Abstract
Advances in cancer treatment have greatly improved survival rates of children with cancer. However, these same chemotherapeutic or radiologic treatments may result in long-term health consequences. Anthracyclines, chemotherapeutic drugs commonly used to treat children with cancer, are known to be cardiotoxic, but the mechanism by which they induce cardiac damage is still not fully understood. A higher cumulative anthracycline dose and a younger age of diagnosis are only a few of the many risk factors that identify the children at increased risk of developing cardiotoxicity. While cardiotoxicity can develop at anytime, starting from treatment initiation and well into adulthood, identifying the best cardioprotective measures to minimize the long-term damage caused by anthracyclines in children is imperative. Dexrazoxane is the only known agent to date, that is associated with less cardiac dysfunction, without reducing the oncologic efficacy of the anthracycline doxorubicin in children. Given the serious long-term health consequences of cancer treatments on survivors of childhood cancers, it is essential to investigate new approaches to improving the safety of cancer treatments.
Keywords: anthracycline, cardiotoxicity, childhood cancer survivors, late effects, monitoring, prevention, risk factors
Background
Children who are diagnosed with cancer are living longer lives due to advancements in detection and treatment. The impact is such that children diagnosed with a malignancy before 15 years of age have a 5-year survival rate of approximately 80%, a substantial improvement from that of less than 50% in the 1970s [1]. As of 2005, there were more than 325,000 survivors of childhood cancers in the USA. Remarkably, approximately 24% of these survivors were treated at least 30 years ago. The relatively high survival rate of these individuals, however, is tempered by the high incidence of treatment-related health complications throughout their lifespan. Such complications include clinical or subclinical cardiovascular damage, lipid abnormalities and an increased incidence of obesity [2], risks that may persist up to 45 years after treatment [3]. More than 7% of childhood cancer survivors will develop heart failure 30 years after diagnosis [4,5]. The lifetime incidence of heart disease in survivors is remarkably higher than their non-affected siblings [6]. In addition to their height-ened risk of cardiomyopathies and cardiovascular disease, children treated with anthracyclines, a chemotherapeutic class of drugs commonly used to treat hematologic cancers and malignant neoplasms, are at increased risk for a range of other complications, including secondary malignancies, infertility, and developmental and functional musculoskeletal impairments that can produce chronic pain [7–11].
More than half of the children with cancer receive an anthracycline as part of their treatment plan [12,13]. Selection of an appropriate anthracycline for a patient's treatment plan largely depends on the specific characteristics of their cancer. Despite the hope that anthracyclines offer to these patients, their potential for inducing cardiovascular damage has been recognized since their introduction in the early 1970s [14,15]. This review summarizes the mechanisms by which anthracyclines may induce cardiac damage, methods for clinically assessing anthracycline-induced cardiotoxicity and the effectiveness of proposed cardioprotective strategies.
Mechanisms of anthracycline-induced cardiotoxicity
Despite more than three decades of use, it is still unclear exactly how anthracyclines exert their chemotherapeutic activity and induce cardiotoxic changes. The antineoplastic activities of doxorubicin seem, at least in part, to be derived from the inhibition of topoisomerase II, free-radical generation, activation of signaling pathways, DNA intercalation and binding and apoptosis [16,17]. Similarly, causes of anthracycline-induced cardiotoxicity appear to be multifactorial. A critical component may be the generation of free radicals and the presence of redox-related damage, which occur through both enzymatic and nonenzymatic pathways and result in the accumulation of iron [18–20]. Anthracycline-generated free radicals induce lipid peroxidation, which ultimately produces membrane damage [16,21]. In addition, mitochondrial damage [22], increased Ca2+ current along with inhibition of sarcoplasmic reticulum function [23,24] and decreased activity of Na, K-ATPase [25], have all been implicated in doxorubicin-induced cardiotoxicity. The down-regulation of myocardial TNF-α [26] and doxorubicin binding to bivalent cations (Ca2+, Mg2+, Cu2+ and Zn2+) may also be contributive [27]. Figure 1 illustrates the possible complex mechanism of anthracycline-induced cardiotoxicity as described above [28].
Figure 1. Mechanism of anthracycline toxicity within the cardiomyocyte.
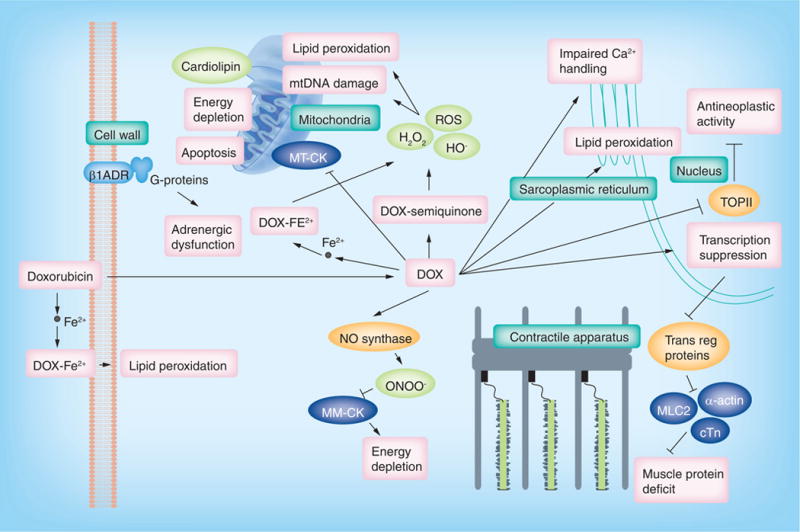
Anthracyclines enter cardiomyocytes by passive diffusion and spur the generation of free radicals, leading to cell damage. Anthracyclines also directly and indirectly inhibit gene transcription, mitochondrial functioning, and energy production within the cell.
β1ADR: β1 adrenergic receptor; Ca: Calcium; cTn: Cardiac troponin; DOX: Doxorubicin; Fe2+: Iron; MLC2: Myosin light chain; MM-CK: Muscular creatine kinase; MT-CK: Mitochondrial creatine kinase; NO: Nitric oxide; ROS: Reactive oxygen species; TOPII: Topoisomerase II; Trans reg: Transcriptional regulatory.
Reproduced with permission from [28].
Compared with other organs, the heart is especially susceptible to anthracycline-induced damage, in part, owing to anthracyclines' high affinity for cardiolipin [29–31]. Cardiolipin is a unique mitochondrial phospholipid involved in various stages of mitochondrial membrane dynamics and the mitochondrial apoptotic process [29]. During the initial stages of apoptosis, seemingly in coordination with death receptor stimulation and the generation of reactive oxygen species (ROS) [32], cardiolipin can become peroxidized [33]. Peroxidation of cardiolipin can interfere with the localization of heme iron of cytochrome C and cause its release, as well as the release of additional apoptogenic factors, from mitochondria [30,34]. While it appears that peroxidation of cardiolipin plays a critical role in releasing cytochrome C from the inner mitochondrial membrane, cytochrome C itself can catalyze cardiolipin peroxidation [30]. Cardiolipin's cellular role is multifaceted and further investigation into its contribution to apoptosis is warranted.
Anthracycline exposure activates both ROS-dependent and ROS-independent pathways that may ultimately lead to a sizeable loss of cardiomyocytes through necrosis and apoptosis [35,36]. Other types of cell death, including senescence and autophagy, may also contribute to anthracycline-induced cardiomyopathy [36]. Myofibrillar loss and cytoplasmic vacuolization, caused by dilation of the sarcoplasmic reticulum in myocardial cells, are the most common histological findings in patients with anthracycline-induced cardiomyopathy [18]. The degree of cellular damage corresponds with the cumulative anthracycline dose administered [37]. The primary compensatory mechanism for anthracycline-induced cardiomyocyte loss is cardiomyocyte hypertrophy, which ultimately results in a series of physiological changes that lead to the development of cardiomyopathy. These changes include decreased left ventricular (LV) contractility, fibrosis and thinning of the LV wall and increased LV afterload, all of which contribute to diminished LV function [37–40].
In addition, the heart's relative lack of strong antioxidant defense mechanisms and high rate of oxidative metabolism make it particularly vulnerable to damage by free radicals and iron, which, in turn, can increase cell membrane permeability [20,41]. Substantial experimental data supports the involvement of iron in anthracycline-induced cardiotoxicity, including the ability of dexrazoxane, an iron chelator, to mitigate anthracycline-induced cardiotoxicity. Cascales et al. investigated whether anthracyclines increase iron concentration in the heart and, if so, whether the HFE genotype helps to regulate iron deposition [42]. They retrospectively examined the HFE genotype, cardiac iron concentrations and cardiac events in 97 consecutive necroscopies from adults with hematological and solid neoplasms. Of these, 48 patients had been treated with anthracyclines. The other 49 had received either some other type of chemotherapy (24 patients) or some nonchemotherapeutic form of therapy (25 patients) and were used as controls. Compared with the control group, a cumulative doxorubicin dose greater than 200 mg/m2 was associated with increased cardiac concentrations of iron (490 vs 240 μg/g; p = 0.01), regardless of the patient's transfusion history or level of iron in the liver. Certain mutated haplotypes were associated with greater iron deposition (282C/63D, p = 0.049; 282Y/63H, p = 0.03), and the haplotype C282Y-Y/H63D-H actually increased cardiac iron accumulation through its interactions with anthracyclines. The investigators concluded that HFE helps regulate iron accumulation after exposure to anthracyclines and that this iron accumulation is independent of a patient's systemic iron load.
Other possible contributors to anthracycline-induced cardiotoxicity include reduced expression of mRNA encoding for the sarcoplasmic reticulum Ca2+-ATPase [43], which results in diminished cardiac contractility and transcriptional changes in intracellular cardiomyocyte ATP production [44]. In addition, prolonged exposure to anthracyclines may also lead to mtDNA damage resulting from depressed cardiac glutathione peroxidase (GSHPx) activity and respiratory chain defects [41]. Respiratory chain defects are, in turn, associated with the generation of free radicals, which may continue to form even after anthracycline treatment, eventually contributing to late anthracycline-induced cardiomyopathy [45]. This production of radicals may also release cytochrome C from mitochondria, ultimately leading to apoptosis [46,47].
Focusing on liver and cardiac tissue, Doroshow et al. examined the changes in concentrations of catalase, superoxide dismutase and GSHPx (all enzymes noted for their ability to detoxify activated oxygen) in response to treatment with doxorubicin administered through intraperitoneal injection [41]. Experiments in murine models suggested that the GSHPx in cardiac tissue was selenium-dependent. Catalase and super-oxidase concentrations in the cardiac tissue were <0.6% and about 27% of that found in the liver tissue, respectively, but GSHPx concentrations were similar in both types of tissues. However, after 6 weeks of selenium depletion, GSHPx activity was reduced to less than 20% of baseline and in this selenium-depleted state, marked doxorubicin-induced toxicity occurred in animals receiving only 15 mg/kg intraperitoneally. By contrast, neither the concentrations of hepatic superoxide dismutase or GSHPx, nor the level of cardiac superoxide dismutase were affected at this dose. The authors concluded that selenium-dependent GSHPx, together with superoxide dismutase, greatly helps detoxify ROS in cardiac tissues, and that doxorubicin- or diet-induced depletion of selenium would impair the heart's ability to efficiently dispose of lipid peroxides and hydrogen peroxide.
Although redox-induced damage is likely involved in anthracycline-induced cardiotoxicity, some evidence suggests that it is not the only mechanism at play. Several studies of various redox inhibitors, or ROS scavengers, given concomitantly with anthracycline treatments have found no statistically significant levels of cardioprotection. The inability of these agents to ameliorate anthracycline-generated cardiotoxic damage does not substantiate redox-induced damage as a stand-alone cause of anthracycline-induced cardiotoxicity [29].
Pointon et al. compared the cardiotoxicity induced by doxorubicin with that induced by 2,3-dimethoxy-1,4-naphtoquinone (DMNQ) in murine models to investigate the adequacy of the redox hypothesis of anthracycline-induced cardiotoxicity [29]. DMNQ has limited ability to stop DNA replication or gene transcription but it is a superior redox cycling agent than doxorubicin (-183 mV compared with doxorubicin's redox potential of -328 mV). The investigators postulated that if redox damage was the primary cause of anthracycline-induced cardiotoxicity, then DMNQ should produce substantially more cardiac damage than doxorubicin. Acute responses to DMNQ or doxorubicin exposure suggest that anthracycline-induced cardiotoxicity is not primarily a result of general redox stress, but rather from the inhibition of the electron transport chain in mitochondria. Pointon et al. reported similar results in a chronic exposure murine model, but they did not include the supporting data [29].
Risk factors for developing anthracycline-induced cardiotoxicity
The risk of developing clinical cardiotoxicity in both children and adults coincides with increases in the cumulative dose of anthracycline [48–52]. While avoiding the use of anthracyclines in the treatment of certain cancers would be the most adequate strategy to prevent cardiotoxicity [53], this may negatively influence tumor response and ultimately survival. The most severe cardiac deficits associated with anthracycline toxicity occur at the highest cumulative doses. However, lower doses are not entirely safe either, as signs of cardiotoxicity have even been reported in patients receiving only low doses of anthracyclines [54]. This variability in susceptibility may be partially explained by genetic polymorphisms of certain genes, but only a few studies have explored this possibility and have reported conflicting results [5,52,55,56].
One study found that children with a cumulative anthracycline dose greater than 550 mg/m2 were more than five-times as likely to experience cardiotoxicity, than children receiving lower cumulative doses [57]. van Dalen et al. reported that the estimated incidence of developing anthracycline-induced clinical heart failure increased with both time since treatment and cumulative anthracycline dose (5.5% at 20 years after the start of anthracycline therapy if treated with a cumulative dose less than 300 mg/m2, and 9.8% if treated with a cumulative dose exceeding 300 mg/m2), over a mean of 8.5 years (median: 7.1 years; range: 0.01–28.4 years) [50]. The incidence of anthracycline-induced clinical heart failure in this cohort was 2.5% and a cumulative anthracycline dose of 300 mg/m2 or more was the only independent risk factor (relative risk [RR]: 8.0). The authors concluded that 10% of children receiving a cumulative anthracycline dose of 300 mg/m2 or more will eventually experience anthracycline-induced clinical heart failure.
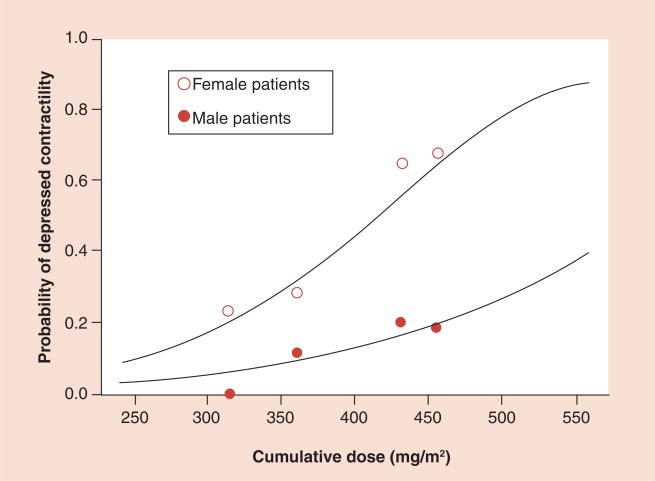
Lipshultz et al. reported that a higher cumulative anthracycline dose was associated with worsened LV structure and function with regard to both afterload and contractility [38]. More precisely, several studies have noted that cardiac dysfunction becomes more noticeable in patients who received a cumulative anthracycline dose that exceeds approximately 250 mg/m2 [58,59]. In addition, a higher dose rate, prior treatment with anthracyclines, longer follow-up, younger age at diagnosis and radiation therapy involving the heart have all been identified as risk factors for developing late cardiotoxicity (Table 1) [38,39,48,54,59–67]. Another risk factor for developing anthracycline-induced clinical heart failure in children is being female. For girls, this risk is approximately four-times as great as it is for male childhood cancer survivors treated with anthracyclines [65]. Lipshultz et al. reported that LV contractility of female childhood cancer survivors 8 years after completing doxorubicin treatment was significantly worse than that of their male counterparts (Figure 2) [60]. A few explanations have been posited for this difference. Doxorubicin clearance is lower in patients with higher BMI, which generally correlates with increases in the percentage of body fat [68]. Girls, on average, have a larger body-fat:lean-mass ratio than males do, which could increase the amount of anthracyclines stored in adipose tissue, subsequently exposing nonadipose tissues to higher concentrations of anthracyclines for a longer period of time [60,64].
Table 1.
Risk factors for anthracycline cardiotoxicity.
| Risk factor | Aspects | Ref. |
|---|---|---|
| Cumulative anthracycline dose | Cumulative doses >500 mg/m2 associated with marked long-term risk | [38, 52, 54,57] |
| Length of post-therapy interval | Incidence of clinically important cardiotoxicity increases progressively after therapy | [38,54,166] |
| Rate of anthracycline administration | Prolonged administration to minimize circulating dose volume may decrease toxicity; results are mixed | [107] |
| Individual anthracycline dose | Higher individual anthracycline doses are associated with increased late cardiotoxicity, even when cumulative doses are limited | [54,60] |
| Type of anthracycline | Liposomal encapsulated preparations may reduce cardiotoxicity. Data detailing anthracycline analogs and cardiotoxicity differences are conflicting | [18,76] |
| Radiation therapy | Cumulative radiation dose >30 Gy; prior or concomitant anthracycline treatment | [37,40] |
| Concomitant therapy | Trastuzamab, cyclophosphamide, bleomycin, vincristine, amsacrine and mitoxantrone, among others, may increase susceptibility or toxicity | [40,76] |
| Pre-existing cardiac risk factors | Hypertension; ischemic, myocardial and valvular heart diseae; prior cardiotoxic treatment | [76] |
| Comorbidities | Diabetes, obesity, renal dysfunction, pulmonary disease, endocrinopathies, electrolyte and metabolic abnormalities, sepsis, infection, pregnancy | [76] |
| Age | Both young and advanced age at treatment are associated with increased risk | [38,60] |
| Sex | Females are at greater risk than males | [60] |
| Additional factors | Trisomy 21; African–American ancestry | [57] |
Adapted from [73], with permission from BMJ Publishing Group Ltd.
Figure 2. Probability of depressed contractility as a function of the cumulative dose of doxorubicin by sex.
Adapted from [60], with permission from Massachusetts Medical Society.
It is important to note that not all of the presented risk factors are identified by all of the studies that assessed them. However, differences in study design and sample size between these studies may partially explain the varied results. For example, some studies only examined the high-risk subgroup of survivors, or those with a more aggressive form of cancer, a priori. These children tend to receive higher doses of doxorubicin in order to treat the cancer more aggressively, while standard-risk children do not receive such high doses [38,54,60].
Pathophysiology of anthracycline-induced cardiotoxicity
Cardiotoxicity is subdivided into three categories based on the time of onset: acute, early onset chronic progressive cardiomyopathy and late-onset progressive cardiomyopathy (Table 2) [18].
Table 2.
Characteristics of the different types of anthracycline-associated cardiotoxicity.
| Characteristic | Acute cardiotoxicity | Early onset, chronic progressive cardiotoxicity | Late onset, chronic progressive cardiotoxicity |
|---|---|---|---|
| Onset | Within the first week of anthracycline treatment | <1 year after completing anthracycline treatment | >1 year after the completion of anthracycline treatment |
| Risk factor dependence | Unknown | Yes | Yes |
| Clinical feature in adults | Transient depression of myocardial contractility; myocardial necrosis (cTnT elevation); arrhythmia | Dilated cardiomyopathy; arrhythmia | Dilated cardiomyopathy; arrhythmia |
| Clinical feature in children | Transient depression of myocardial contractility; myocardial necrosis (cTnT elevation); arrhythmia | Restrictive cardiomyopathy or dilated cardiomyopathy; arrhythmia | Restrictive cardiomyopathy or dilated cardiomyopathy; arrhythmia |
| Course | Usually reversible on discontinuation of anthracycline | Can be progressive | Can be progressive |
cTNT: Cardiac troponin T.
Adapted from [37], with permission from John Wiley and Sons, Inc.
Acute anthracycline-induced cardiotoxicity
Acute anthracycline-induced cardiotoxicity occurs in less than 1% of childhood cancer patients and is defined as an acute, but transient, decrease in LV contractility seen immediately after anthracycline administration [40]. Symptoms usually occur within 1 week of treatment and may range from arrhythmias and electrocardiographic abnormalities to myocarditis-pericarditis syndrome or congestive heart failure [69]. Sinus tachycardia, possibly as a result of autonomic dysfunction, is the most common arrhythmia, but decreased QRS amplitude, prolonged QTc interval, and non-specific ST segment and T-wave changes may also occur [40,57]. Serum cardiac biomarker elevations are considerably more common during this window [70].
Discontinuing therapy generally reduces the symptoms initially, but many patients, especially those who received a greater cumulative anthracycline dose, experience permanent cardiac damage and are more likely to develop late signs of cardiotoxicity [57].
Early onset anthracycline-induced cardiotoxicity
Anthracycline-induced cardiotoxicity that develops during therapy or within the first year after treatment and persists, is referred to as early onset chronic progressive cardiotoxicity and is observed in 1.6% [68] to 2.1% [66] of all anthracycline-treated children. In early onset cardiotoxicity, LV contractility is diminished, presumably from anthracycline-induced damage or death of cardiomyocytes [18,57,66].
In addition to the positive correlation between higher cumulative anthracycline doses and the incidence of cardiotoxicity, other factors are associated with a markedly increased risk of developing early onset anthracycline-induced cardiotoxicity. These factors include female sex, black race and trisomy 21, in addition to concomitant mediastinal irradiation, or treatment with amsacrine [57,60].
Late-onset anthracycline-induced cardiotoxicity
Cardiotoxicity presenting at least a year after the completion of anthracycline therapy is classified as late-onset cardiotoxicity and typically follows a chronic, progressive course [38,54]. Lipshultz et al. noted that 6 years after the end of anthracycline treatment approximately 65% of survivors of childhood cancer have detectable left ventricular abnormalities, either structural or functional [38,60].
Late-onset anthracycline-induced cardiac failure may evolve from the inability of the remaining cardiomyocytes to meet the demands of normal growth or other cardiac stresses, such as pregnancy or acute viral infection [37,71]. The resulting cardiomyopathy may depend on the age during which the survivor was treated with anthracyclines [38]. Treatment before the completion of physiological growth and development would leave fewer cardiomyocytes available for compensatory hypertrophic remodeling of the LV.
Clinical presentations of cardiotoxicity
Interestingly, although adult cancer survivors who experience cardiomyopathy as a result of their exposure to anthracyclines generally have dilated cardiomyopathic disease, survivors of childhood cancer often present with a combination of both dilated and restrictive cardiomyopathy [40,48]. The type of cardiomyopathy that develops in these young patients is influenced by whether they also received radiation treatment that included the heart. Dilated cardiomyopathy, which leads to LV systolic dysfunction, is more common among patients who only received anthracyclines, but it may also occur in the later stages of patients in whom cardiomyopathy developed from exposure to both anthracyclines and cardiac radiation. Initially, most patients who received both cardiac radiation and anthracyclines present with restrictive cardiomyopathy and thus diastolic dysfunction, which much later may progress to systolic dysfunction [37,39,72].
Diagnosis & monitoring
Currently, there is no consistently used, precise definition for anthracycline-induced cardiotoxicity. Part of the difficulty concerns the identification of practical biological markers or imaging techniques that are sensitive and specific for chemotherapeutic-induced cardiac damage. Evidence of anthracycline-induced cardiotoxicity can manifest as either clinical or subclinical heart failure. Clinical heart failure is defined as abnormal cardiac findings in a patient with signs and symptoms such as exercise intolerance, dyspnea and peripheral or pulmonary edema confirmed by a diagnostic exam [53,66]. Subclinical heart failure is defined as atypical cardiac findings in an asymptomatic patient [53]. These abnormal findings can be detected as asymptomatic LV dysfunction with several diagnostic tests.
In children at risk for anthracycline-induced cardiotoxicity, which can later lead to more serious cardiac complications, cardiac function must be monitored [73]. Given the vast range of abnormalities associated with anthracycline treatment, many different screening methods would be required to identify cardiovascular abnormalities (Table 3). For example, elevated N-terminal proBNP (NT-proBNP) and serum cardiac troponin T (cTnT) levels during therapy may predict late LV remodeling in this population [70]. However, presently, there are no evidence-based guidelines using cardiac biomarkers that have been validated as predictors of late cardiovascular events in this population to indicate how often this population needs cardiac monitoring and which tests are most sensitive [11,18,37,40,57,74–79]. Furthermore, the evidence supporting any guidelines for using any monitoring modality is insufficient to determine when anthracycline treatment should be stopped [11,18,37,40,57,74–79]. This uncertainty regarding the strengths and weaknesses of different monitoring modalities, and the lack of uniformity in monitoring schedules and methods, means that any monitoring could have serious safety or financial consequences from tests that are unnecessarily frequent or not frequent enough to detect early cardiac dysfunction in asymptomatic patients. Consequently, it is important to study the more promising modalities more extensively in larger clinical trials.
Table 3.
Potentially useful screening modalities for patients at risk for cardiotoxicity.
| Screening modality | Late effects | Indication |
|---|---|---|
| Echocardiogram | Cardiomyopathy | Anthracycline therapy and radiation therapy† |
|
| ||
| LV shortening fraction, contractility, wall stress and wall thickness | Systolic dysfunction | |
|
| ||
| Systolic indices, LV chamber size, LV mass and peak E/A ratio‡ | Diastolic dysfunction | Anthracycline therapy and radiation therapy† |
|
| ||
| Doppler echocardiography | Valvular disease | Anthracycline therapy and radiation therapy† |
|
| ||
| Wall thickness and visualization of pericardium | Pericarditis | Anthracycline therapy and radiation therapy† |
|
| ||
| RNA | Cardiomyopathy | Anthracycline therapy and radiation therapy† |
|
| ||
| LV ejection fraction | Systolic dysfunction | |
|
| ||
| Peak flow rate of filling | Diastolic dysfunction | |
|
| ||
| Exercise stress test | Anthracycline therapy and radiation therapy† | |
|
| ||
| With or without imaging (echocardiogram or RNA) | Coronary artery disease | |
|
| ||
| With imaging | Cardiomyopathy (systolic dysfunction only) | |
|
| ||
| ECG and 24-h Holter ECG | Arrhythmia and conduction disturbances | Anthracycline therapy and radiation therapy† |
|
| ||
| Lipid profile | Dyslipidemia | All survivors, but especially those with known cardiac dysfunction and with radiation to the heart, head or neck |
Radiation therapy that potentially included the heart in the treatment field.
E/A ratio, peak early-to-peak late filling of the LV during diastole.
E/A ratio: Ratio between early and late ventricular filling velocity; LV: Left ventricle; RNA: Radionuclide ventriculography.
Adapted from [37], with permission from John Wiley and Sons Inc.
Monitoring cardiac function: imaging studies
As previously mentioned, evidence-based guidelines for monitoring cardiac function in anthracycline-treated children are still being investigated. Furthermore, there are currently no studies in childhood cancer survivors designed to explore the accuracy of diagnostic testing as a primary objective. However, major cardiology organizations have made recommendations to assist in the clinical application of echocardiography. In 1992, the Cardiology Committee of the Children's Cancer Study Group created guidelines for monitoring cardiotoxicity in order to modify therapy [80]; however, after further review of the sources from which they based the decisions for treatment modification, none of the studies cited were properly designed to test the efficacy of such an approach [78]. The American Heart Association's class I recommendation for children receiving anthracycline treatment is serial monitoring by echocardiography starting at diagnosis and throughout treatment, using either M mode echocardiography, Doppler M analysis, 2D transthoracic echocardiography, or, if appropriate, transesophageal echocardiography [76]. Similarly, the Children's Oncology Group recommends the use of serial echocardiography to monitor cardiac status in this population. However, although their guidelines take into account the patient's age at diagnosis, radiation received and cumulative anthracycline dose for the recommended frequency of monitoring [201], they do not capture the full spectrum of potential cardiac risk factors that should be considered [78].
Thus far, no monitoring protocol has been specifically evaluated for either its cost–effectiveness or efficacy in detecting cardiac dysfunction [37,80–83,201]. Most critically, no guidelines for monitoring have demonstrated that the balance of oncologic efficacy and toxicity/late effects as assessed by lifespan quality of life are improved by any current recommendation, and this is the goal that is most important for this issue. Although there is consensus for the need of at least a baseline measurement and some kind of serial monitoring thereafter [76,201], there is little agreement on the most appropriate mode and frequency of monitoring [78,81]. The establishment of a monitoring protocol in the population is challenged by the progressive nature of anthracycline-induced cardiomyopathy [54] and the unknown stresses on the heart from various lifestyle changes and normal growth. Intuitively, however, the monitoring intervals and protocol may benefit by taking into account several variables, including symptoms, cardiac risk factors, cumulative dose of anthracyclines, other therapies received and any abnormal findings from previous studies [37,78,82], but even this is untested and speculative.
Lipshultz et al. demonstrated that echocardiograms measured during therapy in children with acute lymphoblastic leukemia (ALL) were not associated with cardiac abnormalities as measured by the cardiac biomarker cTnT, a validated marker for myocyte cell death [84]. Thus, although some studies may support the use of echocardiographic monitoring during therapy in these patients [85], others do not concur [84].
Anthracycline-induced cardiac dysfunction has been reported in asymptomatic childhood cancer survivors, by measure of echocardiography, after a median of 5.2 years (range: 2–15 years) from treatment completion of cumulative anthracycline doses less than 300 mg/m2 [86]. Furthermore, echocardiograms measured at the end of therapy have been associated with abnormal echocardiographic status 11.8 years after therapy [54]. Together, these findings may potentially suggest the usefulness of echocardiographic monitoring at the end of therapy, but again, no study has looked at individualized echocardiographic or other cardiac biomarker-guided changes in chemotherapy in terms of whether it results in an improvement in the balance of oncologic efficacy and toxicity/late effects in survivors. Hence, its exact role during and after anthracycline treatment requires further evaluation.
The type and reliability of the data collected from different imaging studies varies. LV fractional shortening (LVFS) depends on heart rate and LV loading conditions and therefore, may fluctuate in ways that do not reflect LV contractility (the health of cardiomyocytes). Nonetheless, it is an accurate echocardiographic measure of global LV systolic performance [87,88]. LV contractility, on the other hand, is a load-independent measure of myocyte vitality and cardiac function, suggesting this as more ideal measurement for longitudinal studies assessing intrinsic cardiac health [87]. Whether one is more important than the other at predicting future clinically significant cardiovascular morbidity or mortality is not know for this population.
Late-onset anthracycline-induced cardiomyopathy is often detectable using either radionuclide ventriculography (radionuclide angiography or equilibrium radionuclide angiography) or transthoracic echocardiography. Each imaging modality may measure LV systolic function accurately and can also assess heart structures, including the valves and pericardium (although these are more easily captured by echocardiography when acceptable echocardiographic windows are present) [37]. Although transthoracic echocardiography is noninvasive and thus safer in patients who may not tolerate anesthesia well, radionuclide ventriculography measures the change in radioactivity between end systole and end diastole, in order that the calculated LV ejection fraction (LVEF) can be determined [89]. Thus, although LV function can be estimated either by measuring LVFS or LVEF using echocardiography or radionuclide ventriculography, these values are not directly interchangeable [37]. Cardiac MRI may also be useful in this setting, but its utility in detecting doxorubicin-induced cardiotoxicity in children has yet to be determined.
No data support the use of radionuclide ventriculography for monitoring asymptomatic survivors. However, some have recommended radionuclide ventriculography studies every 5 years for all survivors of childhood cancers who were treated with anthracyclines. In addition, regular, more frequent, echocardiographic studies are also recommended for these individuals [83,90]. Assessment of LV diastolic function is important for the assessment of cardiotoxicity in survivors. Unfortunately, LV diastolic dysfunction is best confirmed with invasive techniques, such as radionuclide ventriculography [37].
Monitoring cardiac status: cardiac biomarkers
Clarifying the relationship between anthracycline dosages and the corresponding degree of cardiotoxicity is continuously challenging. Clinically significant cardiac damage may not present for months or years after the cessation of chemotherapy, yet clinically asymptomatic, detrimental changes in cardiac function and structure may be detectable much earlier. In addition, physicians often must extrapolate the dosage data collected from anthracycline-based studies of adults to children and there is a fine line between treating cancer aggressively enough and overwhelming patients with the toxic side effects of these drugs. Children are particularly susceptible to the detrimental effects of anthracyclines, which can greatly alter their developmental maturation.
A reliable assessment of a patients' tumor response to their dosage of chemotherapeutic drugs would reduce some of the guesswork involved their treatment. Several biomarkers and clinical procedures have been investigated with mixed results. The most promising tools for monitoring cardiac health include cardiac troponins, NT-proBNP, and high sensitivity C reactive protein (hsCRP) [70].
Serum cardiac troponins are proteins mostly found in cardiomyocyte sarcomeres. In patients with normal renal clearance, the degree of cardiac damage appears to be directly related to elevations in serum cardiac troponin concentrations [11,18,37,74–76,84,91].
Lipshultz et al. found that even small increases in serum concentrations of cTnT in children after the first dose of doxorubicin increased the risk of LV wall thinning and dilation [70]. In addition to this use as an independent predictor of certain LV characteristics, the authors also found that in children, cTnT concentrations seemed to provide a more sensitive measurement of myocardial damage than CK did, CK-MB, or myoglobin concentrations.
BNP, a neurohormone involved in fluid homeostasis, is released by ventricular myocytes in response to stretching of the myocardium [92]. Its levels are elevated in patients with volume-overloaded ventricles, as observed in congestive heart failure [93]. NT-proBNP is a biomarker for distressed cardiomyocytes. It independently predicts mortality and cardiovascular events in the general population [94] and in patients with chronic heart failure, vascular disease and acute coronary syndromes [95]. Furthermore, elevations in NT-proBNP within the first 90 days of anthracycline treatment were associated with LV remodeling 4 years post-diagnosis in a cohort of children with high-risk ALL, which suggests it may be able to detect cardiac stress in children receiving anthracyclines before irreversible damage occurs [70].
Similarly, hsCRP, which detects generalized inflammation, may be useful for monitoring cardiovascular changes in these vulnerable children [70]. Some studies indicate that hsCRP independently predicts outcomes in adults with nonischemic and ischemic cardiomyopathy and could perhaps provide a general sense of cardiovascular health, both during and after anthracycline treatment [76].
Despite the promise of cardiac biomarkers for monitoring children exposed to anthracyclines, there is still a need for clinical studies designed to specifically test whether these biomarkers can accurately indicate short-term changes in cardiac function or predict the development of late cardiotoxicity [18,73,76,96,97].
Monitoring cardiac function: exercise stress testing
Exercise stress testing may detect asymptomatic cardiac dysfunction in patients treated with anthracyclines or mediastinal radiation, but its application in monitoring cardiac function of childhood survivors is uncertain. Although these patients may have adequate cardiac function at rest, they may decompensate if cardiac demands are increased [98–100]. Stress testing assesses maximum oxygen consumption, which closely corresponds with prognosis in patients with congestive heart failure. Adams et al. noted that maximum oxygen consumption values were low (<20 ml/kg/m2) in long-term survivors of Hodgkin's disease who had received mediastinal radiotherapy as part of their treatment [101].
Hogenhuis et al. asked 229 adults hospitalized for chronic heart failure to complete two quality of life surveys: the Minnesota Living with Heart Failure Questionnaire and the RAND-36ph [102]. They determined the LVEF and New York Heart Association (NYHA) class, measured their BNP and assessed their performance on a 6-min walk test, a common exercise stress test. Although walk-test results did not correlate with LVEF (r = -0.15, p = 0.05), they were significantly correlated with Minnesota quality-of-life scores (r = -0.23, p < 0.01), RAND-36ph scores (r = 0.52, p < 0.01) and NYHA class (r = -0.46, p < 0.01). On the other hand, BNP concentrations were not correlated with Minnesota quality-of-life or RAND-36ph scores and were only weakly correlated with LVEF (r = -0.29, p < 0.01) and NYHA class (r = 0.20, p < 0.01). Hogenhuis et al. suggested that the 6-min walk test provides a better measure of quality of life and functional capacity, while plasma concentrations of BNP correspond more closely to cardiac function [102]. It remains to be seen how these findings in adults with chronic heart failure help interpret the results of exercise stress tests in survivors of childhood cancers treated with anthracyclines.
Cardioprotection
Limiting the cumulative dose of anthracyclines
Not using anthracyclines at all, so long as it does not negatively influence tumor response and survival, would be the most adequate strategy to prevent cardiotoxicity [53]. However, the lack of randomized trials that assess the risk or benefits from limiting cumulative anthracycline dose precludes clinicians from making such changes to treatment protocols without knowing if it impedes oncologic efficacy. Other studies, not specifically designed to assess limiting cumulative doses, have found significant associations between higher cumulative doses and increased risk of cardiotoxicity. However, lower doses are not entirely safe or effective for cardioprotection. Even patients receiving only low doses of anthracyclines may show signs of cardiotoxicity [54]. Moreover, the clinical signs of cardiotoxicity may present much later and be both chronic and progressive. Such delayed presentations require long-term follow-up and make it difficult to establish doses of anthracyclines that achieve their therapeutic goals without causing marked cardiac damage. Despite this uncertainty, many cancer treatment protocols for both adults and children use a maximum cumulative dose of 450–550 mg/m2 for doxorubicin [48,57] and 900 mg/m2 for epirubicin [103].
Altering anthracycline administration
The intended goal of continuous infusion is to reduce peak drug concentrations by prolonging the duration of infusion without reducing the dose. In adults, continuous infusion of anthracyclines may reduce acute cardiotoxicity, but the same cannot be said in children. In women with breast cancer, continuous infusions given over 48 or 96 h results in less cardiotoxicity than bolus administration. Moreover, administering anthracycline through continuous infusion in these women allows them to receive almost double the cumulative dose of the drug without diminishing its oncological properties [104–106]. However, in children receiving treatment for ALL, Lipshultz et al. found no difference in event-free survival or in cardiac manifestations of anthracycline toxicity between patients treated with bolus infusion or those treated with continuous infusion over 48 h [107]. Similarly, Levitt et al. reported that 6 h of continuous infusion did not attenuate the degree of late subclinical cardiotoxicity in patients who received moderate doses of anthracyclines [108].
Use of anthracycline analogs & anthracenediones
Novel formulations of anthracycline analogs have been developed, but few have less cardiotoxicity or better cytotoxicity than that of doxorubicin [109,110]. Those that do appear to be less cardiotoxic, at least in preclinical studies, include mitoxantrone, epirubicin and idarubicin [111–114]. However, results from clinical studies remain inconclusive [62,115–117].
Liposomal anthracyclines
The distribution of liposomal anthracyclines, by their nature, is relatively confined within the areas of vasculature lined by tight junctions, which include the heart muscle, but can extend to tissues at sites where the junctions are not as tight or where the vascular wall has been disrupted [118]. Tumors, with their angiogenic properties, can disrupt the integrity of the capillary endothelium. Consequently, liposomal anthracyclines, when administered intravenously, are capable of more selectively permeating their target tissues than traditional anthracyclines are. This selectivity alters the volume of their distribution and results in less cardiotoxicity than that of traditionally formulated anthracyclines but without reducing anti-tumor effects. Most randomized trials in adults comparing liposomal doxorubicin with the standard formulation have concluded that they are about equally effective but that liposomal anthracyclines are less cardiotoxic [119–121].
A further advantage of liposomal anthracyclines over traditional formulations is that their release is slower. This property, in turn, may result in lower peak concentrations, which may help lower their cardiotoxicity [122].
Currently, liposomal doxorubicin, pegylated liposomal doxorubicin and liposomal daunorubicin are the only liposomal anthracycline formulas being assessed in the USA. Although higher cumulative doses of liposomal daunorubicin (600–900 mg/m2) are cardiotoxic, early clinical studies suggest that liposomal daunorubicin is less cardiotoxic than traditionally formulated daunorubicin [123,124].
One randomized trial of 509 patients with metastatic breast cancer compared pegylated liposomal doxorubicin with the traditional formulation. The cumulative dose was at least 500 mg/m2. Although overall survival rates were similar, the group given pegylated liposomal doxorubicin had significantly less cardiotoxicity (hazard ratio: 3.2; 95% CI: 1.6–6.3) [125].
Despite the positive outlook of liposomal anthracyclines, these results are based on adult studies and therefore cannot be extrapolated to children. There is a lack of randomized trials in children with cancer [126], and until there is more evidence, these children should proactively continue to undergo cardiac monitoring.
Probucol
Probucol, a cardioprotectant for use with doxorubicin, has been studied in preclinical settings. It is an antioxidant and lipid-lowering agent [127] that may help reduce atherosclerotic cardiovascular disease [128,129], but its use has been limited because of its propensity to lower HDLs more than LDLs [127]. Despite this drawback, studies in rats have shown that pretreatment with probucol negates the toxic impact of doxorubicin therapy on cardiomyocytes. Moreover, these studies suggest that probucol does not interfere with the anti-tumor activity of doxorubicin [130].
The exact mechanism behind probucol's protection is not well understood, but it may be related to either its lipid-lowering or antioxidant properties [131,132]. Several studies in rats found that the activity of GSHPx, an antioxidant enzyme, was significantly lowered by a cumulative dose of 15 mg/kg body weight of doxorubicin given over six equal injections, but was not altered in the rats pretreated with probucol before receiving doxorubicin. One study found that pretreatment with probucol prevented an increase in a marker of oxidant stress, thiobarbituric acid [133–135]. Another study reported that pretreatment with probucol before doxorubicin therapy increased the levels of superoxide dismutase, whereas doxorubicin administration without probucol pretreatment slightly decreased the levels of this antioxidant enzyme [133,134]. Although the benefits of probucol administration before doxorubicin treatment could be important, its viability as a cardioprotectant in humans remains to be evaluated.
N-acetylcystein
N-acetylcystein is a mucolytic agent with both anticarcinogenic and antigenotoxic properties. In addition, it promotes intracellular glutathione synthesis, thereby acting as an antioxidant [136]. However, despite these properties, a randomized trial concluded that N-acetylcystein added to doxorubicin was not cardioprotective [137].
Carvedilol
Carvedilol is a β-adrenergic antagonist widely used to treat CHF, hypertension and angina. It has α1-blocking vasodilatory effects and [138] is a potent antioxidant, which may explain its cardioprotective effects [139,140]. Several studies have reported that carvedilol and several of its metabolites can prevent lipid peroxidation and the depletion of endogenous antioxidants, including intracellular glutathione and vitamin E [140,141]. Carvedilol both scavenges for and impedes the formation of reactive oxygen free radicals [140,141] and studies in rats have shown that it markedly reduces doxorubicin-induced cardiomyopathy [142]. It is currently used to improve prognosis, alleviate symptoms, and stop the decline of LV function in patients with established doxorubicin cardiomyopathy [142,143]. In vitro studies by Spallarossa et al. found that carvedilol could inhibit apoptosis [144] by substantially improving the Bax:Bcl2 ratio and significantly reducing the production of hydroperoxide and superperoxide anions. The authors also established that the cardioprotective activity of carvedilol did not stem from its properties as a β-blocker, but rather as an antioxidant. Although carvedilol is promising, we found no randomized trials testing its efficacy as a cardioprotectant in children. In addition, whether or not it interferes with the antineoplastic activity of doxorubicin must be assessed.
Inhibition of the renin-angiotensin-aldosterone system
Several studies involving angiotensin-converting enzyme (ACE) inhibitors administered after anthracycline treatment have found that the renin-angiotensin-aldosterone system is important in creating anthracycline-induced cardiac dysfunction. Nakamae et al. investigated whether valsartan, an angiotensin II blocker, could reduce doxorubicin-induced cardiomyopathy in a randomized trial of 40 adults with non-Hodgkin's lymphoma [145]. One group received standard chemotherapy with cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) and the experimental group received an additional daily dose of 80 mg of valsartan. Valsartan significantly reduced chemotherapy-induced transient increases in the QTc interval (p < 0.001), QTc dispersion (p = 0.02) and the LV end-diastolic diameter (p = 0.01). In addition, valsartan appeared to prevent increases in BNP (p = 0.001). CHOP-induced increases in these various physiological values nearly returned to normal in the control group within a week after the end of chemotherapy (p < 0.001). Mean blood pressure and heart rate values did not differ significantly between the two groups. Consequently, the authors concluded that valsartan reduced doxorubicin-induced cardiac damage by its role as an angiotensin II blocker.
In a similar fashion, various ACE inhibitors, including captopril and enalapril, have been assessed as chemotherapy adjuvants to reduce oxidative stress, downregulate the activation of renin-angiotensin-aldosterone system and minimize the generation of free radicals [146]. As observed with the angiotensin II blocker valsartan, concomitant administration of ACE inhibitors seems to reduce cardiac damage. Unfortunately, these studies do not adequately address how children might respond to adjuvant treatment with an angiotensin II blocker or an ACE inhibitor during anthracycline-based chemotherapy or whether treatment with valsartan provides lasting cardiac benefits. These findings are preliminary and so require further investigation, particularly in long-term survivors.
Sildenafil
Fischer et al. determined whether sildenafil, a phosphodiesterase-5 inhibitor proven to reduce ischemic- and reperfusion-related cardiac injury, could also protect against doxorubicin-induced cardiotoxicity [147]. Mice were randomly assigned to one of four treatment groups: saline, sildenafil, doxorubicin (5 mg/kg intraperitoneally), or doxorubicin plus sildenafil (0.7 mg/kg intraperitoneally). The sildenafil-treated mice had lower apoptosis and desmin disruption than mice treated only with doxorubicin. LV pressure and rate pressure product was also lower in sildenafil-treated mice than in mice treated only with doxorubicin. Furthermore, during the 8-week trial, mean ST intervals only increased significantly from baseline in mice treated with doxorubicin and not in mice treated with both doxorubicin and sildenafil. The cardioprotection provided by sildenafil against doxorubicin-induced apoptosis in cardiomyocytes was completely prevented by L-NAME, a nitric oxide synthase inhibitor and 5-hydroxydecanoate [148], which blocks both ischemic and pharmacological preconditioning and is thought to specifically inhibit mitochondrial ATP-sensitive channels. These results illustrate the dependence of this pathway on nitric oxide synthase and indicate that sildenafil's effect on the opening of mitochondrial ATP-sensitive channels channels helps prevent doxorubicin cardiotoxicity.
Di et al. examined the effects of sildenafil in one murine breast tumor line and in four human breast tumor cell lines that were grown in mice subsequently treated with doxorubicin [149]. Adding sildenafil to cisplatin, taxol, camptothecin or doxorubicin did not reduce the antineoplastic activities of these chemotherapeutic agents. The effects of sildenafil on treatment with doxorubicin were increased DNA damage and apoptosis in MDA-MB231 cells. In addition, sildenafil treatment notably increased the sensitivity of the p53 mutant MDA-MB231 and p53 null MCF-7/E6 cells to doxorubicin and mildly increased the sensitivity in the MCF-7/caspase 3 and 4T1 cell lines. Furthermore, sildenafil did not change the sensitivity of bone marrow cells or macrophages to doxorubicin, nor did it increase doxorubicin-associated toxicity or immunosuppression. This finding was particularly noteworthy because phosphodiesterase type 5 is expressed in macrophages [150] and other cells involved in the immune system and because there was concern that sildenafil would exacerbate doxorubicin's immunosuppressive effects [151,152].
Although more studies are needed to further examine the pharmacokinetics of sildenafil in children, it has already demonstrated benefit in children treated for pulmonary hypertension related to congenital heart disease [153,154] and in persistent pulmonary hypertension in newborns [155,156]. The findings of both Fischer et al. [147] and Di et al. [149] suggest that sildenafil may be useful as a cardioprotectant against the harms of doxorubicin, but further research is necessary to determine whether or not its effects are transient and whether it protects against the development of late cardiomyopathy.
Adiponectin
Adiponectin, a hormone involved in a several metabolic processes [157], appears to confer some degree of protection against ventricular hypertrophy and ischemia-reperfusion injury. Konishi et al. in an in vivo study in mice, assessed whether adiponectin also protects against doxorubicin-induced cardiomyopathy [158]. Their results suggest that adiponectin may reduce doxorubicin-induced cardiomyopathy and thus ameliorate cardiac function, through antiapoptotic effects achieved by upregulating AMP-activated protein kinase. Whether or not adiponectin is an appropriate cardioprotective agent in children treated with anthracyclines remains to be established.
Erythropoietin
Erythropoietin (EPO) a cytokine produced by the adult kidney and is known for its role in hematopoiesis. Li et al. note that EPO receptors are also found in nonhematopoietic tissues, such as cardiac, brain, spinal cord and skeletal muscle and that recent studies in adults indicate that EPO may play a protective role in these tissues [159]. Cardiac tissue that received EPO prior to or during ischemic insult appeared to demonstrate improved LV contractility and cardiac recovery. Wanting to determine if EPO offered additional protection against cardiac disease of nonischemic origin, specifically, doxorubicin-induced cardiomyopathy, Li et al. used murine models to study the effects of administering EPO at various durations following doxorubicin administration compared with a control group that was given the chemotherapeutic agent only [159]. They reported that the administration of EPO prior to the appearance of cardiac dysfunction resulted in less LV dilation and dysfunction and appeared to provide some degree of protection against such doxorubicin-associated complications [159].
While EPO use may be a valuable adjunct to therapy in those treated with doxorubicin, the actual effects of EPO therapy in patients, with regard to survival outcomes and possible side effects remains to be thoroughly established, especially in children.
Dexrazoxane
Dexrazoxane inhibits DNA topoisomerase II and is converted by the cells to an open-ring derivative (ADR-925), which chelates iron. Its activity as a chelator enables dexrazoxane to either bind free iron or remove iron from the doxorubicin–iron complex, thereby avoiding oxygen radical formation. This property may be essential for dexrazoxane to mitigate some of the cardiotoxic effects of doxorubicin treatment [160]. The cardioprotective benefit of inhibiting DNA topoisomerase II, on the other hand, is less certain and requires further evaluation since cardiac tissue lacks topoisomerase IIα. Regardless of whether or not it is important in cardioprotection, dexrazoxane's inhibition of DNA toposisomerase II is thought to contribute fundamentally to dexrazoxane's antineoplastic activity [115].
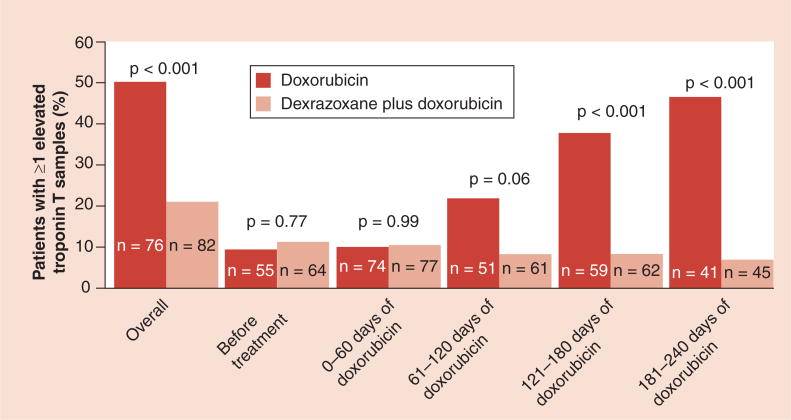
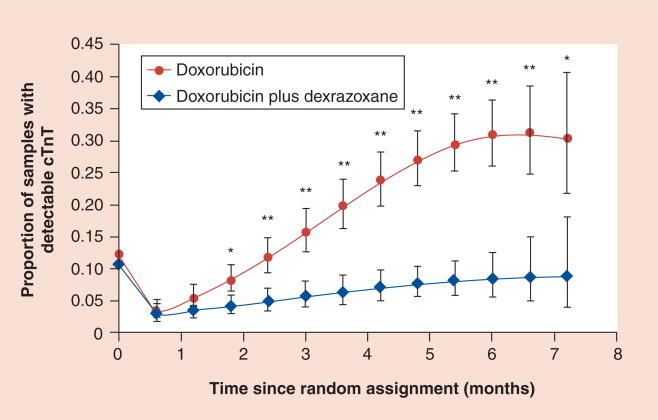
In patients with breast cancer, higher cumulative doses of doxorubicin are well tolerated when patients are pretreated with dexrazoxane [161,162]. Several randomized trials in both children and adults have all concluded that dexrazoxane is an effective cardioprotectant against anthracycline-induced toxicity [91,161,163–166] and all but one concluded that pretreatment with dexrazoxane did not impair tumor response to anthracycline treatment. Results of the Dana–Farber Cancer Institute Childhood (DFCI) ALL protocol 1995–2001 showed that dexrazoxane reduced or prevented doxorubicin-induced cardiac injury in patients with high-risk ALL, as indicated by elevations in cardiac troponin T, after a median follow-up of 2.7 years (Figure 3) [84,91]. An additional study showed that at the end of therapy, cardiac troponin T (a biomarker that indicates cardiac injury) was elevated in more children who received doxorubicin alone than in those who also received dexrazoxane (Figure 4) [70]. After a median follow-up of 8.7 years in this same 1995–2001 protocol, the cardioprotective effect of dexrazoxane still remained and no differences were found in event-free survival between the group who received doxorubicin alone and the dexrazoxane plus doxorubicin group (event-free survival: 77% for both; p = 0.99) [166].
Figure 3. Percentage of patients with at least one elevated cardiac troponin T level overall, before treatment with doxorubicin and during treatment.
An elevated level of troponin T was defined as one that exceeded 0.01 ng/ml. The number of patients in whom troponin T was measured at least once during the specified intervals is shown in each bar.
Adapted with permission from [84].
Figure 4. Model-based estimated probability of having an increased cardiac troponin T level at each depicted time point in patients treated with doxorubicin, with or without dexrazoxane.
Vertical bars show 95% CIs. Increased cTnT is defined as a value greater than 0.01 ng/ml.
*p-value versus dexrazoxane group ≤0.05; **p-value versus dexrazoxane group ≤0.001. An overall test for dexrazoxane effect during treatment was significant (p < 0.001).
cTnT: Cardiac troponin T.
Adapted with permission from [70].
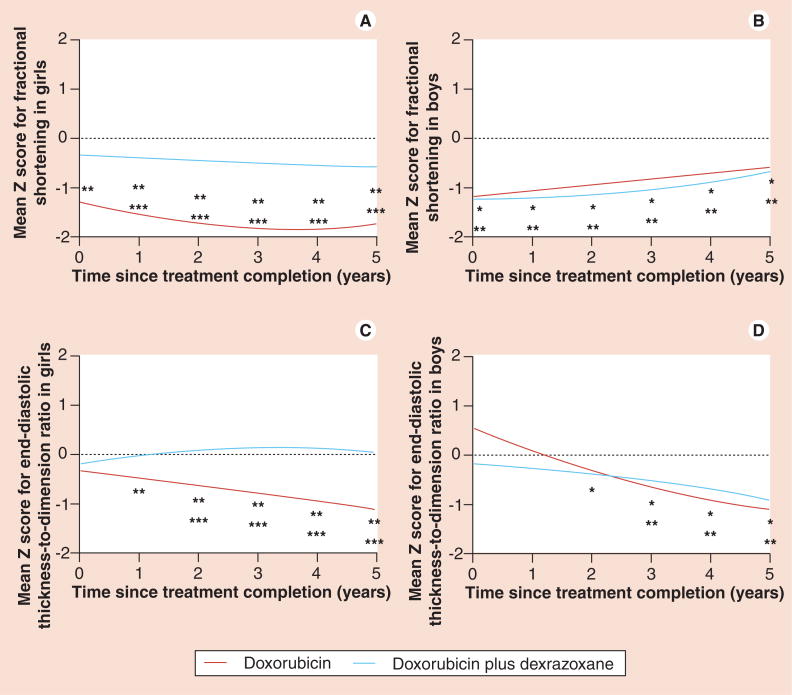
The long-term cardioprotective effect of dexrazoxane is further concluded in the analysis of echocardiographic measurements from 66 and 68 high-risk ALL survivors who received doxorubicin or doxorubicin plus dexrazoxane, respectively. 5 years after completing doxorubicin chemotherapy, LVFS and end-systolic dimension Z scores were significantly worse than normal for children who received doxorubicin alone (LVFS: -0.82; end-systolic dimension: 0.57) as compared with children who also received dexrazoxane (LVFS: -0.41; end-systolic dimension: 0.15). Girls given dexrazoxane rather than doxorubicin alone had significantly better LVFS than boys (Figure 5A & B). Mean Z scores in boys were significantly lower than zero from 2 years onwards in the doxorubicin plus dexrazoxane group and 3 years onwards in the doxorubicin alone group (Figure 5D). In girls, mean Z score differed significantly between treatment groups, with the doxorubicin plus dexrazoxane group close to zero (Figure 5C). Left ventricular wall thickness and thickness-to-dimension ratio were the only statistically significant characteristics for the protective effect of dexrazoxane [166].
Figure 5. Mean left ventricular echocardiographic Z scores in boys and girls (n = 134).
Plots are adjusted for age *p ≤ 0.05 for comparison of the mean Z score of the doxorubicin plus dexrazoxane group with zero. **p ≤ 0.05 for comparison of the mean Z score for the doxorubicin group with zero. ***p ≤ 0.05 for comparisons of mean Z scores between the doxorubicin and doxorubicin plus dexrazoxane groups.
Adapted with permission from [166].
Fears that dexrazoxane may protect cancer cells, in addition to cardiomyocytes, or increase the risk of second malignancies have raised concern of its use among investigators [167]. A meta-analysis by van Dalen et al. on cardioprotective strategies for anthracycline-treated children and adults, assessed dexrazoxane's effectiveness as a cardioprotectant [53]. The findings of this study implied that there was a significant benefit in favor of using dexrazoxane, with regard to the prevention of clinical heart failure (RR: 0.18; 95% CI: 0.10–1.32; p < 0.001) and clinical and subclinical heart failure combined (RR: 0.29; 95% CI: 0.20–0.41; p < 0.0001). Furthermore, they noted that there was no difference in the tumor response rate between the dexrazoxane treatment group and the control group (RR: 0.89; 95% CI: 0.78–1.02; p = 0.08). In addition, a total of 113 (36%) of the 315 patients who did not receive dexrazoxane with their anthracycline treatment eventually experienced either clinical or subclinical heart failure, whereas heart failure occurred in only 34 (10%) of the 328 patients pretreated with dexrazoxane (RR: 0.29; 95% CI: 0.20–0.41; p < 0.0001). Herman et al. found that adding the cardioprotectant dexrazoxane in rats treated with doxorubicin lowered cTnT concentrations below that in controls [168]. Furthermore, they found no significant differences in overall survival between patients treated with and without dexrazoxane [53]. Barry et al. found no association between dexrazoxane and second malignant neoplasms among the 205 high-risk ALL patients in the DFCI 1995–2001 protocol after a median follow-up of 6.2 years [169]. Furthermore, Vrooman et al. expanded the study to include 553 patients treated with dexrazoxane in three consecutive multicenter trials (DFCI protocols 1995–2001, 2000–2001, 2005–2010). The overall 5-year cumulative incidence of second malignant neoplasms in this study was 0.24 ± 0.24%. Of the 553 patients, the only case was that of an acute myelogenous leukemia, which occurred in a patient with mixed-lineage leukemia-rearranged ALL approximately 2 years after initial diagnosis [170].
Dexrazoxane is thought to be the only cardioprotectant proven to be effective against anthracycline treatment in randomized trials [53,166]. Despite this fact, the American Society of Clinical Oncology currently recommends limiting its use to adults with metastatic breast cancer who would benefit from additional doxorubicin treatment but in who the cumulative dose of doxorubicin is already above 300 mg/m2. The findings from Schuchter et al. in adults treated with anthracyclines for nonbreast cancer malignancies also suggest that dexrazoxane may be used cautiously in these patients who would benefit from further doxorubicin treatment but whose cumulative dose of doxorubicin already exceeds 300 mg/m2 [171].
Dexrazoxane is an important component of cardioprotection without compromising oncologic activity, but its use may be insufficient to fully prevent long-term LV dysfunction, at least in some groups of patients [166]. A Cochrane review recommends the use of dexrazoxane if substantial cardiac damage is anticipated, but the clinician must weigh dexrazoxane's cardioprotective effect with the poorly substantiated risk of moderating tumor response rates [53].
Amifostine
Amifostine, a membrane-bound alkaline phosphatase, is a cytoprotective agent with a concentration 275-times greater in normal tissue than in tumor tissue [172]. Conversion to its active metabolite, WR-1065, is favored by the neutral pH of normal tissues and not by the more acidic pH of neoplastic tissue [173]. The favorable pH of normal tissue, combined with the markedly higher concentration of amifostine in normal tissue results in a much higher rate of conversion to the active metabolite in normal tissue than in neoplastic tissue. The higher level of WR-1065 in normal tissue would, therefore, confer selective protection during anthracycline treatment. Although in vivo studies in rats indicated that amifostine was somewhat cardioprotective [174,175], Herman et al. concluded that dexrazoxane was the better cardioprotectant [176].
Treatment of cardiovascular disease
Despite all efforts to prevent anthracycline-induced cardiomyopathy, it is still a prevalent complication in survivors with serious implications on mortality and quality of life [177]. Both symptomatic and asymptomatic presentations of LV dysfunction are associated with poor prognosis. Based on the NYHA criteria, ambulatory adults with doxorubicin-induced NYHA class II–IV heart failure have an estimated 40% 1-year mortality rate and a 60% 2-year mortality rate [178]. Adults with incidentally discovered asymptomatic idiopathic dilated cardiomyopathy have a similarly poor prognosis, with an estimated 7-year survival rate of 50% [179]. These grim statistics underscore the imperative nature of reducing anthracycline-induced cardiotoxicity. Since nothing completely prevents the damage induced by anthracycline-treatments, survivors of childhood cancers must be monitored carefully and continuously for both asymptomatic and clinical indicators of cardiac distress. Symptomatic and asymptomatic LV dysfunction in particular should be treated aggressively to prevent progression to heart failure. However, very few studies on treatment of anthracycline-induced cardiotoxicity exist in the pediatric population [180].
Growth hormone (GH) has been investigated as a means of restoring LV function following treatment with anthracyclines. It has been proposed that GH directly, or perhaps indirectly through IGF-1, may decrease LV systolic wall stress and thereby improve LV performance by inducing LV hypertrophy. In a 10-year longitudinal study, Lipshultz et al. evaluated whether or not treatment with GH ameliorated certain types of LV dysfunction [181]. Serial cardiac findings in two groups of childhood cancer survivors were compared; one group who received GH following treatment with anthracyclines and another group with similar baseline cardiac function that did not receive GH. While LV wall thickness for the GH-treated group was greater than that of the control group during therapy, the effects of treatment were transient and dissipated after GH therapy was discontinued. Additionally, excess afterload did not improve during GH therapy, despite decreases in end systolic blood pressure and increases in wall thickness and LV mass. Noting that wall stress decreases with increasing LV wall thickness and increases with increasing LV pressure or dimension, Lipshultz et al. concluded that patients treated with GH continued to experience a progressive decrease in contractility, which resulted in a secondary increase in afterload due to the subsequent decrease in the thickness:dimension ratio [181].
Another treatment targeted at minimizing LV dysfunction associated with anthracycline-induced cardiac damage is the use of ACE inhibitors. Silber et al. performed a double-blind, placebo-controlled study of enalapril, an ACE inhibitor, in patients exposed to anthracyclines during childhood to determine if the use of an ACE inhibitor would inhibit the progression of cardiac dysfunction by decreasing left ventricular end-diastolic wall stress [182]. Although ACE inhibitors did lower left ventricular end-diastolic wall stress, the authors failed to find an effect on the progression of cardiac dysfunction after a follow-up of 2.8 years [182]. In contrast to Silber et al., a retrospective study of 18 childhood cancer survivors treated with doxorubicin who underwent regular echocardiographic examinations during enalapril therapy, showed progressive improvement in LVFS, afterload, LV dimension and mass during the first 6 years of treatment with enalapril [183]. However, these gains deteriorated between 6 and 10 years of treatment and became not significantly different from pre-enalapril LV structure and function measurements. LV contractility, systolic blood pressure, and to a lesser extent, diastolic blood pressure declined throughout the study. This study demonstrated that the short-term benefit observed with enalapril therapy was primarily attributable to the decreased diastolic pressure, and that children with higher diastolic blood pressure are more likely to experience a beneficial response to enalapril therapy [183]. LV wall thickness, the primary cardiac abnormality seen in these patients, continued to progressively decline on enalapril therapy, even during the period of treatment in which LV dilation declined. These findings suggest that the continued decline in LV thickness might be attributable to enalapril-induced impairment of both pathologic and physiologic hypertrophy, a well-known effect of ACE inhibition. The inhibitory effect of enalapril on physiologic hypertrophy may explain why the younger children in this study fared worse than their older counterparts, with more depression of LVFS and more increases in LV afterload, dilation and mass during the first 2.4 years of enalapril therapy. Overall, regardless of whether or not they were treated with enalapril, doxorubicin-treated children continued to experience progressive LV dysfunction. However, this study concluded that enalapril treatment did provide a 6–10-year respite before asymptomatic LV dysfunction returns to baseline [183].
Conclusion
Anthracycline-induced cardiotoxicity is extremely costly at many levels. In addition to greatly increased medical costs [71,75], treatment options are often limited with regard to the patients' cumulative dose and their treatment protocols in the event of relapse or lack of response to their first chemotherapy drug. Additionally, childhood cancer survivors are at increased risk for early mortality and substantial reductions in their quality of life and often require long-term treatment for anthracycline-related complications [18]. The cardiac damage clearly linked to anthracycline exposure provides ample reason to search for effective cardioprotective measures and to carefully assess the evidence for administering the cumulative doses currently used in various treatment protocols.
Although preventing doxorubicin-induced cardiomyopathy should remain a priority, it is also necessary to investigate postchemotherapeutic treatments that can ameliorate the damage of anthracycline-induced cardiomyopathy and impede the progression of cardiac dysfunction in these young and vulnerable patients.
Future perspective
Currently, dexrazoxane is potentially the only cardioprotectant for targeted prevention of children receiving anthracycline treatment whose efficacy has been established in a large randomized trial [91,166]. Although it is currently only approved for use in a small subset of adult cancer patients, the critical demand for a safe and effective adjuvant treatment that reduces cardiac damage while preserving the antineoplastic effects of anthracyclines makes a strong argument for administering dexrazoxane as part of the standard of care in children treated with anthracyclines.
Aside from dexrazoxane and other cardioprotective measures [53], the use of complementary and alternative medicine therapies, such as use of yoga, dietary supplements, acupuncture [184] and adopting a heart-healthy lifestyle may hold promise in reducing the physiological changes that accompany anthracycline-induced cardiomyopathy. However, further large-scale studies are needed to assess whether any of these therapies can be recommended in children treated with anthracyclines.
Unfortunately, dexrazoxane does not prevent all anthracycline-related cardiac damage and thousands of survivors of childhood cancers have already completed treatment with anthracycline-based chemotherapy. Thus, we foresee continued investigation into the use of traditional heart failure medications, such as ACE inhibitors and β-blockers, in children, as well as experimentation with novel therapies, such as safer formulations of anthracyclines and even stem cell therapy, to repair damaged cardiomyocytes.
Ibsen et al. developed a prodrug form of doxorubicin (DOX-PBC), which blocks the free amine group in doxorubicin, whose activation can be controlled with exposure to ultraviolet light at 350 nm [185]. While traditional doxorubicin quickly entered cells and almost completely localizes to the nucleus, DOX-PBC did not stain the exposed DNA during mitosis and remained in the cytoplasm of the cell, even though it, too, rapidly entered cells. Furthermore, DOX-PCB was relatively stable against metabolic breakdown in the liver and showed a 200-fold decrease in cytotoxicity compared with free DOX [185].
Developing novel formulations of anthracyclines may help prevent many anthracycline-induced cardiomyopathies, but for children who currently have anthracycline-related cardiac damage, advances in stem cell therapy may potentially be able to reverse or repair such damage, improving the quality of life for these children. The use of cardiac stem cells to help repair myocardial damage in adults is already underway [186]. Although stem cell therapy in adults with ischemic cardiomyopathy has proven beneficial in several clinical trials, similar trials have not been conducted in children [187–191]. Regardless of the attractiveness of stem cell therapy, little is known regarding its long-term effects on patients, especially in children who have yet to complete their physical and mental growth and development. As such, it is imperative that the primary focus on future studies should be on protecting these children from the toxic effects of anthracycline treatment, without reducing the antineoplastic activity of these therapies.
Executive summary.
Background
Anthracyclines are commonly used and are successful, in treating hematologic cancers and malignant neoplasms in both adults and children, but are tempered by their toxic-related health complications throughout life.
Mechanisms of anthracycline-induced cardiotoxicity
Despite more than three decades of use, it is still unclear exactly how anthracyclines exert their chemotherapeutic activity and induce cardiotoxic changes.
Risk factors for developing anthracycline-induced cardiotoxicity
Some of the known risk factors for developing anthracycline-induced cardiotoxicity include cumulative dose, female gender, younger age at diagnosis, longer follow-up and radiation therapy involving the heart.
Pathophysiology of anthracycline-induced cardiotoxicity
Although there is no consistently used, precise definition for anthracycline-induced cardiotoxicity, it is generally referred to in three categories on the basis of time-of-onset: acute changes, early onset chronic progressive cardiomyopathy and late-onset progressive cardiomyopathy.
Diagnosis & monitoring
The uncertainty regarding the strengths and weaknesses of different monitoring modalities and the lack of uniform monitoring schedules and methods can cause tests to detect early cardiac dysfunction in asymptomatic patients to become unnecessarily frequent or not frequent enough potentially resulting in serious safety or financial consequences.
Cardioprotection
Many cardioprotective strategies have been tested, but none have managed to protect the heart entirely from the cardiotoxic effects of anthracycline treatment.
Dexrazoxane is thought to be the only cardioprotectant proven to be effective against anthracycline treatment in randomized trials.
Treatment of cardiovascular disease
Growth hormone and angiotensin-converting enzyme inhibitors have each been investigated as a means to treat anthracycline-induced cardiomyopathy, but the cardiac improvements resulting from these treatments were only transient.
Conclusion
The association between anthracycline treatment and cardiotoxicity is apparent based on the evidence to date.
Further research is needed in developing evidence-based guidelines for monitoring cardiac dysfunction in patients treated with anthracyclines.
Given the success, but toxic nature, of anthracyclines in the treatment of cancer, there is an urgent need to find efficient cardioprotective strategies that can balance toxicity and oncologic efficacy.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 2.Hinkle AS, Proukou C, French CA, et al. A clinic-based, comprehensive care model for studying late effects in long-term survivors of pediatric illnesses. Pediatrics. 2004;113(Suppl 4):1141–1145. [PubMed] [Google Scholar]
- 3.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304(2):172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 4.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30(13):1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 6 ▪▪.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28(8):1276–1281. doi: 10.1200/JCO.2009.26.5751. Critically analyzes various aspects of the association between specific treatments and specific cardiovascular causes of death in childhood cancer treatment. It raises several critical questions in determining which screening modalities could be most useful. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the childhood cancer survivor study. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 8.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor study. Blood. 2008;111(12):5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9 ▪.Miller TL, Lipsitz SR, Lopez-Mitnik G, et al. Characteristics and determinants of adiposity in pediatric cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19(8):2013–2022. doi: 10.1158/1055-9965.EPI-10-0163. Determined that emerging long-term complications of childhood cancer survivors, such as adiposity, should be closely monitored. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Research Council. Chapter 4: late effects of childhood cancer. In: Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. National Academic Press; DC, USA: 2003. pp. 48–49. [PubMed] [Google Scholar]
- 11.Alvarez JA, Scully RE, Miller TL, et al. Long-term effects of treatments for childhood cancers. Curr Opin Pediatr. 2007;19:23–31. doi: 10.1097/MOP.0b013e328013c89e. [DOI] [PubMed] [Google Scholar]
- 12.van Dalen EC, Raphaël MF, Caron HN, Kremer LC. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev. 2009;1:CD006647. doi: 10.1002/14651858.CD006647.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Fulbright JM, Huh W, Anderson P, Chandra J. Can anthracycline therapy for pediatric malignancies be less cardiotoxic? Curr Oncol Rep. 2010;12(6):411–419. doi: 10.1007/s11912-010-0129-9. [DOI] [PubMed] [Google Scholar]
- 14.Herman EH, Ferrans VJ, Jordan W, Ardalan B. Reduction of chronic daunorubicin cardiotoxicity by ICRF-187 in rabbits. Res Commun Chem Pathol Pharmacol. 1981;31(1):85–97. [PubMed] [Google Scholar]
- 15.Herman EH, el-Hage AN, Ferrans VJ, Ardalan B. Comparison of the severity of the chronic cardiotoxicity produced by doxorubicin in normotensive and hypertensive rats. Toxicol Appl Pharmacol. 1985;78(2):202–214. doi: 10.1016/0041-008x(85)90284-4. [DOI] [PubMed] [Google Scholar]
- 16.Outomuro D, Grana DR, Azzato F, Milei J. Adriamycin-induced myocardial toxicity: new solutions for an old problem? Int J Cardiol. 2007;117(1):6–15. doi: 10.1016/j.ijcard.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Mordente A, Meucci E, Martorana GE, Giardina B, Minotti G. Human heart cytosolic reductases and anthracycline cardiotoxicity. IUBMB Life. 2001;52:83–88. doi: 10.1080/15216540252774829. [DOI] [PubMed] [Google Scholar]
- 18.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131(5):561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 19.Gianni L, Zweier JL, Levy A, Myers CE. Characterization of the cycle of iron-mediated electron transfer from adriamycin to molecular oxygen. J Biol Chem. 1985;260(11):6820–6826. [PubMed] [Google Scholar]
- 20.Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypothesis. FASEB J. 1990;4(13):3076–3086. [PubMed] [Google Scholar]
- 21.Milei J, Boveris A, Llesuy S, et al. Amelioration of adriamycin-induced cardiotoxicity in rabbits by prenylamine and vitamins A and E. Am Heart J. 1986;111:95–102. doi: 10.1016/0002-8703(86)90559-4. [DOI] [PubMed] [Google Scholar]
- 22.Ferrero ME, Ferrero E, Gaja U. Adriamycin: energy metabolism and mitochondrial oxidations in the heart of treated rabbits. Biochem Pharmacol. 1976;25(2):125–130. doi: 10.1016/0006-2952(76)90278-1. [DOI] [PubMed] [Google Scholar]
- 23.Earm YE, Ho WK, So I. Effects of adriamycin on ionic currents in single cardiac myocytes of the rabbit. J Mol Cell Cardiol. 1994;26(2):163–172. doi: 10.1006/jmcc.1994.1019. [DOI] [PubMed] [Google Scholar]
- 24.Olson RD, Li X, Palade P, et al. Sarcoplasmic reticulum calcium release is stimulated and inhibited by daunorubicin and daunorubicinol. Toxicol Appl Pharmacol. 2000;169(2):168–176. doi: 10.1006/taap.2000.9065. [DOI] [PubMed] [Google Scholar]
- 25.Gozalvez M, Blanco M. Inhibition of NA-K ATPase by the antitumor antibiotic adriamycin. 5th International Biophysics Congress, Copenhagen. Cancer Res. 1979;39(1):257–261. [PubMed] [Google Scholar]
- 26.Lou H, Danelisen I, Singal PK. Cytokines are not upregulated in adriamycin-induced cardiomyopathy and heart failure. J Mol Cell Cardiol. 2004;36(5):683–690. doi: 10.1016/j.yjmcc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Lenaz L, Page JA. Cardiotoxicity of adriamycin and related anthracyclines. Cancer Treat Rev. 1976;3(3):111–120. doi: 10.1016/s0305-7372(76)80018-7. [DOI] [PubMed] [Google Scholar]
- 28.Lipshultz SE, Rusconi P, Scully RE. Chapter 18: assessment of cardiotoxicity during anti-cancer therapy. In: Januzzi JL, Bayes-Genis A, editors. NT-proBNP as a Biomarker in Cardiovascular Diseases. Prous Science SA; Barcelona, Spain: 2007. pp. 193–198. [Google Scholar]
- 29.Pointon AV, Walker TM, Phillips KM, et al. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE. 2010;5(9):e12733. doi: 10.1371/journal.pone.0012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12(5):877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 31.Goormaghtigh E, Brasseur R, Huart P, Ruysschaert JM. Study of the adriamycin cardiolipin complex structure using attenuated total reflection infrared spectroscopy. Biochemistry. 1987;26(6):1789–1794. doi: 10.1021/bi00380a043. [DOI] [PubMed] [Google Scholar]
- 32.Garcia Fernandez M, Troiano L, Moretti L. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ. 2002;13(9):449–455. [PubMed] [Google Scholar]
- 33.Aguilar L, Ortega-Pierres G, Campos B, et al. Phospholipid membranes form specific nonbilayer molecular arrangements that are antigenic. J Biol Chem. 1999;274(36):25193–25196. doi: 10.1074/jbc.274.36.25193. [DOI] [PubMed] [Google Scholar]
- 34.Vlasova II, Tyurin VA, Kapralov AA, et al. Nitric oxide inhibits peroxidase activity of cytochrome C cardiolipin complex and blocks cardioliopin oxidation. J Biol Chem. 2006;281(21):14554–14562. doi: 10.1074/jbc.M509507200. [DOI] [PubMed] [Google Scholar]
- 35.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocte apoptosis. Cancer Res. 2000;60(7):1789–1792. [PubMed] [Google Scholar]
- 36.Zhang YQ, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp. 2009;57(6):435–445. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44(7):600–606. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 38 ▪.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324(12):808–815. doi: 10.1056/NEJM199103213241205. Established that that doxorubicin impairs myocardial growth resulting in reduced contractility in a dose-dependent manner. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen K, Levitt G, Bull C, Chessells J, Sullivan I. Anthracycline dose in childhood acute lymphoblastic leukemia: issues of early survival versus late cardiotoxicity. J Clin Oncol. 1997;15(1):61–68. doi: 10.1200/JCO.1997.15.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Giantris A, Abdurrahman L, Hinkle A, et al. Anthracycline-induced cardiotoxicity in children and young adults. Crit Rev Oncol Hematol. 1998;27:53–68. doi: 10.1016/s1040-8428(97)10007-5. [DOI] [PubMed] [Google Scholar]
- 41.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65(1):128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascales A, Sanchez-Vega B, Navarro N, Pastor-Quirante F, Corral J, Vicente V, de la Pena FA. Clinical and genetic determinants of anthracycline-induced cardiac iron accumulation. Int J Cardiol. 2010;154(3):282–286. doi: 10.1016/j.ijcard.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 43.Arai M, Yoguchi A, Takizawa T, et al. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca2+-ATPase gene transcription. Circ Res. 2000;86(1):8–14. doi: 10.1161/01.res.86.1.8. [DOI] [PubMed] [Google Scholar]
- 44.Jeyaseelan R, Poizat C, Wu HY, Kedes L. Molecular mechanisms of doxorubicin-induced cardiomyopathy. Selective suppression of Reiske iron-sulfur protein, ADP/ATP translocase, and phosphofructokinase genes is associated with ATP depletion in rat cardiomyocytes. J Biol Chem. 1997;272(9):5828–5832. doi: 10.1074/jbc.272.9.5828. [DOI] [PubMed] [Google Scholar]
- 45.Lebrecht DMS, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. Time-dependant and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003;108(19):2423–2429. doi: 10.1161/01.CIR.0000093196.59829.DF. [DOI] [PubMed] [Google Scholar]
- 46.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocte apoptosis. Cancer Res. 2000;60(7):1789–1792. [PubMed] [Google Scholar]
- 47.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62(16):4592–4598. [PubMed] [Google Scholar]
- 48.Von Hoff DD, Layard M, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 49.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 50.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42(18):3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 51.van der Pal HJ, van Dalen EC, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170(14):1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 52.Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes – a report from the Children's Oncology Group. J Clin Oncol. 2012;30(13):1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Dalen EC, Caron HN, Dickinson HO, Kremer LCM. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2005;25(1):CD003917. doi: 10.1002/14651858.CD003917.pub2. Update in: Cochrane Database Syst. Rev. 2011(6), CD003917. [DOI] [PubMed] [Google Scholar]
- 54 ▪▪.Lipshultz SE, Lipsitz SR, Sallen SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629–2636. doi: 10.1200/JCO.2005.12.121. Cardiac dysfunction can be persistent and progressive in childhood survivors of acute lymphoblastic leukemia. Dilated cardiomyopathy can develop in these children after treatment; however, this study also found that as time passed the children expressed a restrictive cardiomyopathy pattern in their measurements. [DOI] [PubMed] [Google Scholar]
- 55.Davies SM. Getting to the heart of the matter. J Clin Oncol. 2012;30(13):1399–1400. doi: 10.1200/JCO.2011.40.1422. [DOI] [PubMed] [Google Scholar]
- 56.Visscher H, Ross CJD, Rassekh SR, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30(13):1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 57.Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 58.Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003;97(8):1991–1998. doi: 10.1002/cncr.11274. [DOI] [PubMed] [Google Scholar]
- 59.Nysom K, Holm K, Lipsitz SR, et al. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;2:545–550. doi: 10.1200/JCO.1998.16.2.545. [DOI] [PubMed] [Google Scholar]
- 60.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 61.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266(12):1672–1677. [PubMed] [Google Scholar]
- 62.Anderlini P, Bengamin RS, Wong FC, et al. Idarubicin cardiotoxicity: a retrospective study in acute myeloid leukemia and myelodysplasia. J Clin Oncol. 1995;13(11):2827–2834. doi: 10.1200/JCO.1995.13.11.2827. [DOI] [PubMed] [Google Scholar]
- 63.Sorensen K, Levitt G, Sebag-Montefore D, Bull C, Sullivan I. Cardiac function in Wilms' tumor survivors. J Clin Oncol. 1995;13(7):1546–1556. doi: 10.1200/JCO.1995.13.7.1546. [DOI] [PubMed] [Google Scholar]
- 64.Nysom K, Colan DC, Lipshultz SE. Late cardiotoxicity following anthracycline therapy for childhood cancer. Prog Pediatr Cardiol. 1998;8:121–138. [Google Scholar]
- 65.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol. 2001;19(7):1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 66.Kremer LCM, van Dalen EC, Offringa M, Ottenkamp J, Voûte PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19(1):191–196. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 67.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28(8):1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 68.Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol. 1998;6(8):1321–1327. doi: 10.1200/JCO.1988.6.8.1321. [DOI] [PubMed] [Google Scholar]
- 69.Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med. 1978;65(5):823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- 70 ▪▪.Lipshultz SE, Miller TM, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30(10):1042–1049. doi: 10.1200/JCO.2010.30.3404. Early detection of damage or death to the cardiomyocytes is crucial to individualization of treatment and effective prevention. This study found that cardiac troponin T and N-terminal probrain natriuretic peptide hold promise as biomarkers of cardiotoxicity for children with high-risk acute lymphoblastic leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali MK, Ewer MS, Gibbs HR, Swafford J, Graff KL. Late doxorubicin-associated cardiotoxicity in children. The possible role of intercurrent viral infection. Cancer. 1994;74(1):182–188. doi: 10.1002/1097-0142(19940701)74:1<182::aid-cncr2820740129>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 72.Tolba KA, Deliargyris EN. Cardiotoxicity of cancer therapy. Cancer Invest. 1999;17(6):408–422. doi: 10.3109/07357909909021433. [DOI] [PubMed] [Google Scholar]
- 73.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 74.Grenier MA, Lipshultz ME. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol. 1998;25(4 Suppl 10):72–85. [PubMed] [Google Scholar]
- 75.Simbre VC, Duffy SA, Dadlani GH, Miller TL, Lipshultz SE. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatr Drugs. 2005;7:187–202. doi: 10.2165/00148581-200507030-00005. [DOI] [PubMed] [Google Scholar]
- 76.Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–1058. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 77.Herman EH, Zhang J, Lipshultz SE, et al. Correlation between serum levels of cardiac troponin-T and the severity of the chronic cardiomyopathy induced by doxorubicin. J Clin Oncol. 1999;17:2237–2243. doi: 10.1200/JCO.1999.17.7.2237. [DOI] [PubMed] [Google Scholar]
- 78.Lipshultz SE, Sanders SP, Goorin AM, Krischer JP, Sallan SE, Colan SD. Monitoring for anthracycline cardiotoxicity. Pediatrics. 1994;93:433–437. [PubMed] [Google Scholar]
- 79.Carver JR, Shapiro CL, NG A, et al. ASCO Cancer Survivorship Exert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 80.Steinhertz LJ, Graham T, Hurwitz R, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: Report of the cardiology committee of the Children's Cancer Study Group. Pediatrics. 1992;89:942–949. [PubMed] [Google Scholar]
- 81.Lipshultz SE, Sanders SP, Colan SD, Goorin AM, Sallan SE, Krischer JP. Letter to the Editor. Pediatrics. 1994;94:781. [PubMed] [Google Scholar]
- 82.Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. 2003;13(3):346–356. doi: 10.1016/S1053-4296(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 83.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45(1):55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 84.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351(2):145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 85.Ganame J, Claus P, Eyskens B, et al. Acute cardiac functional and morphological changes after anthracycline infusions in children. Am J Cardiol. 2007;99(7):974–977. doi: 10.1016/j.amjcard.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 86.Ganame J, Claus P, Uyttebroeck A, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. 2007;20(12):1351–1358. doi: 10.1016/j.echo.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Colan SD, Borrow KM, Neuman A. Left ventricular end-systolic wall stress-velocity of fiber shortening relation: a load independent index of myocardial contractility. J Am Coll Cardiol. 1984;4:715–724. doi: 10.1016/s0735-1097(84)80397-6. [DOI] [PubMed] [Google Scholar]
- 88.Lipshultz SE, Colan SD. Cardiac Toxicity After Treatment for Childhood Cancer. Wiley-Liss; NY, USA: 1993. The use of echocardiography and holter monitoring in the assessment of anthracycline-treated patients; pp. 45–62. [Google Scholar]
- 89.Mitani I, Jain D, Joska TM, Burtness B, Zaret BL. Doxorubicin cardiotoxicity: Prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol. 2003;10(2):132–139. doi: 10.1067/mnc.2003.7. [DOI] [PubMed] [Google Scholar]
- 90.Glanzmann C, Huguenin P, Lutolf UM, Maire R, Jenni R, Gumppenberg V. Cardiac lesions after mediastinal radiation for Hodgkin's disease. Radiother Oncol. 1994;30(1):43–54. doi: 10.1016/0167-8140(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 91.Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997;96(8):2641–2648. doi: 10.1161/01.cir.96.8.2641. [DOI] [PubMed] [Google Scholar]
- 92.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339(5):321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 93.Cowie MR, Mendez GF. BNP and congestive heart failure. Prog Cardiovasc Dis. 2002;44(4):293–321. doi: 10.1053/pcad.2002.24599. [DOI] [PubMed] [Google Scholar]
- 94.Linssen GC, Bakker SJ, Voors AA, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J. 2010;31(1):120–127. doi: 10.1093/eurheartj/ehp420. [DOI] [PubMed] [Google Scholar]
- 95.Bibbins-Domingo K, Gupta R, Na B, et al. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trachtenberg BH, Landy DC, Franco VI, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. 2011;32:342–353. doi: 10.1007/s00246-010-9878-3. [DOI] [PubMed] [Google Scholar]
- 97.Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiovascular effects in childhood cancer survivors treated with anthracyclines. Cardiol Res Pract. 2011;10:134679. doi: 10.4061/2011/134679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwartz CL, Hobbie WL, Truesdell S, Constine LC, Clark EB. Corrected QT interval prolongation in anthracycline-treated survivors of childhood cancer. J Clin Oncol. 1993;11(10):1906–1910. doi: 10.1200/JCO.1993.11.10.1906. [DOI] [PubMed] [Google Scholar]
- 99.Larsen RL, Barber G, Heise CT, August CS. Exercise assessment of cardiac function in children and young adults before and after bone marrow transplantation. Pediatrics. 1992;89(4 Pt 2):722–729. [PubMed] [Google Scholar]
- 100.Weesner KM. Exercise echocardiography in the detection of anthracycline cardiotoxicity. Cancer. 1991;68(2):435–438. doi: 10.1002/1097-0142(19910715)68:2<435::aid-cncr2820680237>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 101 ▪.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22(15):3139–3148. doi: 10.1200/JCO.2004.09.109. Identifies several, often unsuspected, cardiovascular abnormalities found in long-term survivors of Hodgkin's disease. [DOI] [PubMed] [Google Scholar]
- 102.Hogenhuis J, Jaarsma T, Voors AA, Hillege HL, Lesman I, van Veldhuisen DJ. Correlates of B-type natriuretic peptide and 6-min walk in heart failure patients. Int J Cardiol. 2006;108(1):63–67. doi: 10.1016/j.ijcard.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Ryberg M, Nielsen D, Skovsgaard T, Hansen J, Jensen BV, Dombernowsky P. Epirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J Clin Oncol. 1998;16(11):3502–3508. doi: 10.1200/JCO.1998.16.11.3502. [DOI] [PubMed] [Google Scholar]
- 104.Legha SS, Benjamin RS, Mackay B, et al. Adriamycin therapy by continuous intravenous infusion in patients with metastatic breast cancer. Cancer. 1982;49(9):1762–1766. doi: 10.1002/1097-0142(19820501)49:9<1762::aid-cncr2820490905>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 105.Hortobagyi GN, Frye D, Buzdar AU, et al. Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer. 1989;63(1):37–45. doi: 10.1002/1097-0142(19890101)63:1<37::aid-cncr2820630106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 106.Shapira J, Gotfried M, Lishner M, Ravid M. Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regiment. A prospective evaluation. Cancer. 1990;65(4):870–873. doi: 10.1002/1097-0142(19900215)65:4<870::aid-cncr2820650407>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 107 ▪▪.Lipshultz SE, Giantris AL, Lipsitz SR, et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91–01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20(6):1677–1682. doi: 10.1200/JCO.2002.20.6.1677. Pediatric protocols have incorporated continuous infusion of anthracyclines as a means of cardioprotection based on adult findings. However, this study found that continuous doxorubicin infusion over 48 h provided no cardioprotective advantage when compared with bolus infusion. Additionally, continuous infusion can result in longer hospital stays and decreased quality of life for the patient. [DOI] [PubMed] [Google Scholar]
- 108.Levitt GA, Dorup I, Sorensen K, Sullivan I. Does anthracycline administration by infusion in children affect late cardiotoxicity? Br J Haematol. 2004;24(4):463–468. doi: 10.1111/j.1365-2141.2004.04803.x. [DOI] [PubMed] [Google Scholar]
- 109.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339(13):900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 110.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19(6):670–686. [PubMed] [Google Scholar]
- 111.Ganzina F. 4′-epi-doxorubicin, a new analogue of doxorubicin: a preliminary overview of preclinical and clinical data. Cancer Treat Rev. 1983;10(1):1–22. doi: 10.1016/s0305-7372(83)80029-2. [DOI] [PubMed] [Google Scholar]
- 112.Lahtinen R, Kuikka J, Nousiainene T, Uusituoa M, Lansimies E. Cardiotoxicity of epirubicin and doxorubicin: a double-blind randomized study. Eur J Haematol. 1991;46(5):301–305. doi: 10.1111/j.1600-0609.1991.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 113.Cottin Y, Touzery C, Dalloz F, et al. Comparison of epirubicin and doxorubicin cardiotoxicity induced by low doses: evolution of the diastolic and systolic parameters studies by radionuclide angiography. Clin Cardiol. 1998;21(9):665–670. doi: 10.1002/clc.4960210911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dorr RT, Shipp NG, Lee KM. Comparison of cytotoxicity in heart cells and tumor cells exposed to DNA intercalating agents in vitro. Anticancer Drugs. 1991;2(1):27–33. doi: 10.1097/00001813-199102000-00003. [DOI] [PubMed] [Google Scholar]
- 115.Alderton PM, Gross J, Green MD. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Res. 1992;52(1):194–201. [PubMed] [Google Scholar]
- 116.Herman EH, Zhang J, Hasinoff BB, Clark JRJ, Ferrans VJ. Comparison of the structural changes induced by doxorubicin and mitoxantrone in the heart, kidney and intestine and characterization of the Fe(III)-mitoxantrone complex. J Mol Cell Cardiol. 1997;29(9):2415–2430. doi: 10.1006/jmcc.1997.0477. [DOI] [PubMed] [Google Scholar]
- 117.Creutzig U, Ritter J, Zimmermann M, et al. Idarubicin improves blast cell clearance during induction therapy in children with AML: results of study AML-BFM 93. AML-BFM Study Group. Leukemia. 2001;15(3):348–354. doi: 10.1038/sj.leu.2402046. [DOI] [PubMed] [Google Scholar]
- 118.Tardi PG, Boman NL, Cullis PR. Liposomal doxorubicin. J Drug Target. 1996;4(3):129–140. doi: 10.3109/10611869609015970. [DOI] [PubMed] [Google Scholar]
- 119.Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19(5):1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 120.Harris L, Batist G, Belt R, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multi-center trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94(1):25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 121.Safra T. Cardiac safety of liposomal anthracyclines. Oncologist. 2003;8(2):17–24. doi: 10.1634/theoncologist.8-suppl_2-17. [DOI] [PubMed] [Google Scholar]
- 122.Gabizon AA. Liposomal anthracyclines. Hematol Oncol Clin North Am. 1994;8(2):431–450. [PubMed] [Google Scholar]
- 123.Money-Kyrle JF, Bates F, Ready J, Gazzard BG, Phillips RH, Boag FC. Liposomal daunorubicin in advanced Kaposi's sarcoma: a Phase II study. Clin Oncol (R Coll Radiol) 1993;5(6):367–371. doi: 10.1016/s0936-6555(05)80088-3. [DOI] [PubMed] [Google Scholar]
- 124.Gill PS, Wernz J, Scadden DT. Randomized Phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J Clin Oncol. 1996;14(8):2353–2364. doi: 10.1200/JCO.1996.14.8.2353. [DOI] [PubMed] [Google Scholar]
- 125.O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a Phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 126.Sieswerda E, Kremer LC, Caron HN, van Dalen EC. The use of liposomal anthracycline analogues for childhood malignancies: a systematic review. Eur J Cancer. 2011;47(13):2000–2008. doi: 10.1016/j.ejca.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 127.Zimethbaum P, Eder H, Frishman W. Probucol: pharmacology and clinical application. J Clin Pharmacol. 1990;30(1):3–9. doi: 10.1002/j.1552-4604.1990.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 128.Carew TE, Schwenke DC, Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci USA. 1987;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuzuya M, Kuzuya F. Probucol as an antioxidant and antiatherogenic drug. Free Radic Biol Med. 1993;14(1):67–77. doi: 10.1016/0891-5849(93)90510-2. [DOI] [PubMed] [Google Scholar]
- 130.Siveski-Iliskovic N, Hill M, Chow DA, Singhal PK. Probucol protects against doxorubicin cardiomyopathy without interfering with its antitumor effect. Circulation. 1995;91(1):10–15. doi: 10.1161/01.cir.91.1.10. [DOI] [PubMed] [Google Scholar]
- 131.Singal PK, Deally CM, Weinberg LE. Subcellular effects of doxorubicin in the heart. A concise review. J Mol Cell Cardiol. 1987;19(8):817–828. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- 132.Iliskovic N, Singal PK. Lipid lowering: an important factor in preventing doxorubicin-induced heart failure. Am J Pathol. 1997;150(2):727–734. [PMC free article] [PubMed] [Google Scholar]
- 133.Iliskovic N, Hasinoff BB, Malisza KL, Li T, Danelisen I, Singal PK. Mechanisms of beneficial effects of probucol in doxorubicin cardiomyopathy. Mol Cell Biochem. 1999;196(1–2):43–49. [PubMed] [Google Scholar]
- 134.Li T, Danelisen I, Bello-Klein A, Singal PK. Effects of probucol on changes of antioxidant enzymes in doxorubicin-induced cardiomyopathy in rats. Cardiovasc Res. 2000;46(3):523–530. doi: 10.1016/s0008-6363(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 135.Li T, Singal PK. Adriamycin-induced early changes in myocardial antioxidant enzymes and their modulation by probucol. Circulation. 2000;102(17):2105–2110. doi: 10.1161/01.cir.102.17.2105. [DOI] [PubMed] [Google Scholar]
- 136.De Flora S, Bennicelli C, Serra D, Izzotti A, Cesarone CF. Role of glutathione and N-acetylcysteine on the mutagenicity and carcinogenesis. In: Friedman M, editor. Absorption and Utilization of Amino Acids (Volume 3) CRC Press; FL, USA: 1989. pp. 19–53. [Google Scholar]
- 137.Myers CE, Bonow R, Palmeri S, et al. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. Semin Oncol. 1983;10(1):53–55. [PubMed] [Google Scholar]
- 138.Frishman WH. Carvedilol. Drug Therapy. 1998;339(24):1759–1765. doi: 10.1056/NEJM199812103392407. [DOI] [PubMed] [Google Scholar]
- 139.Yue TL, Cheng HY, Lysko PG, et al. Carvedilol, a new vasodilator and beta adrenoreceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exper Therap. 1992;263(1):92–98. [PubMed] [Google Scholar]
- 140.Feuerstein GZ, Ruffolo RR., Jr Carvedilol, a novel multiple action antihypertensive agent with antioxidant activity and the potential for myocardial and vascular protection. Eur Heart J. 1995;16:38–42. doi: 10.1093/eurheartj/16.suppl_f.38. [DOI] [PubMed] [Google Scholar]
- 141.Noguchi N, Nishino K, Niki E. Antioxidant action of the antihypertensive drug, carvedilol, against lipid peroxidation. Biochem Pharmacol. 2000;59(9):1069–1076. doi: 10.1016/s0006-2952(99)00417-7. [DOI] [PubMed] [Google Scholar]
- 142.Matsui H, Morishima I, Numaguchi Y, Toki Y, Okumura K, Hayakawa T. Protective effects of carvedilol against doxorubicin-induced cardiomyopathy in rats. Life Sci. 1999;65(12):1265–1274. doi: 10.1016/s0024-3205(99)00362-8. [DOI] [PubMed] [Google Scholar]
- 143.Fazio S, Palmieri EA, Ferravante B, Bone F, Biondi B, Sacca L. Doxorubicin-induced cardiomyopathy treated with carvedilol. Clin Cardiol. 1998;21(10):777–779. doi: 10.1002/clc.4960211017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spallarossa P, Garibaldi S, Altieri P, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37(4):837–846. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 145.Nakamae H, Tsumura K, Terada Y, et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer. 2005;104(11):2492–2498. doi: 10.1002/cncr.21478. [DOI] [PubMed] [Google Scholar]
- 146.Tokudome T, Mizushige K, Noma T, et al. Prevention of doxorubicin (adriamycin)-induced cardiomyopathy by simultaneous administration of angiotensin-converting enzyme inhibitor assessed by acoustic densitometry. J Cardiovasc Pharmacol. 2000;36(3):361–368. doi: 10.1097/00005344-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 147.Fischer PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with Sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111(13):1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 148.Hanley PJ, Dröse S, Brandt U, et al. 5-hydroxydecanoate is metabolised in mitochondria and creates a rate-limiting bottleneck for beta-oxidation of fatty acids. J Physiol. 2005;562(2):307–318. doi: 10.1113/jphysiol.2004.073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Di X, Gennings C, Bear HD, et al. Influence of the phosphodiesterase-5 inhibitor, Sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat. 2010;124(2):349–360. doi: 10.1007/s10549-010-0765-7. [DOI] [PubMed] [Google Scholar]
- 150.Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001;108(5):671–680. doi: 10.1067/mai.2001.119555. [DOI] [PubMed] [Google Scholar]
- 151.Asmis R, Wang Y, Xu L, Ksgati M, Begley JG, Mieyal JJ. A novel thiol oxidation-based mechanism for adriamycin-induced cell injury in human macrophages. FASEB J. 2005;19(13):1866–1868. doi: 10.1096/fj.04-2991fje. [DOI] [PubMed] [Google Scholar]
- 152.Li W, Lam MS, Birkeland A, et al. Cell-based assays for profiling activity and safety properties of cancer drugs. J Pharmacol Toxicol Methods. 2006;54(3):313–319. doi: 10.1016/j.vascn.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 153.Raja SG, Danton MD, MacArthur KJ, Pollock JC. Effects of escalating doses of sildenafil on hemodynamics and gas exchange in children with pulmonary hypertension and congenital cardiac defects. J Cardiothorac Vasc Anesth. 2007;21(2):203–207. doi: 10.1053/j.jvca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 154.Karatza AA, Bush A, Magee AG. Safety and efficacy of sildenafil therapy in children with pulmonary hypertension. Int J Cardiol. 2005;100(2):267–273. doi: 10.1016/j.ijcard.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 155.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117(4):1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 156.Juliana AE, Abbad FCB. Severe persistent pulmonary hypertension of the newborn in a setting where limited resources exclude the use of inhaled nitric oxide: successful treatment with sildenafil. Eur J Pediatr. 2005;164(10):626–629. doi: 10.1007/s00431-005-1724-x. [DOI] [PubMed] [Google Scholar]
- 157.Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148(3):293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 158.Konishi M, Haraguchi G, Ohigashi H, et al. Adiponectin protects against doxorubicin-induced cardiomyopathy by anti-apoptotic effects through AMPK upregulation. Cardiovasc Res. 2011;89(2):309–319. doi: 10.1093/cvr/cvq335. [DOI] [PubMed] [Google Scholar]
- 159.Li L, Takemura G, Li Y, et al. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113(4):535–543. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- 160.Hasinoff BB. The interaction of the cardioprotective agent ICRF-187 [+)-1, 2-bis(3, 5 dioxopiperazinyl-1-yL)propane); its hydrolysis product (ICRF-198); and other chelating agents with the Fe(III) and Cu(II) complexes of doxorubicin. Agents Actions. 1989;26(3–4):378–385. doi: 10.1007/BF01967305. [DOI] [PubMed] [Google Scholar]
- 161.Speyer JL, Green MD, Zeleniuch-Jacquotte A, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol. 1992;10(1):117–127. doi: 10.1200/JCO.1992.10.1.117. [DOI] [PubMed] [Google Scholar]
- 162.Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol. 1997;15(4):1333–1340. doi: 10.1200/JCO.1997.15.4.1333. [DOI] [PubMed] [Google Scholar]
- 163.Wexler LH, Andrich MP, Venzon D, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14(2):362–372. doi: 10.1200/JCO.1996.14.2.362. [DOI] [PubMed] [Google Scholar]
- 164.Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15(4):1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 165.Lopez M, Vici P, Di Lauro L, et al. Randomized prospective clinical trial to evaluate cardioprotection of dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998;16(1):86–92. doi: 10.1200/JCO.1998.16.1.86. [DOI] [PubMed] [Google Scholar]
- 166▪▪.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11(10):950–961. doi: 10.1016/S1470-2045(10)70204-7. Dexrazoxane is a clinically important cardioprotectant. This study found that dexrazoxane provides long-term cardioprotection in children with high-risk acute lymphoblastic leukemia without compromising the oncological efficacy of doxorubicin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 168.Herman EH, Zhang J, Rifai N, et al. The use of serum levels of cardiac troponin T to compare the protective activity of dexrazoxane against doxorubicin- and mitoxantrone-induced cardiotoxicity. Cancer Chemother Pharmacol. 2001;48(4):297–304. doi: 10.1007/s002800100348. [DOI] [PubMed] [Google Scholar]
- 169▪▪.Barry EV, Vrooman LM, Dahlberg SE, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008;26:1106–1111. doi: 10.1200/JCO.2007.12.2481. It has been hypothesized that dexrazoxane treatment increases risk of secondary malignant neoplasms. This study found that dexrazoxane was not associated with increased risk of secondary malignant neoplasms in children treated for high-risk acute lymphoblastic leukemia. [DOI] [PubMed] [Google Scholar]
- 170.Vrooman LM, Neuber DS, Stevenson KE, et al. The low incidence of secondary acutemyelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47(9):1373–1379. doi: 10.1016/j.ejca.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schuchter LM, Hensley ML, Meropol NJ, Winer EP. Update of recommendations for the use of chemotherapy and radiotherapy protectants: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2002;20(12):2895–2903. doi: 10.1200/JCO.2002.04.178. [DOI] [PubMed] [Google Scholar]
- 172.Yang JL, Fernandes DJ, Speicher L, Capizzi RL. Biochemical determinants of the cytoprotective effect of amifostine. Proc Am Cancer Res. 1995;36:3725. [Google Scholar]
- 173.Calabro-Jones PM, Aguilera JA, Ward JF, Smoluk GD, Fahey RC. Uptake of WR-2721 derivatives by cells in cultures: identification of the transported form of the drug. Cancer Res. 1988;48(13):3634–3640. [PubMed] [Google Scholar]
- 174.Nazeyrollas P, Frances C, Prevost A, et al. Efficiency of amifostine as a protection against doxorubicin toxicity in rats during a 12-day treatment. Anticancer Res. 2003;23(1A):405–409. [PubMed] [Google Scholar]
- 175.Dragojevic-Simic VM, Dobric SL, Bokonjic DR, et al. Amifostine protection against doxorubicin cardiotoxicity in rats. Anticancer Drugs. 2004;15(2):169–178. doi: 10.1097/00001813-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 176.Herman EH, Ferrans VJ. Reduction of chronic doxorubicin cardiotoxicity in dogs by pretreatment with (+/-)-1, 2-bis (3, 5-dioxopipreazine-1-yl) propane (ICRF-187) Cancer Res. 1981;41(9 Pt 1):3436–3440. [PubMed] [Google Scholar]
- 177.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 178.Haq MM, Legha SS, Choski J, et al. Doxorubicin-induced congestive heart failure in adults. Cancer. 1985;56(6):1361–1365. doi: 10.1002/1097-0142(19850915)56:6<1361::aid-cncr2820560624>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 179.Redfeld MM, Gersh BJ, Bailey KR, Rodeheffer RJ. Natural history of incidentally discovered asymptomatic idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;74(7):737–739. doi: 10.1016/0002-9149(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 180.Sieswerda E, van Dalen EC, Postma A, Cheuk DK, Caron HN, Kremer LC. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2011;7(9):CD008011. doi: 10.1002/14651858.CD008011.pub2. [DOI] [PubMed] [Google Scholar]
- 181.Lipshultz SE, Vlach SA, Lipsitz SR, et al. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics. 2005;115:1613–1622. doi: 10.1542/peds.2004-1004. [DOI] [PubMed] [Google Scholar]
- 182.Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22(5):820–828. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 183.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood. Cancer J Clin Oncol. 2002;20:4517–4522. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 184.Mertens AC, Sencer S, Myers CD, et al. Complementary and alternative therapy use in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor study. Pediatr Blood Cancer. 2008;50:90–97. doi: 10.1002/pbc.21177. [DOI] [PubMed] [Google Scholar]
- 185.Ibsen S, Zahavy E, Wrasdilo W, Berns M, Chan M, Esener S. A novel doxorubicin prodrug with controllable photolysis activation for cancer chemotherapy. Pharm Res. 2010;27(9):1848–1860. doi: 10.1007/s11095-010-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Young K, Hare JM. Stem cells in cardiopulmonary development: Implications for novel approaches to therapy for pediatric cardiopulmonary diseases. Progr Pediatr Cardiol. 2008;25(3):37–49. [Google Scholar]
- 187.Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 188.Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol. 2004;44(8):1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 189.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomized controlled clinical trial. Lancet. 2004;364:10–16. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 190.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOnemarrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 191.Pillekamp F, Reppel M, Brockmeier K, Hescheler J. Stem cells and their potential relevance to paediatric cardiology. Cardiol Young. 2006;16(2):117–124. doi: 10.1017/S1047951106000023. [DOI] [PubMed] [Google Scholar]
- 201.Childrens Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. 2008 www.survivorshipguidelines.org.