Abstract
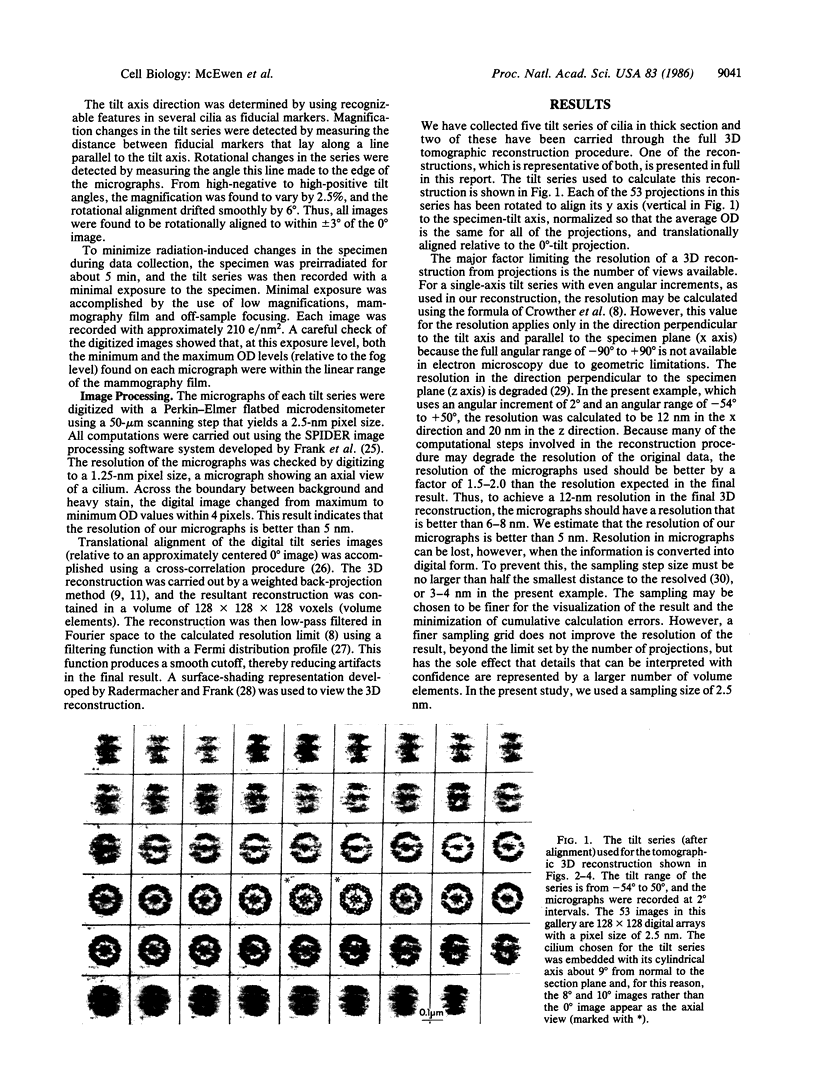
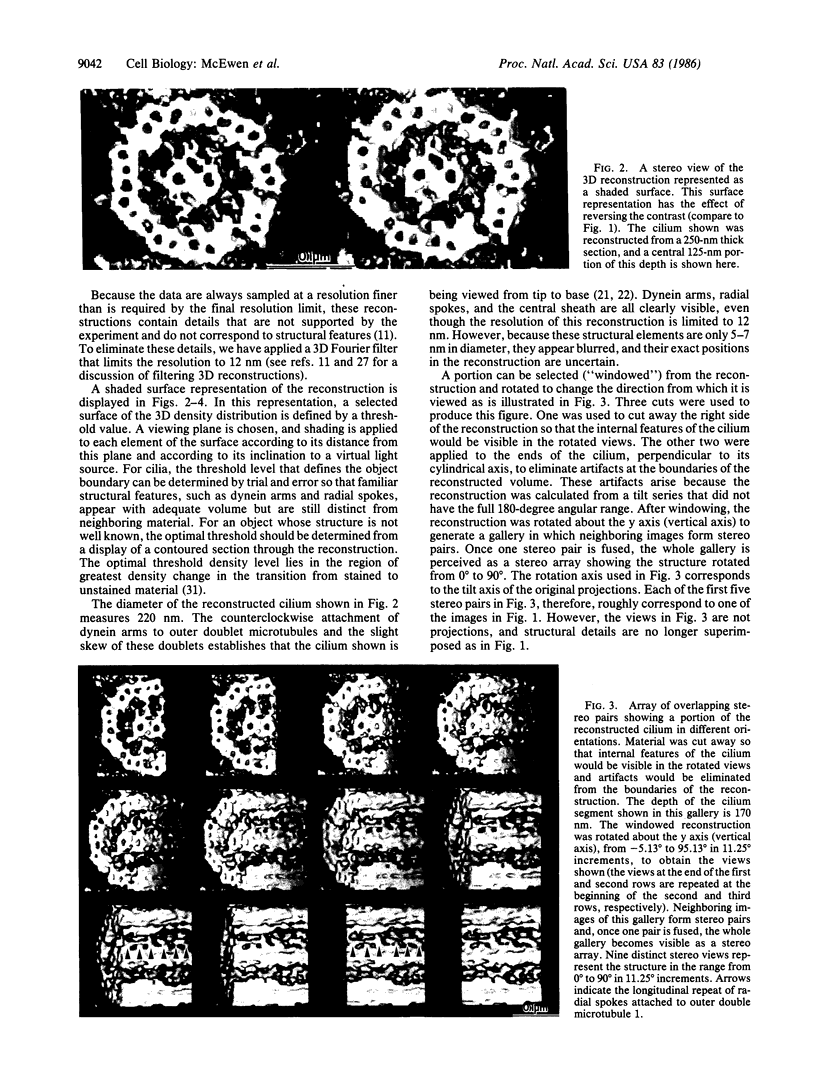
We have applied a computer-based tomographic technique to reconstruct the three-dimensional ultrastructure of newt lung cilia. Epon-embedded samples were cut into 0.25-micron-thick sections that were imaged at 1 MV with a high-voltage electron microscope. For the reconstruction shown, a tilt series of 53 micrographs was taken at tilt angles between -54 degrees and +50 degrees. The reconstruction was accomplished from these projections using a weighted back-projection algorithm. The 12-nm resolution of the reconstruction was sufficient to resolve the outer doublet and central pair microtubules, dynein arms, radial spokes, and central sheath structures. The reconstruction can be viewed from various angles and with appropriate parts cut away to reveal structural features of interest. The sense of depth in these views can be enhanced by stereo viewing of shaded surface images. From this reconstruction, we determined that newt lung cilia contain the more common triplet grouping of radial spokes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avolio J., Lebduska S., Satir P. Dynein arm substructure and the orientation of arm-microtubule attachments. J Mol Biol. 1984 Mar 5;173(3):389–401. doi: 10.1016/0022-2836(84)90127-x. [DOI] [PubMed] [Google Scholar]
- Baccetti B., Porter K. R., Ulrich M. High voltage electron microscopy of sperm axoneme. J Submicrosc Cytol. 1985 Apr;17(2):171–176. [PubMed] [Google Scholar]
- Bennett P. M. Decrease in section thickness on exposure to the electron beam; the use of tilted sections in estimating the amount of shrinkage. J Cell Sci. 1974 Aug;15(3):693–701. doi: 10.1242/jcs.15.3.693. [DOI] [PubMed] [Google Scholar]
- Crewe A. V., Crewe D. A., Kapp O. H. Inexact three-dimensional reconstruction of a biological macromolecule from a restricted number of projections. Ultramicroscopy. 1984;13(4):365–371. doi: 10.1016/0304-3991(84)90002-0. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R. Cilia and flagella of eukaryotes. J Cell Biol. 1981 Dec;91(3 Pt 2):107s–124s. doi: 10.1083/jcb.91.3.107s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. F. The reconstruction of a three-dimensional structure from projections and its application to electron microscopy. II. Direct methods. Proc R Soc Lond B Biol Sci. 1972 Jul 25;182(1066):89–102. doi: 10.1098/rspb.1972.0068. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Heuser J. E. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol. 1985 Jun;100(6):2008–2018. doi: 10.1083/jcb.100.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U., Heuser J. Structural comparison of purified dynein proteins with in situ dynein arms. J Mol Biol. 1984 Dec 25;180(4):1083–1118. doi: 10.1016/0022-2836(84)90272-9. [DOI] [PubMed] [Google Scholar]
- Hard R., Rieder C. L. Muciliary transport in newt lungs: the ultrastructure of the ciliary apparatus in isolated epithelial sheets and in functional triton-extracted models. Tissue Cell. 1983;15(2):227–243. doi: 10.1016/0040-8166(83)90019-8. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Wall J. S. Structure and molecular weight of the dynein ATPase. J Cell Biol. 1983 Mar;96(3):669–678. doi: 10.1083/jcb.96.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. V., Parsons D. F. Design features of a photographic film optimized for the high-voltage electron microscope. Ultramicroscopy. 1977 Aug;2(4):371–384. doi: 10.1016/s0304-3991(76)92150-1. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E., Levy H. A., Durfee R. C., Margle S. M., Tinnel E. P., Hingerty B. E., Dover S. D., Fuchs H. Modeling Balbiani Ring gene transcription with electron microscope tomography. Eur J Cell Biol. 1984 Sep;35(1):129–142. [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Levy H. A., Durfee R. C., Margle S. M., Tinnel E. P., Dover S. D. Electron microscope tomography: transcription in three dimensions. Science. 1983 Apr 29;220(4596):498–500. doi: 10.1126/science.6836293. [DOI] [PubMed] [Google Scholar]
- Radermacher M., Frank J. Representation of three-dimensionally reconstructed objects in electron microscopy by surfaces of equal density. J Microsc. 1984 Oct;136(Pt 1):77–85. doi: 10.1111/j.1365-2818.1984.tb02547.x. [DOI] [PubMed] [Google Scholar]
- Reiser M., Rupp N., Lukas P., Heller H. J., Allgayer B., Petsch R. Die normalen anatomischen Strukturen des Körpers im MR-Tomogramm: Untersuchungen mit einem 0,35 T supraleitenden Magneten. Teil 2: Abdomen, Becken, Haltungs- und Bewegungsapparat. Rontgenpraxis. 1985 Jan;38(1):11–18. [PubMed] [Google Scholar]
- Rieder C. L. Thick and thin serial sectioning for the three-dimensional reconstruction of biological ultrastructure. Methods Cell Biol. 1981;22:215–249. doi: 10.1016/s0091-679x(08)61879-8. [DOI] [PubMed] [Google Scholar]
- Subirana J. A., Muñoz-Guerra S., Aymamí J., Radermacher M., Frank J. The layered organization of nucleosomes in 30 nm chromatin fibers. Chromosoma. 1985;91(5):377–390. doi: 10.1007/BF00291012. [DOI] [PubMed] [Google Scholar]
- Subirana J. A., Muñoz-Guerra S., Radermacher M., Frank J. Three-dimensional reconstruction of chromatin fibers. J Biomol Struct Dyn. 1983 Dec;1(3):705–714. doi: 10.1080/07391102.1983.10507476. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Scanning electron microscopy of intracellular structures. Int Rev Cytol. 1980;68:97–125. doi: 10.1016/s0074-7696(08)62308-6. [DOI] [PubMed] [Google Scholar]
- Verschoor A., Frank J., Radermacher M., Wagenknecht T., Boublik M. Three-dimensional reconstruction of the 30 S ribosomal subunit from randomly oriented particles. J Mol Biol. 1984 Sep 25;178(3):677–698. doi: 10.1016/0022-2836(84)90245-6. [DOI] [PubMed] [Google Scholar]