Abstract
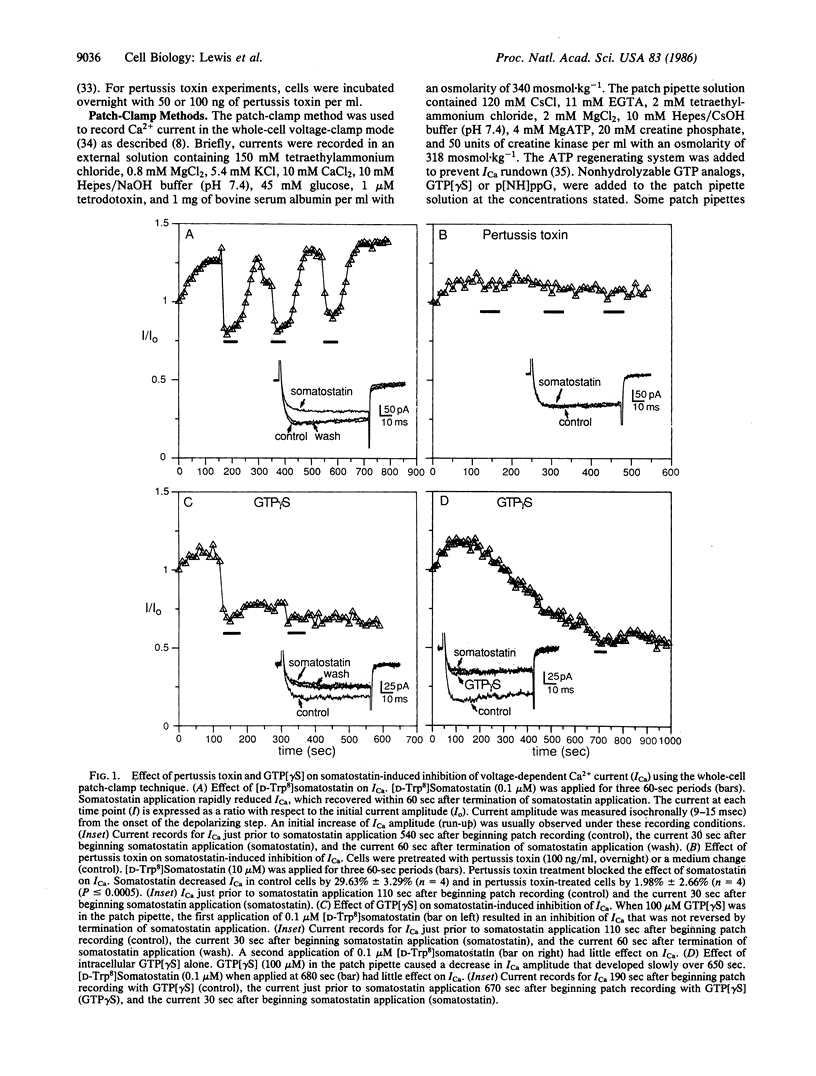
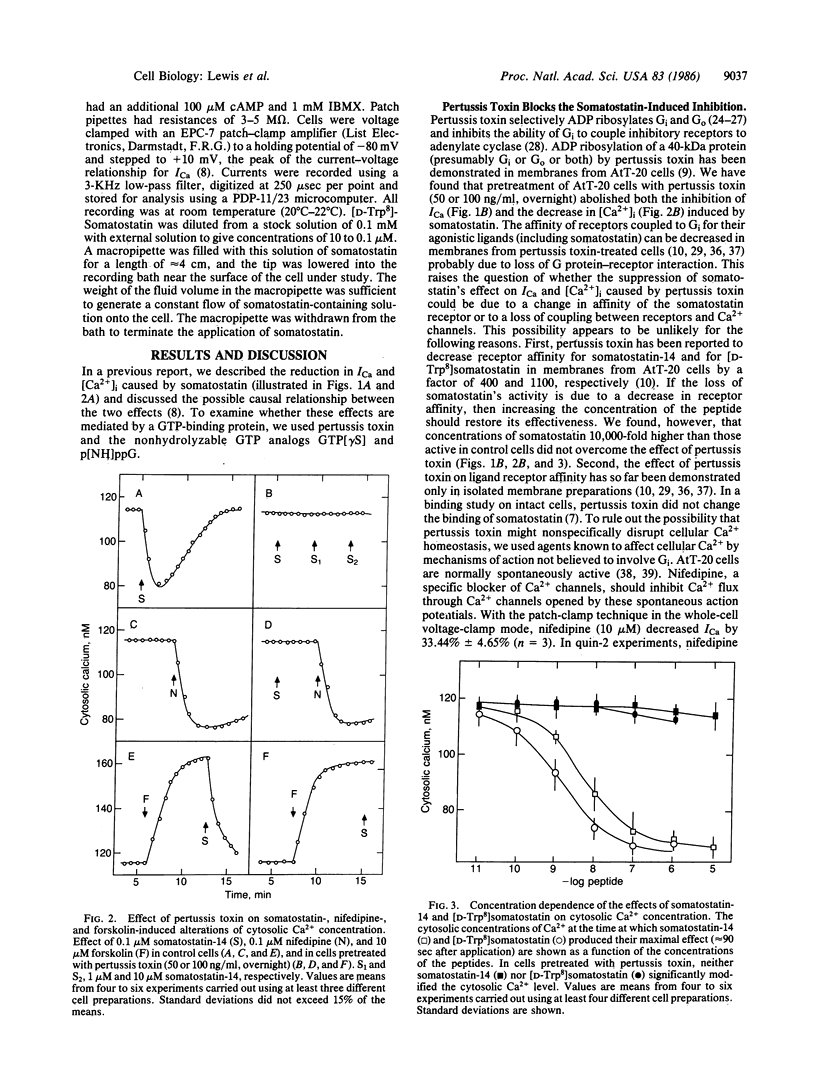
Somatostatin reduces voltage-dependent Ca2+ current (ICa) and intracellular free Ca2+ concentration in the AtT-20/D16-16 pituitary cell line. We tested whether guanine nucleotide-binding proteins (G or N proteins) are involved in the signal transduction mechanism between the somatostatin receptor and voltage-dependent Ca2+ channels. Treatment of the cells with pertussis toxin, which selectively ADP ribosylates the GTP binding proteins Gi and Go and suppresses the ability of Gi to couple inhibitory receptors to adenylate cyclase, abolished the action of somatostatin on both ICa and intracellular free Ca2+. Intracellular application of the nonhydrolyzable guanine nucleotide analog guanosine 5'-[gamma-thio]triphosphate (GTP[gamma S]), which irreversibly activates G proteins, changed the somatostatin effect on ICa from a reversible to an irreversible inhibition. Intracellular GTP[gamma S] alone caused a very slowly developing inhibition of ICa. When ICa was inhibited by GTP[gamma S] (alone or with somatostatin), it failed to respond to subsequent applications of somatostatin. The effect of GTP[gamma S] on the inhibition of ICa by somatostatin was not altered by the intracellular application of cAMP and 3-isobutyl-1-methylxanthine. The results suggest that a GTP-binding protein is directly involved in the cAMP-independent receptor-mediated inhibition of voltage-dependent Ca2+ channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Wong B. S., Sabol S. L., Busis N., Jackson M. B., Weight F. F. Action potentials and membrane ion channels in clonal anterior pituitary cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2086–2090. doi: 10.1073/pnas.80.7.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Codina J., Mattera R., Cerione R. A., Hildebrandt J. D., Sunyer T., Rojas F. J., Caron M. G., Lefkowitz R. J., Iyengar R. Regulation of hormone receptors and adenylyl cyclases by guanine nucleotide binding N proteins. Recent Prog Horm Res. 1985;41:41–99. doi: 10.1016/b978-0-12-571141-8.50006-8. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Cote T. E., Frey E. A., Sekura R. D. Altered activity of the inhibitory guanyl nucleotide-binding component (Ni) induced by pertussis toxin. Uncoupling of Ni from receptor with continued coupling of Ni to the catalytic unit. J Biol Chem. 1984 Jul 25;259(14):8693–8698. [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Florio V. A., Sternweis P. C. Reconstitution of resolved muscarinic cholinergic receptors with purified GTP-binding proteins. J Biol Chem. 1985 Mar 25;260(6):3477–3483. [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Schultz G. Occurrence of a hormone-sensitive inhibitory coupling component of the adenylate cyclase in S49 lymphoma cyc- variants. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3899–3902. doi: 10.1073/pnas.80.13.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Smigel M. D., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and the inhibition of adenylate cyclase in S49 lymphoma cyc- and wild type membranes. J Biol Chem. 1984 Mar 25;259(6):3586–3595. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Koch B. D., Dorflinger L. J., Schonbrunn A. Pertussis toxin blocks both cyclic AMP-mediated and cyclic AMP-independent actions of somatostatin. Evidence for coupling of Ni to decreases in intracellular free calcium. J Biol Chem. 1985 Oct 25;260(24):13138–13145. [PubMed] [Google Scholar]
- Kurose H., Katada T., Amano T., Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J Biol Chem. 1983 Apr 25;258(8):4870–4875. [PubMed] [Google Scholar]
- Kurose H., Katada T., Haga T., Haga K., Ichiyama A., Ui M. Functional interaction of purified muscarinic receptors with purified inhibitory guanine nucleotide regulatory proteins reconstituted in phospholipid vesicles. J Biol Chem. 1986 May 15;261(14):6423–6428. [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Corda D., Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotropin-secreting cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8034–8038. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Schofield G., Weight F. Somatostatin, an inhibitor of ACTH secretion, decreases cytosolic free calcium and voltage-dependent calcium current in a pituitary cell line. J Neurosci. 1986 Nov;6(11):3128–3132. doi: 10.1523/JNEUROSCI.06-11-03128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Reisine T., Kebabian J. W. Adenosine 3',5'-monophosphate (cAMP)-dependent protein kinase activity in rodent pituitary tissue: possible role in cAMP-dependent hormone secretion. Endocrinology. 1984 Nov;115(5):1933–1945. doi: 10.1210/endo-115-5-1933. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T. Somatostatin: mechanism of action in pancreatic islet beta-cells. Diabetes. 1981 Oct;30(10):836–842. doi: 10.2337/diab.30.10.836. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N Engl J Med. 1983 Dec 15;309(24):1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- Reisine T., Guild S. Pertussis toxin blocks somatostatin inhibition of calcium mobilization and reduces the affinity of somatostatin receptors for agonists. J Pharmacol Exp Ther. 1985 Dec;235(3):551–557. [PubMed] [Google Scholar]
- Reisine T., Zhang Y. L., Sekura R. Pertussis toxin treatment blocks the inhibition of somatostatin and increases the stimulation by forskolin of cyclic AMP accumulation and adrenocorticotropin secretion from mouse anterior pituitary tumor cells. J Pharmacol Exp Ther. 1985 Jan;232(1):275–282. [PubMed] [Google Scholar]
- Richardson U. I. Adrenocorticotropin secretion by mouse pituitary tumor cells in culture: the role of Ca+2 in stimulated and somatostatin-inhibited secretion. Endocrinology. 1983 Jul;113(1):62–68. doi: 10.1210/endo-113-1-62. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Wuarin F., Wollheim C. B., Zahnd G. R. Somatostatin lowers the cytosolic free Ca2+ concentration in clonal rat pituitary cells (GH3 cells). Cell Calcium. 1984 Jun;5(3):223–236. doi: 10.1016/0143-4160(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Surprenant A. Correlation between electrical activity and ACTH/beta-endorphin secretion in mouse pituitary tumor cells. J Cell Biol. 1982 Nov;95(2 Pt 1):559–566. doi: 10.1083/jcb.95.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhing R. J., Jiang H., Prpic V., Exton J. H. Regulation of a liver plasma membrane phosphoinositide phosphodiesterase by guanine nucleotides and calcium. FEBS Lett. 1985 Sep 2;188(2):317–320. doi: 10.1016/0014-5793(85)80394-x. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M. Regulatory peptides of the hypothalamus. Annu Rev Physiol. 1977;39:473–527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]
- Wallace M. A., Fain J. N. Guanosine 5'-O-thiotriphosphate stimulates phospholipase C activity in plasma membranes of rat hepatocytes. J Biol Chem. 1985 Aug 15;260(17):9527–9530. [PubMed] [Google Scholar]



