Significance
Hummingbirds have exceedingly high oxygen demands because of their elevated rates of aerobic metabolism, and yet they thrive in high-altitude environments in the Andes where oxygen is scarce. Here we report the finding that when hummingbird species colonized new elevational zones, evolutionary changes in the respiratory properties of hemoglobin were repeatedly mediated by the same amino acid replacements. Specifically, ancestral sequence reconstruction and protein engineering experiments revealed that parallel adaptation of hemoglobin function in multiple species is attributable to repeated amino acid replacements at a single pair of interacting sites. This striking parallelism at the molecular level suggests a surprising degree of reproducibility and predictability in adaptive protein evolution.
Keywords: high-altitude adaptation, hypoxia, parallel evolution, protein evolution, epistasis
Abstract
Animals that sustain high levels of aerobic activity under hypoxic conditions (e.g., birds that fly at high altitude) face the physiological challenge of jointly optimizing blood-O2 affinity for O2 loading in the pulmonary circulation and O2 unloading in the systemic circulation. At high altitude, this challenge is especially acute for small endotherms like hummingbirds that have exceedingly high mass-specific metabolic rates. Here we report an experimental analysis of hemoglobin (Hb) function in South American hummingbirds that revealed a positive correlation between Hb-O2 affinity and native elevation. Protein engineering experiments and ancestral-state reconstructions revealed that this correlation is attributable to derived increases in Hb-O2 affinity in highland lineages, as well as derived reductions in Hb-O2 affinity in lowland lineages. Site-directed mutagenesis experiments demonstrated that repeated evolutionary transitions in biochemical phenotype are mainly attributable to repeated amino acid replacements at two epistatically interacting sites that alter the allosteric regulation of Hb-O2 affinity. These results demonstrate that repeated changes in biochemical phenotype involve parallelism at the molecular level, and that mutations with indirect, second-order effects on Hb allostery play key roles in biochemical adaptation.
In air-breathing vertebrates, the optimal Hb-O2 affinity varies according to the partial pressure of atmospheric O2 (PO2) because of the trade-off between the need to maximize arterial O2 saturation under hypoxia while simultaneously ensuring adequate O2 unloading in the tissue capillaries (1–8). However, in species that are native to high-altitude environments, it is not known how often and to what extent physiological adaptation to hypoxia is mediated by genetically based modifications of Hb-O2 affinity (9). Such questions can be resolved by conducting systematic comparative studies of Hb function among species with known phylogenetic relationships and contrasting altitudinal distributions.
In cases where multiple species have adapted independently to high-altitude hypoxia, replicated changes in Hb function may be instructive about the relative accessibility of different design solutions to natural selection. If repeated changes in Hb-O2 affinity involve parallel amino acid substitutions, then this suggests that adaptive protein evolution may be predisposed to follow particular mutational pathways. If, by contrast, myriad different mutational changes can produce the same functional outcome, then particular design solutions may be selectively accessible from a diverse range of ancestral starting points, and pathways of protein evolution may be highly idiosyncratic.
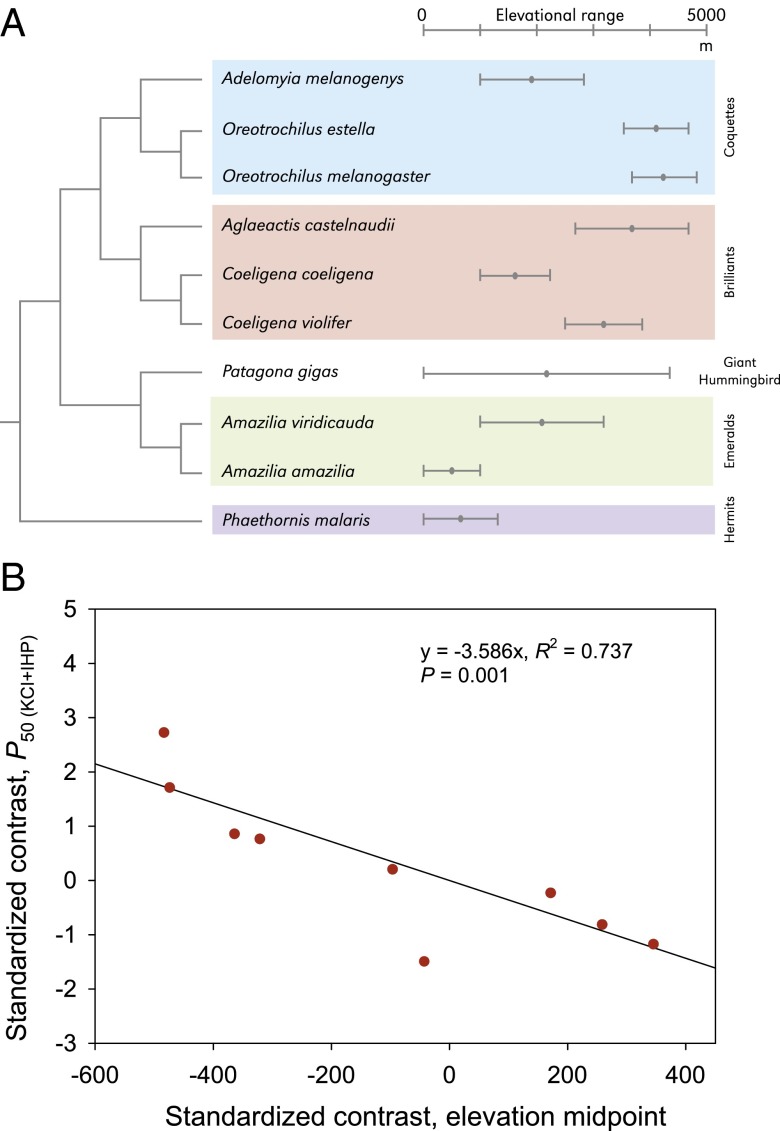
Among vertebrates, hummingbirds have some of the highest basal metabolic rates and the highest metabolic scopes for activity, and are therefore especially compelling subjects for studies of Hb function and blood-O2 transport under hypoxia (10–13). We conducted an experimental analysis of Hb function in 10 species of Andean hummingbirds that have dramatically different altitudinal distributions. The species included in this study fall into three main clades: the Coquettes, the Brilliants, and the Emeralds + Giant Hummingbird (Patagona gigas) (Fig. 1A). Each of these three clades contains species that are restricted to low or moderate elevations, as well as independently derived high-elevation species that routinely occur at elevations >4,200 m. We also collected data for one outgroup species from the hermit subfamily (Phaethornithinae), a primarily lowland clade that represents the likely ancestral elevational distribution for hummingbirds (14).
Fig. 1.
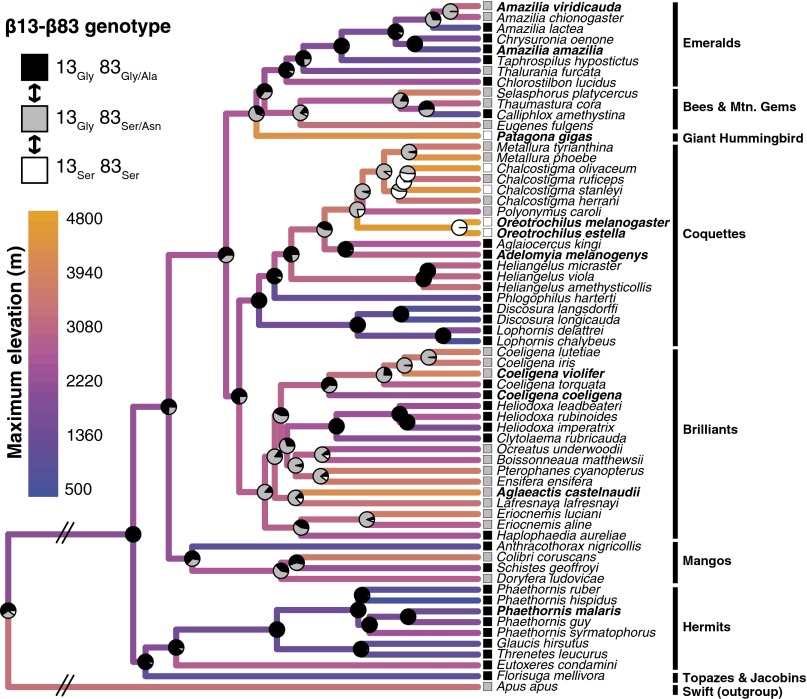
Phylogenetically independent contrasts reveal a positive association between Hb-O2 affinity and native elevation in Andean hummingbirds. (A) Phylogenetic relationships (25) and elevational distributions (ranges and midpoints) of 10 hummingbird species included in the analysis of Hb function. (B) Least-squares regression of phylogenetically independent contrasts revealed a significant negative relationship between P50(KCl+IHP) and native elevation (i.e., a positive relationship between Hb-O2 affinity and elevation). See Table S2 for full results.
Results and Discussion
Hb Isoform Composition.
The Hbs of birds and other jawed vertebrates are heterotetramers, composed of two α-chain and two β-chain subunits (15, 16). During postnatal life, most bird species express two main Hb isoforms in circulating red blood cells: a major isoform, HbA 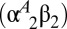 , with α-chain subunits encoded by the αA-globin gene, and a minor isoform, HbD
, with α-chain subunits encoded by the αA-globin gene, and a minor isoform, HbD 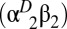 , with α-chain subunits encoded by the αD-globin gene (17) (Fig. S1). Given that avian HbD has a consistently higher O2-affinity relative to HbA (17), changes in the intracellular HbA/HbD ratio could substantially alter blood-O2 affinity, and it has been suggested that regulatory changes in Hb isoform composition may contribute to adaptive changes in blood-O2 transport in high-altitude species (18–20). To test this hypothesis we conducted a proteomic analysis of red cell lysates from each of the 10 hummingbird study species. Results of this analysis revealed that each of the hummingbird species express both HbA and HbD isoforms, and the relative concentration of the minor HbD isoform ranged from 1.6 to 24.2% (mean ± SD = 13.3 ± 6.2%). However, phylogenetically independent contrasts revealed no clear association between HbA/HbD isoform ratio and native elevation (R2 = 0.026, P = 0.657).
, with α-chain subunits encoded by the αD-globin gene (17) (Fig. S1). Given that avian HbD has a consistently higher O2-affinity relative to HbA (17), changes in the intracellular HbA/HbD ratio could substantially alter blood-O2 affinity, and it has been suggested that regulatory changes in Hb isoform composition may contribute to adaptive changes in blood-O2 transport in high-altitude species (18–20). To test this hypothesis we conducted a proteomic analysis of red cell lysates from each of the 10 hummingbird study species. Results of this analysis revealed that each of the hummingbird species express both HbA and HbD isoforms, and the relative concentration of the minor HbD isoform ranged from 1.6 to 24.2% (mean ± SD = 13.3 ± 6.2%). However, phylogenetically independent contrasts revealed no clear association between HbA/HbD isoform ratio and native elevation (R2 = 0.026, P = 0.657).
Altitudinal Variation in Hb-O2 Affinity.
Evolutionary adjustments in Hb-O2 affinity can be achieved via changes in intrinsic O2 affinity or changes in the sensitivity of Hb to the modulating effects of physiological allosteric cofactors, such as Cl− ions and organic phosphates (8, 15). The allosteric regulation of Hb-O2 affinity involves the oxygenation-linked binding of nonheme ligands that indirectly modulate heme reactivity by shifting the equilibrium between a low-affinity “T-state” and a high-affinity “R-state.” Allosteric cofactor molecules typically reduce Hb-O2 affinity by preferentially binding and stabilizing deoxygenated Hb, thereby displacing the R↔T equilibrium in favor of the low-affinity T-state conformation (Fig. S2). After isolating and purifying the HbA and HbD isoforms from each hummingbird species, we measured oxygenation properties in the presence and absence of the two main allosteric effectors that regulate Hb-O2 affinity: inositol hexaphosphate (IHP, a chemical analog of the naturally occurring inositol pentaphosphate in avian red cells, at twofold molar excess over tetrameric Hb) and Cl− ions (added as KCl; 0.1 mol⋅L−1). Hb-O2 affinity was indexed by P50, the PO2 at which Hb is half-saturated. O2-equilibrium measurements revealed that the major HbA isoforms of the high-altitude hummingbird species were generally characterized by elevated O2-affinities in the absence of allosteric effectors (“stripped” Hb) and the difference in P50 values between highland and lowland species was amplified in the presence of IHP alone and in the simultaneous presence of both IHP and Cl− ions (Table 1 and Fig. S3).
Table 1.
Functional properties of hummingbird HbA isoforms
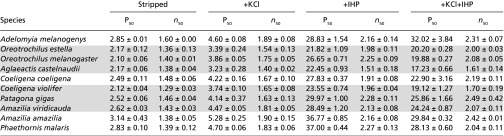 |
O2-affinities (P50, torr) and cooperativity coefficients (n50; mean ± SEM) of purified HbA isoforms measured in 0.1 M Hepes buffer at pH 7.40 (± 0.01), 37 °C, in the absence of allosteric effectors (stripped), in the presence of KCl (0.1 M) or IHP (IHP/Hb tetramer ratio = 2.0), and in the presence of both allosteric effectors. [Heme], 0.3 mM. As explained in the text, P50 is an inverse measure of Hb-O2 affinity. High-altitude species with maximum elevational ranges of >3,000 m are denoted by gray shading.
Regressions based on phylogenetically independent contrasts (PICs) revealed a significantly negative relationship between HbA P50 values and native elevation (i.e., a positive relationship between Hb-O2 affinity and elevation) (Fig. 1B and Table S1). Similarly, for five species that expressed the HbD isoform at levels sufficient for experimental analysis, regressions based on PICs revealed significantly negative relationships between P50 values and native elevation, both for HbD alone and for the weighted average of HbA and HbD in their naturally occurring relative concentrations (Table S1). From this point onward, we primarily focus on oxygenation properties of Hb in the presence of IHP and Cl− ions, the experimental treatment that is most relevant to in vivo conditions in avian red cells.
Causative Substitutions and the Structural Mechanisms Underlying Evolutionary Transitions in Hb-O2 Affinity.
Inspection of the genotypic and phenotypic data suggested that phylogenetically replicated changes in Hb-O2 affinity were largely attributable to repeated amino acid replacements at two sites: β13 (position 10 in the A helix) and β83 (position 7 in the EF interhelical segment) (Fig. 2 and Fig. S4). Within each clade, the species with the highest Hb-O2 affinities in the presence of allosteric effectors always possessed the two-site genotype β13Ser-β83Ser (Oreotrochilus estella, Oreotrochilus melanogaster, and P. gigas, all of which are predominantly highland species) or β13Gly-β83Ser (Aglaeactis castelnaudii, Coeligena violifer, and Amazilia viridicauda, all of which are predominantly highland species), whereas the species with the lowest Hb-O2 affinities always possessed β13Gly-β83Gly (Adelomyia melanogenys, Coeligena coeligena, Amazilia amazilia, and Phaethornis malaris, all of which are predominantly lowland species) (Fig. 2 and Table 1). Comparisons among HbD isoforms are also informative about the effects of these substitutions because HbA and HbD isoforms of the same species share identical β-chain subunits. Among the five species in which HbD was examined, the only species with the β13Ser-β83Ser genotype (the predominantly highland P. gigas) had the highest HbD O2 affinity [P50(KCl+IHP) = 16.56 ± 0.56 torr], and the two species that shared the alternative β13Gly-β83Gly genotype (the exclusively lowland A. amazilia and P. malaris) had the two lowest affinities [P50(KCl+IHP) = 23.20 ± 1.21 and 24.92 ± 0.52 torr].
Fig. 2.
Variable residue positions in a multiple alignment of hummingbird βA-globin sequences. The estimated sequence for the common ancestor of hummingbirds (Anc1) is included for comparison, and derived Ser residues at β13 and β83 are shown in red boxes. High-altitude species with maximum elevational ranges of >3,000 m are denoted by shading. Sequences represent the most common haplotypes for each species.
To identify the structural basis of variation in Hb-O2 affinity, a comparison between the HbA isoforms of A. melanogenys (an exclusively lowland species) and O. estella (a high-altitude specialist) is especially informative because they exhibit pronounced differences in O2-affinity [P50(KCl+IHP) = 32.02 ± 3.84 vs. 20.20 ± 0.28 torr, respectively] (Table 1), and yet they differ by just one conservative α-chain substitution (α8Thr→Ser) and two polarity-changing β-chain substitutions (β13Gly→Ser and β83Gly→Ser) (Fig. 2). To isolate the functional effects of the two β-chain substitutions, we used a recombinant expression vector (21) and site-directed mutagenesis to synthesize the reconstructed ancestral Coquette HbA (β13Gly-β83Gly, identical to wild-type A. melanogenys β-globin), the derived double-mutant genotype that is identical to wild-type O. estella β-globin (β13Ser-β83Ser), and each of the alternative single-mutant intermediates (β13Ser-β83Gly and β13Gly-β83Ser). Consistent with measurements of the native Hbs in the presence of allosteric effectors, P50(KCl+IHP) for the recombinant O. estella Hb was significantly lower (i.e., O2-affinity was higher) than that of A. melanogenys (Table S2), confirming the affinity-enhancing effect of the β13Gly→Ser and β83Gly→Ser substitutions in combination.
Epistasis for Hb-O2 Affinity.
Analysis of the alternative single- and double-mutant recombinant Hbs (rHbs) revealed that phenotypic effects of mutations at β13 and β83 are highly context-dependent; P50(KCl+IHP) values exhibited a significant epistatic deviation from expectations of an additive model (ε = 12.94, 95% confidence interval = 10.05–15.82). In the presence of allosteric effectors, the β13Gly→Ser substitution increased O2-affinity on the ancestral Coquette background (in the presence of β83Gly) and reduced O2-affinity in the presence of the derived β83Ser. Similarly, the β83Gly→Ser substitution increased O2-affinity on the ancestral Coquette background (in the presence of β13Gly) and reduced O2-affinity in the presence of the derived β13Ser (Table S2). This is an example of sign epistasis (22, 23), where the sign of the phenotypic effect of a mutation is conditional on the genetic background in which it occurs.
Structural Basis of Species Differences in Hb Function.
To determine the structural mechanisms responsible for the additive and epistatic effects of substitutions at β13 and β83, we conducted homology-based modeling analyses of hummingbird Hb (Methods). These analyses revealed that Gly→Ser replacements at β13 and β83 produce localized changes in secondary structure of the A and F helices, respectively (Table S3), which impinge indirectly on the allosteric regulatory control of Hb-O2 affinity. Site β83 is located within a segment of the β-subunit main chain (residues 81–84) that alternates between helical and nonhelical secondary structure in the allosteric transition between the oxy and deoxy states, respectively (Fig. 3 A–C). At β83, either Gly or Ser can donate a helix-capping, amide H-bond to the carbonyl oxygen of β85Phe (the N-terminal residue of the F-helix), but the polar -OH side-chain of β83Ser forms additional intermolecular H-bonds that alter the torsion angle of the F-helix, thereby constraining allosteric movement. When nonpolar Gly is replaced by polar, hydrophilic Ser at β83 (as in the predominantly highland Oreotrochilus, A. castelnaudii, C. violifer, P. gigas, and A. viridicauda) (Fig. 2), the effect on Hb allostery is contingent on the presence of Gly or Ser at β13. Changes in the network of atomic contacts involving β13 and β83 (Table S3) alter the favorability of alternative conformation states for IHP-binding in the central cavity (Fig. 3D), and the resultant changes in the location of IHP-binding account for the observed epistasis for Hb-O2 affinity in the presence of IHP (Table S2). Our experimental results for the hummingbird rHb mutants are consistent with functional studies of a naturally occurring human Hb mutant, Hb Pyrgos (β83Gly→Asp), which is also characterized by an increased O2-affinity in the presence of organic phosphates (24).
Fig. 3.
Amino acid replacements at β13 and β83 produce second-order perturbations of tertiary structure that affect the allosteric regulation of O2-binding by IHP. (A) Homology-based structural model of hummingbird Hb showing the locations of amino acid replacements at sites 13 and 83 in the β1 subunit (shown in cyan). β-Chain residues 18–22, 42–45, and 81–84 (including the variable site 83, the penultimate C-terminal residue in the EF interhelical loop) alternate between helical and nonhelical secondary structure in the allosteric transition between the oxy (B) and deoxy (C) states. (D) Structural model of hummingbird Hb (with the β1 subunit removed) showing alternative IHP-binding sites in the central cavity. Polarity-changing amino acid replacements at β13 and β83 produce nonadditive changes in the favorability of alternative conformation states for polyphosphate-binding: IHP preferentially binds at “site 1” in the β13Gly-β83Gly and β13Ser-β83Ser mutants (representing wild-type β-globin genotypes for A. melanogenys and O. estella, respectively) and at “site 2” in the β13Ser-β83Gly and β13Gly-β83Ser mutants.
Parallelism of β-Chain Substitutions Among Species.
We sequenced βA-globin in 63 hummingbird species and we then used maximum-likelihood and parsimony to map the β13 and β83 replacements onto an independently derived and well-resolved phylogeny (25). This analysis revealed that the substitutions (and, by implication, the associated changes in Hb-O2 affinity) occurred at least 17 times independently (≥4 and ≥13 transitions between Gly and Ser at β13 and β83, respectively). Maximum-likelihood ancestral-state estimates for native elevation indicated that hummingbird species have shifted upwards and downward during the evolution of the group, in conjunction with repeated substitutions and back-substitutions at β13 and β83 (Fig. 4 and Fig. S5). Hence, the negative correlation between P50 and native elevation (Fig. 1B) is attributable to derived increases in Hb-O2 affinity in highland lineages, as well as derived reductions in Hb-O2 affinity in lowland lineages. For example, the common ancestor of the highland genus Oreotrochilus (β13Ser-β83Ser) evolved a derived increase in Hb-O2 affinity relative to the likely ancestral state of the Coquette clade (β13Gly-β83Gly). In contrast, in the Brilliants the lowland C. coeligena (β13Gly-β83Gly) evolved a derived reduction in Hb-O2 affinity relative to the likely ancestral state for that clade (β13Gly-β83Ser) (Fig. 4). Species’ maximum elevation was strongly associated with β13-β83 genotype in a phylogenetic general linear model (R2 = 0.53; P < 10−11) (Fig. 4).
Fig. 4.
Ancestral state estimates for β13 and β83 in hummingbirds. Pie diagrams at the nodes indicate the probability of each genotype based on a stepwise, single-rate maximum-likelihood model with two reversible transitions, as indicated in the inset diagram. Terminal branches of the phylogenetic tree are color-coded according to the upper limit of the species' elevational range, and internal branches are color-coded based on maximum-likelihood estimates of the ancestral states. The phylogenetically corrected association between β13-β83 genotype and native elevation was highly significant (see text for details). Parsimony analysis revealed a minimum of 17 changes in genotype across the tree (Fig. S5). β83Asn was observed in a single species, Doryfera ludoviciae, and was therefore binned with the β83Ser character state because side-chains of the two residues have the same polarity and the underlying codons are connected by a single mutational step. Similarly, β83Ala was observed in a single species, Phlogophilus harterti, and was binned with the β83Gly character state in this analysis. Branch lengths are proportional to relative time, except where indicated. Species names in bold are those that were included in the experimental analysis of Hb function.
Among distantly related species, parallel substitutions at sites β13 and β83 are likely attributable to the repeated fixation of identical-by-state alleles that had independent mutational origins. Among some of the more closely related species, the sorting of ancestral polymorphism may produce the same pattern of parallelism because of the repeated fixation of identical-by-descent alleles in recently diverged lineages (26). Further work is needed to elucidate the mutational origins of the β13 and β83 variants, but it is clear that repeated changes at both sites have contributed to the repeated elevational shifts in Hb function among different lineages. Aside from the variation at sites β13 and β83, no other substitutions in the αA-, αD-, or βA-globin genes exhibited any obvious association with species differences in P50 values for HbA or HbD, although it is likely that particular lineage-specific substitutions (Fig. S4) account for residual variation in Hb-O2 affinity among species.
Possible Adaptive Significance of Altitudinal Differences in Hb-O2 Affinity.
The evolution of divergent Hb-O2 affinities between highland and lowland hummingbirds is consistent with theoretical predictions (1–6). At low altitude, a low Hb-O2 affinity is expected to be physiologically advantageous for hummingbirds and other animals with high mass-specific metabolic rates because O2 unloading in the peripheral circulation can occur at relatively high PO2, thereby optimizing tissue oxygenation by increasing the O2 diffusion gradient between capillary blood and tissue mitochondria. At low altitude, the trade-off with pulmonary O2 loading is alleviated because arterial O2 saturation will still be near-maximal. However, under conditions of severe environmental hypoxia at very high altitudes, an increased Hb-O2 affinity becomes advantageous because tissue O2 delivery can be preserved more effectively by safeguarding arterial O2 saturation than by maximizing O2 unloading from partially desaturated blood (1–6, 8, 9).
Mechanisms of Hb Adaptation and Causes of Parallelism at the Sequence Level.
Comparative studies of Hb function in different animal species and experimental studies of naturally occurring or recombinant human Hb mutants have demonstrated that genetically based changes in Hb-O2 affinity can be produced by numerous possible structural changes (27–29). In Andean hummingbirds, amino acid replacements at β13 and β83 contribute to species differences in Hb-O2 affinity, but it is certainly not because they represent the only possible mutational changes that are capable of producing the observed changes in protein function. Although there may be numerous possible mutations that can produce identical changes in Hb-O2 affinity, many of those changes are known to have deleterious pleiotropic effects. For example, active site mutations that alter the polarity or hydrophobicity of the distal heme pocket can produce direct changes in the association constant for O2 binding, but such mutations typically compromise structural stability or increase the susceptibility to heme autoxidation (the spontaneous oxidation of the heme iron from the ferrous Fe2+ state to the ferric Fe3+ state, which renders Hb functionally inert as an O2-transport molecule) (29). In contrast, mutations remote from the active site—like those at β13 and β83—can potentially produce fine-tuned changes in O2-affinity with minimal pleiotropic effects through subtle displacements of the allosteric equilibrium (28–30). Within the set of all possible mutations that produce functionally equivalent effects on Hb-O2 affinity, those that incur a lesser magnitude of deleterious pleiotropy are predicted to have a higher fixation probability, and such mutations may therefore contribute disproportionately to biochemical adaptation (31–33). When such changes are driven by positive directional selection, theory predicts that they are especially likely to evolve in parallel (34).
The parallel β13 and β83 substitutions that we have documented in hummingbirds have not been implicated in the adaptation of Hb function in other high-altitude birds or mammals (35–39), although a survey of sequence variation in the globin genes of Andean waterfowl documented a shared β13Gly/Ser polymorphism in speckled teals (Anas flavirostris) and yellow-billed pintails (Anas georgica), and in both species the derived Ser variant was present at high frequency in high-altitude populations (40). The phenotypic effects of the β13Gly/Ser variants in these waterfowl species have not yet been investigated, but the similar altitudinal patterns in Andean ducks and hummingbirds suggest parallel mechanisms of Hb evolution. At β13 and β83 in Andean hummingbirds, it may be that recurrent mutation and retention of ancestral polymorphism both contributed to variation in Hb function—variation that was then recruited when selection favored fine-tuned adjustments in blood-O2 transport (e.g., during elevational range shifts). When closely related species independently adapt to a shared environmental challenge, natural selection may be predisposed to hit upon the same design solution in different lineages if one particularly accessible (and minimally pleiotropic) solution happens to be located within striking distance from the same ancestral starting point.
Methods
Specimen Collection.
We preserved blood and tissue samples from voucher specimens of hummingbirds that were collected from numerous Andean localities spanning an elevational range of ∼4,500 m (Table S4). Our analysis of Hb function was based on blood samples from 70 hummingbird specimens (n = 3–8 individuals per species). All hummingbirds were live-trapped in mistnets and were bled and killed in accordance with guidelines of the Ornithological Council (41), and protocols approved by the University of New Mexico Institutional Animal Care and Use Committee (Protocol number 08UNM033-TR-100117; Animal Welfare Assurance number A4023-01). All fieldwork was carried out under permits issued by the management authorities of Peru (76-2006-INRENA-IFFS-DCB, 087–2007-INRENA-IFFS-DCB, and 135–2009-AG-DGFFS-DGEFFS).
For each individual bird, we collected 0.03–0.20 mL of whole blood from the brachial or ulnar vein using heparinized microcapillary tubes. Red blood cells were separated from the plasma fraction by centrifugation, and the packed red cells were then snap-frozen in liquid nitrogen and were stored at −80 °C before use as a source of Hb for experimental studies. We collected liver and pectoral muscle from each specimen as sources of genomic DNA and globin mRNA, respectively. Muscle samples were snap-frozen or preserved using RNAlater and were subsequently stored at −80 °C before RNA isolation. Voucher specimens were preserved along with ancillary data and were deposited in the collections of the Museum of Southwestern Biology of the University of New Mexico and the Centro de Ornitología y Biodiversidad (CORBIDI), Lima, Peru. Complete specimen data are available via the ARCTOS online database (Table S4).
Molecular Cloning and Sequencing.
We cloned and sequenced the adult globin genes (αA-, αD-, and βA-globin) from at least two specimens per species. We used the RNeasy Mini Kit (Qiagen) to isolate RNA, and we used 5′ and 3′ RACE (Invitrogen Life Technologies) to obtain cDNA sequence for the 5′ and 3′ UTRs of each adult-expressed globin gene. After designing paralog-specific PCR primers with annealing sites in the 5′ and 3′ UTRs, complete cDNAs were synthesized for each gene by reverse transcription using the OneStep RT-PCR kit (Qiagen). We cloned gel-purified RT-PCR products into pCR4-TOPO vector using the TOPO TA Cloning Kit (Invitrogen Life Technologies). All new sequences were deposited in GenBank under accession nos. KF222496, KF222499, KF222501, KF222503, KF222506, and KF222510–KF222539.
Characterization of Hb Isoform Composition.
We used isoelectric focusing (IEF; PhastSystem, GE Healthcare Bio-Sciences) to characterize Hb isoform composition in red cell lysates from each of the 70 hummingbird specimens. After separating native Hbs by means of IEF, gel bands were excised and digested with trypsin. The resultant peptides were then identified by means of tandem mass spectrometry (MS/MS). Database searches of the resultant MS/MS spectra were performed using Mascot (Matrix Science, v1.9.0), whereby peptide mass fingerprints were used to query a custom database of avian α- and β-chain sequences (17, 42–44), including αA-, αD-, and βA-globin sequences from each of the surveyed hummingbird species. After separating the HbA and HbD isoforms by native gel IEF and identifying each of the constituent subunits by MS/MS, the relative abundance of the different isoforms in the hemolysates of each individual was quantified densitometrically using ImageJ (45).
Protein Purification and Measurement of Hb-O2 Equilibria.
The HbA and HbD isoforms (isoelectric points = 8.9–9.1 and 6.8–7.3, respectively) were separated and stripped of organic phosphates by means of ion-exchange chromatography. O2 equilibria of purified Hb solutions [3 μL thin-layer samples, (heme) 0.3 mM] were measured at 37 °C in the presence of 0.1 M Hepes buffer (pH 7.4). To characterize the allosteric regulation of Hb-O2 affinity, we measured O2-equilibrium curves in the absence of allosteric effectors (stripped), in the presence of Cl− ions (0.1 M KCl), in the presence of IHP (IHP/Hb tetramer ratio = 2.0), and in the simultaneous presence of both effectors. For details of the purification protocol and the measurement of Hb-O2 equilibrium curves, see SI Methods.
Vector Construction, Site-Directed Mutagenesis, and Synthesis of rHbs.
To produce rHbs for the protein engineering experiments, the αA- and βA-globin genes of A. melanogenys were synthesized by Genscript after optimizing nucleotide sequences with respect to Escherichia coli codon preferences. Gene cassettes for the αA- and βA-globin genes and the methionine aminopeptidase (MAP) gene were tandemly cloned into the custom pGM expression plasmid described by Natarajan et al. (21). All rHbs were expressed in the JM109 (DE3) E. coli strain. See SI Methods for details regarding the site-directed mutagenesis experiments, the expression and purification of the hummingbird rHb mutants, the measurement of rHb oxygenation properties, and the measurement of epistasis.
Ancestral State Estimates.
To infer the polarity of character-state changes at β13 and β83, we sequenced the βA-globin gene of 63 hummingbird species with known phylogenetic relationships. Orthologous sequence from the common swift (Apus apus) was used as an outgroup. Fifty-six of the 63 nodes in the independently derived phylogeny were resolved with >95% posterior probability (Dataset S1). We estimated ancestral states of the β13-β83 genotypes using maximum-likelihood and parsimony with the APE package in R (46). Two of the observed genotypes included rare variants at β83 (β13Gly-β83Asn and β13Gly-β83Ala) that differed by a single codon change from physiochemically similar alternative states (β83Ser and β83Gly, respectively). We binned each of these singleton changes with the related codon state, resulting in three classes of two-site β13-β83 genotypes. In the maximum-likelihood model, we allowed only the two reversible transitions that each comprised a single nucleotide change. We applied a model with one rate for all transitions because likelihood ratio tests indicated that models with two to four rate parameters were not justified (46) (Fig. 4). For details regarding the phylogenetic topology and phylogenetic comparative methods, see SI Methods.
Structural Modeling and Molecular Docking.
Homology-models of hummingbird Hb were built by the SWISS-MODEL server in the automated model (47), using Anas platyrhynchos Hb (PDB ID code 3EOK) as template. For each of the four rHb mutants, the root-mean-square-deviation was 0.74 Ǻ between model and template and the QMEAN value remained between 0.70 and 0.78 for all models. Molecular docking of IHP in the Hb central cavity was performed using AutoDock Vina (48). Internal molecular contacts were identified by the Frustratometer program (49).
Supplementary Material
Acknowledgments
For assistance in the field, we thank F. Angulo P., E. Bautista O., E. J. Beckman, P. M. Benham, D. Blanco, Centro de Ornitología y Biodiversidad (Lima, Peru), R. W. Dickerman, S. G. DuBay, L. M. Flores, A. B. Johnson, K. G. McCracken, J. A. Otero, A. Quiñonez Z., C. J. Schmitt, D. C. Schmitt, C. G. Schmitt, T. Valqui, W. Vargas C., B. Walker, and N. A. Wright; for assistance in the lab, we thank A. Bang (Aarhus) and A. Rutherford (Lincoln); and we thank K. G. McCracken and two anonymous reviewers for constructive comments on the manuscript. This study was supported by National Institutes of Health Grants R01 HL087216 and HL087216-S1 (to J.F.S); National Science Foundation Grants IOS-0949931 (to J.F.S.), DEB-0543556 (to J.A.M. and R.D.), and DEB-1146491 (to C.C.W.); the Danish Council for Independent Research, Natural Sciences Grant 10-084565 (to A.F.); the Center for Evolutionary and Theoretical Immunology at the University of New Mexico; and the Faculty of Science and Technology at Aarhus University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Complete information for all specimens used in this study is archived on the ARCTOS online database (Table S4). The sequences reported in this paper have been deposited in the GenBank database (accession nos. KF222496, KF222499, KF222501, KF222503, KF222506, KF222510–KF222539).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315456110/-/DCSupplemental.
References
- 1.Turek Z, Kreuzer F, Hoofd LJ. Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia. A theoretical study comparing man and rat. Pflugers Arch. 1973;342(3):185–197. doi: 10.1007/BF00591367. [DOI] [PubMed] [Google Scholar]
- 2.Turek Z, Kreuzer F. Effect of a shift of the oxygen dissociation curve on myocardial oxygenation at hypoxia. Adv Exp Med Biol. 1976;75:657–662. doi: 10.1007/978-1-4684-3273-2_76. [DOI] [PubMed] [Google Scholar]
- 3.Turek Z, Kreuzer F, Ringnalda BEM. Blood gases at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch. 1978;376(1):7–13. doi: 10.1007/BF00585241. [DOI] [PubMed] [Google Scholar]
- 4.Turek Z, Kreuzer F, Turek-Maischeider M, Ringnalda BEM. Blood O2 content, cardiac output, and flow to organs at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch. 1978;376(3):201–207. doi: 10.1007/BF00584951. [DOI] [PubMed] [Google Scholar]
- 5.Bencowitz HZ, Wagner PD, West JB. Effect of change in P50 on exercise tolerance at high altitude: A theoretical study. J Appl Physiol. 1982;53(6):1487–1495. doi: 10.1152/jappl.1982.53.6.1487. [DOI] [PubMed] [Google Scholar]
- 6.Willford DC, Hill EP, Moores WY. Theoretical analysis of optimal P50. J Appl Physiol. 1982;52(4):1043–1048. doi: 10.1152/jappl.1982.52.4.1043. [DOI] [PubMed] [Google Scholar]
- 7.Scott GR, Milsom WK. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir Physiol Neurobiol. 2006;154(1-2):284–301. doi: 10.1016/j.resp.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Mairbäurl H, Weber RE. Oxygen transport by hemoglobin. Compr Physiol. 2012;2(2):1463–1489. doi: 10.1002/cphy.c080113. [DOI] [PubMed] [Google Scholar]
- 9.Storz JF, Scott GR, Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol. 2010;213(Pt 24):4125–4136. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suarez RK, Lighton JRB, Brown GS, Mathieu-Costello O. Mitochondrial respiration in hummingbird flight muscles. Proc Natl Acad Sci USA. 1991;88(11):4870–4873. doi: 10.1073/pnas.88.11.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez RK. Hummingbird flight: Sustaining the highest mass-specific metabolic rates among vertebrates. Experientia. 1992;48(6):565–570. doi: 10.1007/BF01920240. [DOI] [PubMed] [Google Scholar]
- 12.Suarez RK. Oxygen and the upper limits to animal design and performance. J Exp Biol. 1998;201(Pt 8):1065–1072. doi: 10.1242/jeb.201.8.1065. [DOI] [PubMed] [Google Scholar]
- 13.Altshuler DL, Dudley R. The ecological and evolutionary interface of hummingbird flight physiology. J Exp Biol. 2002;205(Pt 16):2325–2336. doi: 10.1242/jeb.205.16.2325. [DOI] [PubMed] [Google Scholar]
- 14.McGuire JA, Witt CC, Remsen JV, Dudley R, Alshuler DL. A higher-level taxonomy for hummingbirds. J Ornithol. 2009;150(1):155–165. [Google Scholar]
- 15.Weber RE, Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir Physiol Neurobiol. 2004;144(2-3):141–159. doi: 10.1016/j.resp.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Storz JF, Opazo JC, Hoffmann FG. Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Mol Phylogenet Evol. 2013;66(2):469–478. doi: 10.1016/j.ympev.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grispo MT, et al. Gene duplication and the evolution of hemoglobin isoform differentiation in birds. J Biol Chem. 2012;287(45):37647–37658. doi: 10.1074/jbc.M112.375600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiebl I, Weber RE, Schneeganss D, Kosters J, Braunitzer G. Structural adaptations in the major and minor hemoglobin components of adult Ruppell’s griffon (Gyps ruepellii, Aegypiinae): A new molecular pattern for hypoxia tolerance. Biol Chem Hoppe Seyler. 1988;369:217–232. doi: 10.1515/bchm3.1988.369.1.217. [DOI] [PubMed] [Google Scholar]
- 19.Weber RE, Hiebl I, Braunitzer G. High altitude and hemoglobin function in the vultures Gyps rueppellii and Aegypius monachus. Biol Chem Hoppe Seyler. 1988;369(4):233–240. [PubMed] [Google Scholar]
- 20.Hoffmann FG, Storz JF. The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol Biol Evol. 2007;24(9):1982–1990. doi: 10.1093/molbev/msm127. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan C, et al. Expression and purification of recombinant hemoglobin in Escherichia coli. PLoS ONE. 2011;6(5):e20176. doi: 10.1371/journal.pone.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinreich DM, Watson RA, Chao L. Sign epistasis and genetic constraint on evolutionary trajectories. Evolution. 2005;59(6):1165–1174. [PubMed] [Google Scholar]
- 23.Natarajan C, et al. Epistasis among adaptive mutations in deer mouse hemoglobin. Science. 2013;340(6138):1324–1327. doi: 10.1126/science.1236862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wajcman H, et al. Hémoglobine Pyrgos beta 83 (EF 7) Gly → Asp chez un Malien: Identification structurale et propriétés fonctionnelles. Nouv Rev Fr Hematol. 1978;20(3):403–411. [PubMed] [Google Scholar]
- 25.McGuire JA, Witt CC, Altshuler DL, Remsen JV., Jr Phylogenetic systematics and biogeography of hummingbirds: Bayesian and maximum likelihood analyses of partitioned data and selection of an appropriate partitioning strategy. Syst Biol. 2007;56(5):837–856. doi: 10.1080/10635150701656360. [DOI] [PubMed] [Google Scholar]
- 26.Clark AG. Neutral behavior of shared polymorphism. Proc Natl Acad Sci USA. 1997;94(15):7730–7734. doi: 10.1073/pnas.94.15.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber RE. High-altitude adaptations in vertebrate hemoglobins. Respir Physiol Neurobiol. 2007;158(2–3):132–142. doi: 10.1016/j.resp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Storz JF, Moriyama H. Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt Med Biol. 2008;9(2):148–157. doi: 10.1089/ham.2007.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varnado CL, et al. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013;18(17):2314–2328. doi: 10.1089/ars.2012.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maillett DH, et al. Interfacial and distal-heme pocket mutations exhibit additive effects on the structure and function of hemoglobin. Biochemistry. 2008;47(40):10551–10563. doi: 10.1021/bi800816v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto SP. Two steps forward, one step back: The pleiotropic effects of favoured alleles. Proc Biol Sci. 2004;271(1540):705–714. doi: 10.1098/rspb.2003.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern DL, Orgogozo V. Is genetic evolution predictable? Science. 2009;323(5915):746–751. doi: 10.1126/science.1158997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streisfeld MA, Rausher MD. Population genetics, pleiotropy, and the preferential fixation of mutations during adaptive evolution. Evolution. 2011;65(3):629–642. doi: 10.1111/j.1558-5646.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- 34.Orr HA. The probability of parallel evolution. Evolution. 2005;59(1):216–220. [PubMed] [Google Scholar]
- 35.Jessen T-H, Weber RE, Fermi G, Tame J, Braunitzer G. Adaptation of bird hemoglobins to high altitudes: Demonstration of molecular mechanism by protein engineering. Proc Natl Acad Sci USA. 1991;88(15):6519–6522. doi: 10.1073/pnas.88.15.6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber RE, Jessen T-H, Malte H, Tame J. Mutant hemoglobins (α 119-Ala and β 55-Ser): functions related to high-altitude respiration in geese. J Appl Physiol (1985) 1993;75(6):2646–2655. doi: 10.1152/jappl.1993.75.6.2646. [DOI] [PubMed] [Google Scholar]
- 37.Storz JF, et al. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci USA. 2009;106(34):14450–14455. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 2010;213(Pt 15):2565–2574. doi: 10.1242/jeb.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Revsbech IG, et al. Hemoglobin function and allosteric regulation in semi-fossorial rodents (family Sciuridae) with different altitudinal ranges. J Exp Biol. 2013;216(Pt 22):4264–4271. doi: 10.1242/jeb.091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCracken KG, et al. Parallel evolution in the major haemoglobin genes of eight species of Andean waterfowl. Mol Ecol. 2009;18(19):3992–4005. doi: 10.1111/j.1365-294X.2009.04352.x. [DOI] [PubMed] [Google Scholar]
- 41.Fair JM, Paul E. In: Guidelines to the Use of Wild Birds in Research. Jones J, editor. Washington, DC: Ornithological Council; 2010. [Google Scholar]
- 42.Hoffmann FG, Storz JF, Gorr TA, Opazo JC. Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol Biol Evol. 2010;27(5):1126–1138. doi: 10.1093/molbev/msp325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann FG, Opazo JC, Storz JF. Differential loss and retention of cytoglobin, myoglobin, and globin-E during the radiation of vertebrates. Genome Biol Evol. 2011;3:588–600. doi: 10.1093/gbe/evr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann FG, Opazo JC, Storz JF. Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol Biol Evol. 2012;29(1):303–312. doi: 10.1093/molbev/msr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with Image J. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 46.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 47.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 48.Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenik M, et al. Protein frustratometer: A tool to localize energetic frustration in protein molecules. Nucleic Acids Res. 2012;40(Web Server issue):W348–W351. doi: 10.1093/nar/gks447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.