL-17A can inhibit the progression of spontaneous intestinal tumorigenesis in ApcMin/+ mice [17]. In this review, we devote to summarize the progress of the current study about the potential role of IL-17 in CRC initiation and progression, as well as its predictive role in clinical prognosis.
2. The Regulation of IL-17 Secretion
IL-17 is an inflammatory cytokine produced by a wide variety of leukocytes, as illustrated in Figure 1, including T cells, natural killer cells (NK cells), lymphoid tissue inducer-like cells (LTi-like cells), and neutrophils [18]. Among these cells, IL-17 is reported to be predominantly produced by activated CD4+ T cells (Th17 cells). It is generally accepted that Th17 cells are induced from naive CD4+ T cells by IL-6, IL-1β, TGF-β, and IL-23, which upregulate the expression of retinoic acid receptor-related orphan receptor-γt (RORγt) via activation of signal transducer and activator of transcription-3 (Stat3) and interferon regulatory factor 4 (IRF4) [19, 20]. Other transcriptional factors such as RORa, basic leucine zipper transcription factor (Batf), Runx1, and aryl hydrocarbon receptor (AHR) can also induce Th17 cell polarization when coordinated with RORγt [21]. Moreover, the regulation mechanism of IL-17 production in Th17 cells is affected by other inflammatory immunocytes and related cytokines. For instance, human inflammatory dendritic cells (infDCs), derived from monocytes, can stimulate autologous memory CD4+ T cells to produce IL-17 [22]. Recently, IL-27 is reported to inhibit IL-17 production in early stage of Th17 cells differentiation through PD-1-PD-L1 interaction [23]. However, the cells or related cytokines negatively regulating IL-17 production are largely unknown.
Figure 1.
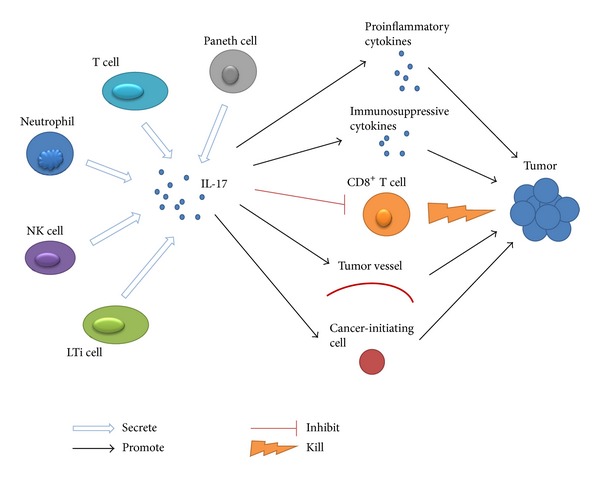
This is an illustration about the protumor activity of IL-17 in CRC microenvironment. Blue colored arrow shows cells producing IL-17. Black arrow shows the latter stimulated by the former. T-shaped arrow shows process inhibited by IL-17 and lightning arrow shows attack on tumor cells.
In addition to Th17 cells, IL-17 secretion can also be induced by IL-6, IL-1β, TGF-β, and IL-23 in other T cells such as CD8+ T cells (Tc17 cells), regulatory T cells (Treg17 cells), gamma delta T cells (γδT17 cells), and invariant natural killer T cells (iNKT cells) [24–26]. Moreover, it has been reported that intestinal Paneth cells (Figure 1) are capable of producing IL-17 [27]. Based on these results, we conclude that IL-17 secretion is regulated by the cooperation of the inflammatory cells, cytokines, and antigens which coexist in the specific inflammatory microenvironment.
3. The Role and Mechanism of IL-17 in Cancer Promotion
IL-17 expression is elevated in several human tumors, such as ovarian cancer, cervical cancer, breast cancer, hepatocellular carcinoma, esophageal cancer, gastric cancer, and CRC [28–34]. But the underlying mechanism of IL-17 in tumor initiation and progression is not completely clear yet. Some researchers propose that IL-17 promotes tumor initiation and progression through suppressing antitumor immune response. For instance, CD8+ T cells are polarized towards an IL-17 secreting (Tc17) fate in the presence of both TGF-β and IL-6, resulting in losing their cytotoxic ability and promoting tumorigenesis [35]. In gastric cancer, activated monocytes promote the development of Tc17 cells via IL-6, IL-1β, and IL-23, resulting in the production of the chemokine CXCL12 by tumor cells, which promotes MDSCs-mediated immunosuppression [33]. Other investigators demonstrate that IL-17 can enhance tumor progression through angiogenesis. IL-17 induces fibroblasts and tumor cells to produce a variety of angiogenic factors, including PGE1, PGE2, VEGF, keratinocyte-derived chemokine (KC), and macrophage inflammatory protein-2 (MIP-2), which promotes angiogenesis in the tumor [36]. In breast cancer, the angiogenic factors CXCL8, MMP-2, MMP-9, and VEGF are induced by IL-17 and associated with poor prognosis [30]. Analogously, IL-17 has been demonstrated to selectively promote the secretion of an array of angiogenic chemokines from NSCLC, such as CXCL1, CXCL5, CXCL6, and CXCL8 [37]. The molecule mechanism involved in the protumor activity of IL-17 is considered to be mediated by inflammation-associated signaling pathways. Transfecting IL-17 into hepatocellular carcinoma cells significantly promotes neoangiogenesis, neutrophils recruitment, and tumor growth via AKT-dependent IL-6/JAK2/STAT3 signaling pathway in vivo [38]. IL-17 induces IL-6 production, which in turn activates Stat3 and promotes cancer cells survival [39]. Further studies on the molecule mechanism of IL-17 inducing tumor promotion are required in the future.
Besides, some studies suggest that IL-17 can inhibit tumor growth. In the tumor initiating model, IL-17 deficient mice are more susceptible to developing lung melanoma, and adoptive T-cell therapy with tumor-specific Th17 cells prevents tumor development by eliciting a remarkable activation of tumor-specific CD8+ T cells [40]. Study in hematopoietic cancer shows that IL-17 inhibits the tumor growth in a CTL-dependent manner [41]. Murine Meth-A fibrosarcoma cells transfected with the hIL-17 gene can promote CD4+ and CD8+ T-cells-mediated antitumor activity [42]. Interestingly, hIL-17-gene-transfected Chinese hamster ovary (CHO) cells show a significant decrease in metastatic potential to the lung by directly reducing the invasiveness of CHO cells and enhancing NK activity [43]. This evidence indicates that IL-17 may have partial antitumor effect through promoting immune response in the tumor initiation stage.
4. IL-17 as a Promoter in CRC Progression
In accordance with studies in other cancers, growing evidence has shown that IL-17 can also promote tumor progression in CRC. In intestinal tumor bearing model, the tumor size is significantly reduced in IL-17 gene-knockout mice compared with wide-type (WT) mice, and anti-IL-17A monoclonal antibody treatment results in decreased tumor size in the WT mice [44]. In vitro, IL-17 and TNF-α synergistically promote carcinogenesis by stimulating glycolysis and growth factor production by CRC cells [45]. Study focused on the intrinsic role of endogenous IL-17 in CRC has demonstrated that CD4+ T-cell-derived IL-17 promotes spontaneous intestinal tumorigenesis in ApcMin/+ mice, suggesting that IL-17 plays an important role in CRC initiation [17]. In colitis-associated cancer model, tumorigenesis and inflammatory cytokines including IL-6, IFN-γ, and TNF-α are markedly decreased in IL-17-deficient mice compared with WT mice, suggesting that IL-7 plays an pivotal role in promoting CRC initiation in colitis-associated cancer [46]. Enterotoxigenic bacteroides fragilis (ETBF), a human colonic commensal bacterium, can promote colonic tumorigenesis in APC mutant mice via Stat3 activation and Th17-cell polarization, and further blocking IL-17 or IL-23R can significantly reduce tumor formation in vivo [47]. It has been reported that epithelial barrier deterioration results in microbial pathogen invasion and microbial products release are driving IL-23/IL-17 axis activation and promoting tumor growth and progression in CPC-APC mice [48]. We have found that tumor-infiltrating γδT17 cells induced by tumor-elicited inflammation can promote tumor progression via secretion of IL-17, IL-8, TNF-α, and GM-CSF to form an immunosuppressive microenvironment in human CRC (unpublished data). Similarly, another study has presented that a subset of Foxp3+ IL-17+ T cells in CRC tissue suppress the tumor-specific CD8+ T cells and attenuate the antitumor immune response [49]. Moreover, IL-17/G-CSF/Bv8 axis has been reported to promote VEGF-independent CRC tumor angiogenesis in vivo [50]. Recently, a study has suggested that CRC tissue-derived Foxp3+ IL-17+ cells have the capacity to induce cancer-initiating cells in vitro [51]. These studies regarding CRC promoting activities of IL-17 are highlighted in Table 1. Based on these findings, we propose that the protumor activity of IL-17 in CRC microenvironment may exert in several aspects: (1) promoting tumor-elicited inflammation which facilitates the proliferation and survival of malignant cells, (2) forming an immunosuppressive tumor microenvironment by chemoattracting immunosuppressive cells and cytokines, (3) suppressing cytotoxic cells-mediated immunosurveillance against tumor, (4) fostering tumor angiogenesis to promote tumor growth and metastasis, and (5) inducing cancer-initiating cells, which facilitates tumor malignant progression and escaping from host immune surveillance as shown in Figure 1.
Table 1.
Role of IL-17 in CRC.
| Species | Mediators | Findings | References |
|---|---|---|---|
| Tumor promoting role | |||
| Human | VEGF | IL-17 induces both CRC cell lines and primary cancer cells to produce VEGF. | [52] |
|
| |||
| Human | HIF-1α and c-myc | IL-17 and TNFα cooperatively stimulate glycolysis in CRC cells via induction of HIF-1α and c-myc expression. | [45] |
|
| |||
| Mouse | Stat 3 | IL-23/IL-17 signaling activated by microbial products promotes STAT3 phosphorylation in CRC epithelial cells. | [48] |
|
| |||
| Mouse | VEGF, KC, and PGE2 | IL-17 promotes angiogenesis via induction of a variety of proangiogenic factors secretion from fibroblasts and tumors. | [36] |
|
| |||
| Mouse | IL-6, IL-23, and IL-1β; KC and Cox-2; CD4 T cells |
IL-6, IL-23, IL-1β, KC, and Cox-2 are decreased and function of CD4 T cells alters in ApcMin/+ mice, resulting in abrogating spontaneous intestinal tumorigenesis. | [17] |
|
| |||
| Mouse | IL-6, STAT3, and TNF-α; cyclin D1, cyclin-dependent kinase 2, and cyclin E |
IL-17A knockout decreases IL-6, STAT3, TNF-α, cyclin-D1, cyclin-dependent kinase 2, and cyclin E and inhibits CAC tumorigenesis. | [46] |
|
| |||
| Mouse | G-CSF, VEGF, and Bv8 NF-κB and ERK signaling |
IL-17 induces the expression of G-CSF through NF-κB and ERK signaling, enhancing proangiogenic function via VEGF and Bv98 and promoting tumor growth. | [50] |
|
| |||
| Tumor inhibiting activity | |||
| Mouse | IFN-γ
NK cells and T cells |
IL-17-deficient decreases IFN-γ + NK and tumor-specific IFN-γ + T cells and promotes tumor growth and lung metastasis. | [53] |
|
| |||
| Human | Claudin ERK MAPK pathway |
IL-17 enhances the development of the tight junctional barrier mediated by claudin of T84-cell monolayers via ERK MAPK pathway in intestine. | [54] |
Although major evidence considers IL-17 as a promoter in CRC progression, there is still controversy. For instance, Kryczek and colleagues have demonstrated that tumor growth is enhanced in subcutaneous transplanted model and lung metastases model in IL-17−/− mice [53]. However, Ngiow et al. fail to reproduce the same results and they conclude that tumor growth has no difference between IL-17-deficient mice and control WT mice after 3 independent sources of MC38 cells inoculated subcutaneously [55]. Analogously, it has been reported that IL-17 promotes the expression of the tight junction protein-claudin in T84 cells via ERK MAPK activation in intestine [54]. Whereas, another study has shown that adenoma-linked barrier deterioration leads to microbial products invasion and triggers IL-23/IL-17-mediated tumor growth in CPC-APC mouse model [48]. In summary, despite the existing controversy presumably derived from the different models, most investigators appreciate IL-17 as a promoter in CRC progression. Table 1 summarizes studies with IL-17 which describe its possible antitumor role in CRC.
5. IL-17 as a Clinical Prognostic Indicator for Human CRC
Tumor progression is affected by the complicated interaction of tumor cells, stromal cells, immune cells, and related cytokines in tumor microenvironment. IL-17 produced by epithelial cells and immune cells plays an important role in CRC development. Increased IL-17 concentration is detected in serum of CRC patients compared with healthy donors, which is inversely correlated with p53 expression. Moreover, it is proposed that IL-17 may act as a valuable tumor marker in patients with CRC and that concomitant expression of p53 and VEGF may provide further information about tumor features [56]. Furthermore, elevated Th17 cells have been observed in more than 80% of human sporadic colon cancer tissues, indicating that IL-17 expression may be one of potential biomarkers for the future development of a new prognostic ‘‘test set” for sporadic CRC [34]. Analogously, IL-17 producing cells induced by microbial dysbiosis are increased in intestinal mucosa of CRC patients, indicating that IL-17 producing cells may be a promising sensitive prognostic indicator for CRC [57]. Confocal microscopic analysis of CRC tissues shows that IL-17 expression is associated with microvessel density. Univariate and multivariate analysis reveal that 5-year survival rate is 72.41% in the 26 cases with lower IL-17 expression and 38.08% in the 26 cases with higher IL-17 expression, proposing that IL-17 is an independent prognostic factor for overall survival and IL-17 producing cells may facilitate development of CRC by fostering angiogenesis via stimulation of VEGF production by cancer cells [52]. Interestingly, an early increase of IL-17 expression in the premalignant stage and its dynamic change in tumor microenvironment throughout the adenoma-carcinoma sequence is associated with the progression of adenomas toward CRC [58]. These clinical studies indicate that IL-17 plays a critical role in the human CRC progression, which deserves to be studied further.
Elevated IL-17 expression level in serum and tissue of CRC patients suggests that it may contribute to predicting cancer prognosis accompanied with an other existing panel of molecular prognosticator. However, more multicentric validated studies are required before considering IL-17 as an authentic prognosticator that can apply to clinical practices. Studies on IL-17 expression level and its significance associated with CRC are summarized in Table 2.
Table 2.
Expression of IL-17 in CRC patients.
| Sample type | Sample size | Findings | References |
|---|---|---|---|
| Serum and tissue | 74 CRC tissues and paired normal mucosa, 61 CRC serum samples, and 78 healthy controls | No significant difference is observed. | [59] |
|
| |||
| Serum and tissue | 59 CRC tissues, 40 CRC serum samples, and 37 healthy controls | IL-17 acts as a valuable tumor marker in CRC patients. | [56] |
|
| |||
| Tissue | 12 CRC patients | Hypoxia induce the expression of IL-17 in Foxp3+ Tregs, which drive cancer cells to be cancer-initiating cells. | [51] |
|
| |||
| Tissue | 125 CRC tissues and 3 normal tissues | IL-17 gene expression level is higher in tumor tissues compared to normal mucosa. | [60] |
|
| |||
| Tissue | 52 CRC patients | IL-17 expression is negatively correlated with OS of CRC patients. | [52] |
|
| |||
| Tissue | 22 CRC patients | Tumor-infiltrating Foxp3+ IL-17+ T cells suppress tumor-specificCD8+T cells response via IL-17. | [49] |
|
| |||
| Serum and tissue | 9 ulcerative colitis-associated CRC tissues | IL-17+ Foxp3+ CD4+ T cells are selectively accumulated in the colitis-associated CRC niche. | [61] |
|
| |||
| Tissue | 50 colorectal adenomas tissues, 50 CRC tissues, and 15 healthy controls | IL-17 level is associated with the severity of dysplastic degree. | [58] |
|
| |||
| Tissue | 23 CRC patients | Tumor-infiltrating TH17 cells and Bv8-expressing neutrophils are associated with poor outcome in CRC. | [50] |
6. Concluding Remarks
In conclusion, accumulative evidence reveals that IL-17 can promote tumor initiation and progression in most cancers. Due to the complexity of tumor microenvironment contents, the underlying mechanism of IL-17 in CRC initiation and progression is still uncovered. Recently, our group has demonstrated that IL-17 promotes polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) mediated immunosuppression in human CRC progression (unpublished data). Thus, some attempts for cancer immunotherapy targeting IL-17, such as neutralizing antibody, and approaches of decreasing IL-17 production or blocking the downstream signaling pathways would come true with the deepening of IL-17-related research in the future. On the other hand, because IL-17 may serve as a promising biomarker with certain prognostic significance and potential clinical application prospect, further studies that focus on IL-17 regulation mechanism and the interaction between IL-17, cancer cells, mesenchymal cells, and immune cells in tumor microenvironment are expected to shed more light both on the exact biological function of IL-17 in CRC development and the clinical applications of IL-17-targeted treatment.
Conflict of Interests
None of the authors have any conflict of interests.
Authors' Contribution
Dang Wu and Pin Wu contributed equally to this work.
Acknowledgments
The authors thank the members of the laboratory for helpful discussions. This work was partly supported by grants from the Ministry of Health of Zhejiang Province (Grant no. 2012ZDA021), Natural Science Foundation of Zhejiang Province (Grants nos. Y2110034, Y2100414, Z2100366, and Y2090386), and Science and Technology Department of Zhejiang Province (Grant no. 2011c13034~1). This work was also funded by the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents (Jian Huang), and the Zhejiang Province Key Discipline of Traditional Chinese Medicine (Grant no. 2012-XK-A27) and Medicine (Grant no. 2011-CX11).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: Cancer Journal for Clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. Journal of Clinical Oncology. 2010;28(26):4045–4051. doi: 10.1200/JCO.2010.27.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klampfer L. Cytokines, inflammation and colon cancer. Current Cancer Drug Targets. 2011;11(4):451–464. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101.e5–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus Saimiri gene. Journal of Immunology. 1993;150(12):5445–5456. [PubMed] [Google Scholar]
- 8.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Kao CY, Chen Y, Thai P, et al. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. Journal of Immunology. 2004;173(5):3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiology and Immunology. 2007;51(12):1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 12.Mays LE, Ammon-Treiber S, Mothes B, et al. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism. Journal of Clinical Investigation. 2013;123(3):1216–1228. doi: 10.1172/JCI65351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Annals of the Rheumatic Diseases. 2011;70(5):727–732. doi: 10.1136/ard.2010.143768. [DOI] [PubMed] [Google Scholar]
- 14.Shilling RA, Wilkes DS. Role of Th17 cells and IL-17 in lung transplant rejection. Seminars in Immunopathology. 2011;33(2):129–134. doi: 10.1007/s00281-011-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Liu S, Ge D, et al. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Research. 2012;72(10):2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. Journal of Immunology. 2009;183(7):4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 17.Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell ALM. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nature Reviews Immunology. 2010;10(7):479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 19.Huber M, Brüstle A, Reinhard K, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(52):20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of Biological Chemistry. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 21.Hirahara K, Ghoreschi K, Laurence A, Yang X, Kanno Y, O’Shea JJ. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine and Growth Factor Reviews. 2010;21(6):425–434. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segura E, Touzot M, Bohineust A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38(2):336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Hirahara K, Ghoreschi K, Yang XP, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36(6):1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raifer H, Mahiny AJ, Bollig N, et al. Unlike αβ T cells, γδ T cells, LTi cells and NKT cells do not require IRF4 for the production of IL-17A and IL-22. European Journal of Immunology. 2012;42(12):3189–3201. doi: 10.1002/eji.201142155. [DOI] [PubMed] [Google Scholar]
- 25.Afzali B, Mitchell PJ, Edozie FC, et al. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. European Journal of Immunology. 2013;43(8):2043–2054. doi: 10.1002/eji.201243296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber M, Heink S, Pagenstecher A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. Journal of Clinical Investigation. 2013;123(1):247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi N, Vanlaere I, de Rycke R, et al. IL-17 produced by Paneth cells drives TNF-induced shock. Journal of Experimental Medicine. 2008;205(8):1755–1761. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang R. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(40):15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tartour E, Fossiez F, Joyeux I, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Research. 1999;59(15):3698–3704. [PubMed] [Google Scholar]
- 30.Benevides L, Cardoso CR, Tiezzi DG, Marana HR, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. European Journal of Immunology. 2013;43(6):1518–1528. doi: 10.1002/eji.201242951. [DOI] [PubMed] [Google Scholar]
- 31.Kuang DM, Peng C, Zhao Q, Wu Y, Chen M, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51(1):154–164. doi: 10.1002/hep.23291. [DOI] [PubMed] [Google Scholar]
- 32.Lv L, Pan K, Li XD, et al. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0018219.e18219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang Y, Peng LS, Zhao YL, et al. CD8+ T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143(4):951.e8–962.e8. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Le Gouvello S, Bastuji-Garin S, Aloulou N, et al. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57(6):772–779. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 35.Nam JS, Terabe M, Kang MJ, et al. Transforming growth factor β subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Research. 2008;68(10):3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 37.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. Journal of Immunology. 2005;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 38.Gu FM, Li QL, Gao Q, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Molecular Cancer. 2011;10, article 150 doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. Journal of Experimental Medicine. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benchetrit F, Ciree A, Vives V, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99(6):2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 42.Hirahara N, Nio Y, Sasaki S, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61(1):79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 43.Hirahara N, Nio Y, Sasaki S, et al. Reduced invasiveness and metastasis of Chinese hamster ovary cells transfected with human interleukin-17 gene. Anticancer Research. 2000;20(5):3137–3142. [PubMed] [Google Scholar]
- 44.Oshiro K, Kohama H, Umemura M, et al. Interleukin-17A is involved in enhancement of tumor progression in murine intestine. Immunobiology. 2012;217(1):54–60. doi: 10.1016/j.imbio.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Straus DS. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Molecular Cancer. 2013;12(article 78) doi: 10.1186/1476-4598-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyun YS, Han DS, Lee AR, Eun CS, Youn J, Kim H. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis. 2012;33(4):931–936. doi: 10.1093/carcin/bgs106. [DOI] [PubMed] [Google Scholar]
- 47.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature Medicine. 2009;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma C, Dong X. Colorectal cancer-derived Foxp3+IL-17+ T cells suppress tumour-specific CD8+ T cells. Scandinavian Journal of Immunology. 2011;74(1):47–51. doi: 10.1111/j.1365-3083.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 50.Chung AS, Wu X, Zhuang G, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nature Medicine. 2013;19(9):1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 51.Yang S, Wang B, Guan C, et al. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. Journal of Leukocyte Biology. 2010;89(1):85–91. doi: 10.1189/jlb.0910506. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Duan Y, Cheng X, et al. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochemical and Biophysical Research Communications. 2011;407(2):348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 53.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114(2):357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinugasa T, Sakaguchi T, Gu X, Reinecker H. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118(6):1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 55.Ngiow SF, Smyth MJ, Teng MWL. Does IL-17 suppress tumor growth? Blood. 2010;115(12):2554–2555. doi: 10.1182/blood-2009-11-254607. [DOI] [PubMed] [Google Scholar]
- 56.Radosavljevic G, Ljujic B, Jovanovic I, et al. Interleukin-17 may be a valuable serum tumor marker in patients with colorectal carcinoma. Neoplasma. 2010;57(2):135–144. doi: 10.4149/neo_2010_02_135. [DOI] [PubMed] [Google Scholar]
- 57.Sobhani I, Tap J, Roudot-Thoraval F, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0016393.e16393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui G, Yuan A, Goll R, Florholmen J. IL-17A in the tumor microenvironment of the human colorectal adenoma-carcinoma sequence. Scandinavian Journal of Gastroenterology. 2012;47(11):1304–1312. doi: 10.3109/00365521.2012.725089. [DOI] [PubMed] [Google Scholar]
- 59.Wågsäter D, Löfgren S, Hugander A, Dimberg J. Expression of interleukin-17 in human colorectal cancer. Anticancer Research. 2006;26(6):4213–4216. [PubMed] [Google Scholar]
- 60.Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Research. 2011;71(4):1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 61.Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. Journal of Immunology. 2011;186(7):4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]


