Significance
Capsules are bacterial surface structures used by many Gram-negative pathogens to evade the host immune system. They are comprised of long carbohydrate chains, called capsular polysaccharides (CPSs), which possess a lipid at one end. The lipid is connected to the CPS through an unusual linker consisting of five to nine residues of an acidic sugar 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo). This study identifies two conserved proteins from CPS assembly systems, KpsC and KpsS, as the enzymes that synthesize the Kdo linker on the terminal lipid. Synthesis of this terminal glycolipid was reconstituted in vitro using purified enzymes and a synthetic lipid acceptor. The data support a unique model for CPS biosynthesis and identify these enzymes as unique targets for antibacterial therapies.
Abstract
Capsular polysaccharides (CPSs) are high-molecular-mass cell-surface polysaccharides, that act as important virulence factors for many pathogenic bacteria. Several clinically important Gram-negative pathogens share similar systems for CPS biosynthesis and export; examples include Escherichia coli, Campylobacter jejuni, Haemophilus influenzae, Neisseria meningitidis, and Pasteurella multocida. Each CPS contains a serotype-specific repeat-unit structure, but the glycans all possess a lipid moiety at their reducing termini. In E. coli and N. meningitidis, the predominant lipid is a lysophosphatidylglycerol moiety that is attached to the repeat-unit domain of the CPS via multiple residues of 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo), referred to as a poly-Kdo linker. The Kdo residues are β-linked, suggesting that they are synthesized by retaining glycosyltransferases. To date, the only characterized Kdo transferases are the inverting enzymes that catalyze the α-linkages found in lipopolysaccharide. Here, we identify two conserved proteins from CPS assembly systems, KpsC and KpsS, as the β-Kdo-transferases and demonstrate in vitro reconstitution of poly-Kdo linker assembly on a fluorescent phosphatidylglycerol acceptor. KpsS adds the first Kdo residue, and this reaction product is then extended by KpsC. Cross-complementation experiments demonstrate that the E. coli and N. meningitidis protein homologs are functionally conserved.
Many bacterial pathogens possess capsules, which are comprised of high-molecular-mass (>100,000 Da) polysaccharides, known as capsular polysaccharides (CPSs) (1, 2). Capsules represent a highly hydrated surface layer, conferring protection against host defenses, primarily phagocytosis. In Gram-negative bacteria, most CPSs are assembled by one of two widely distributed systems: the Wzy-dependent and ATP-binding cassette (ABC) transporter-dependent pathways (1). Escherichia coli CPSs formed by the Wzy-dependent pathway have been called “group 1,” distinguishing them from the “group 2” ABC transporter-dependent CPSs. The ABC transporter-dependent pathway is the focus of this study.
The E. coli K1 system provides an influential model for the ABC transporter-dependent pathway. K1 is one of more than 80 K (CPS) serotypes in E. coli (3), and its repeat-unit structure is composed of α-2,8-linked N-acetylneuraminic acid (polysialic acid; PSA) (4). The genetic locus for K1 biosynthesis and assembly was the first polysaccharide biosynthesis gene cluster cloned and expressed in E. coli, and it paved the way for understanding of this system and other systems (5). The K1 locus encodes serotype-specific proteins that synthesize the K1 repeat-unit glycan and several conserved (serotype-independent) proteins found in all E. coli group 2 capsule loci. Most of the serotype-independent proteins are also found in other bacteria with CPSs synthesized by this pathway. Examples include Campylobacter jejuni, Haemophilus influenzae, Neisseria meningitidis and Pasteurella multocida (2). Four of the conserved proteins are involved in the transport of CPS from the cytoplasm, where it is synthesized, to the cell surface (reviewed in refs. 2 and 6–8). These proteins include the system-defining ABC transporter (KpsMT in E. coli nomenclature), an inner-membrane polysaccharide copolymerase (PCP-3) protein, designated KpsE, and KpsD, and an outer-membrane polysaccharide export (OPX) protein. Together, the KpsMTED proteins are thought to form a transenvelope complex analogous to the one proposed for tripartite efflux pumps (1, 2, 8–10).
We recently reported that CPSs from E. coli and N. meningitidis contain the same reducing terminal (lyso)phosphatidylglycerol moiety, which is attached to the CPS via a poly-Kdo linker (11). The poly-Kdo linker is proposed to be a unifying feature of CPSs synthesized via the ABC transporter-dependent pathway (11). It has long been known that the ABC transporter proteins display no specificity for the CPS repeat unit (1, 12, 13) and that the conserved reducing terminal glycolipid provides an attractive candidate for an export signal. Although the enzymes and processes involved in biosynthesis of the poly-Kdo linker are unknown, the linker consists of β-linked Kdo, and the donor sugar is likely CMP-β-Kdo (14), so the corresponding glycosyltransferase enzyme(s) are predicted to be retaining Kdo transferases (2). All Kdo transferases characterized to date are inverting enzymes that add α-linked Kdo (or Kdo derivatives) to the inner core region of all lipopolysaccharides (LPSs) (15). The highly conserved WaaA α-Kdo transferase provides the best-characterized example (16). In contrast, β-Kdo is relatively rare. It has been found in nature as part of the repeat units of some LPS O antigens in Providencia alcalifaciens and as the nonreducing chain terminating residue in the O12 antigen from Klebsiella pneumoniae (17, 18). It has also been identified in CPS repeat units from E. coli serotype K12 (19), N. meningitidis group E (20), and Actinobacillus pleuropneumoniae 5a and 5b (21, 22). However, the β-Kdo transferases required for synthesis of these structures have not been identified.
The genetic loci for model ABC transporter-dependent CPSs encode two additional conserved (serotype-independent) proteins, designated KpsC and KpsS in E. coli (23) and C. jejuni (24), HcsA and HcsB in H. influenzae (25), LipA and LipB in N. meningitidis (26), and PhyA and PhyB in P. multocida (27). The roles of these proteins in CPS assembly have been debated. In E. coli K1 and K5 and N. meningitidis group B, mutations in either kpsC or kpsS (or their homologs) result in intracellular accumulation of CPS (28–30). This CPS is not lipidated (11, 26, 28), suggesting that KpsC and KpsS may be involved in either synthesizing the phospholipid terminus, or in transferring the CPS to a phospholipid. A reducing terminal Kdo residue was proposed in E. coli K5, and CPS chains from kpsC and kpsS mutants in this serotype were devoid of Kdo (28), suggesting that these proteins may function in assembly of the conserved glycolipid terminus. In contrast, studies involving E. coli K1 kpsC and kpsS and N. meningitidis group b ΔlipAB mutants suggested that their intracellular CPS still possessed a lipid terminus (29, 30). This observation contributed to the proposal that KpsC and KpsS coupled synthesis and export of CPS (8). KpsC and KpsS are known to be integral parts of a multiprotein CPS assembly complex that contains export machinery (9, 29). In H. influenzae group b, hcsAB mutants are reported to accumulate polymer in the periplasm, leading to the suggestion that the proteins facilitate transport through the outer membrane (31). However, their sequences predict them to be cytoplasmic proteins, and this location has been confirmed experimentally for the E. coli homologs (1, 9).
Following the discovery of the poly-Kdo linker, and acknowledging the known distribution of conserved KpsC and KpsS proteins in ABC transporter-dependent CPS-assembly processes, we hypothesized that KpsC and KpsS are β-Kdo transferases, despite having no obvious similarity to known glycosyltransferases (32). The proposed activity was established in an in vitro assay system with purified proteins that reconstitutes the biosynthesis of the poly-Kdo linker.
Results
The KpsC and KpsS Proteins from E. coli and LipA and LipB Proteins from N. meningitidis Are Functionally Exchangeable.
Previous reports noted sequence similarities shared by KpsC and LipA (45% identity and 61% similarity) and between KpsS and LipB (36% and 56%) (9, 30). However, differences have been reported in the corresponding mutant phenotypes (2), so the question of their functional identity was assessed in genetic complementation studies. Insertionally inactivated kpsC and kpsS mutants of E. coli K1 strain EV36 synthesized but did not export CPS (8, 29, 33). We previously constructed complete deletions of kpsC and kpsS genes in EV36 and confirmed similar mutant phenotypes (Table S1 and Fig. S1) (11). The mutants are resistant to bacteriophage K1F, which requires PSA CPS as a receptor for infection (34). However, only the ΔkpsC mutant accumulated intracellular electron-transparent domains (Fig. S1A) that have been interpreted as aggregates of unexported PSA (29, 30). The domains in the ΔkpsC mutant reported here were significantly smaller than those observed in previous studies. Regardless of their EM morphologies, the ΔkpsC and ΔkpsS mutants both contained nonlipidated CPS based on the ability to bind to positively charged nylon membranes but not to PVDF (Fig. S1B). Surface CPS was restored when the ∆kpsC and ∆kpsS mutants were transformed with plasmids expressing the corresponding genes from either E. coli or N. meningitidis (Fig. 1). The activities of the two enzymes are distinct; i.e., expression of KpsC could not complement an E. coli ΔkpsS mutant, or vice versa.
Fig. 1.
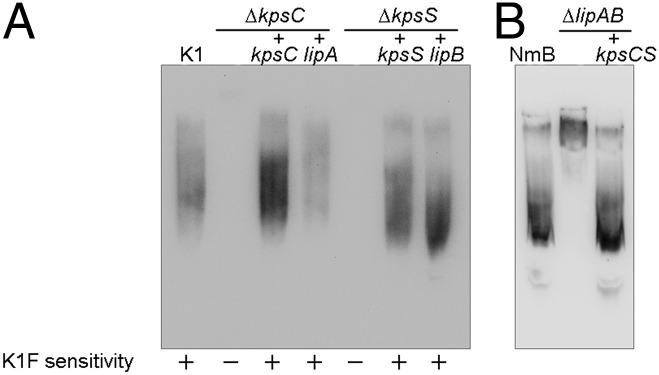
Heterologous complementation of kpsC and kpsS mutations in E. coli and N. meningitidis. E. coli ΔkpsC and ΔkpsS mutants (A) were transformed with plasmids carrying the genes indicated, and protein expression was induced with 0.1% l-arabinose. N. meningitidis strains were analyzed using chromosomally encoded expression of PSA in a strain constructed by allelic replacement (B). PSA production was examined in immunoblots on PVDF membrane, probed with mAb 2.2B. The inability of nonlipidated CPS produced by ΔkpsC and ΔkpsS mutants was reported previously (14). Small variations in apparent chain length may be due to different levels of expression of plasmid-encoded proteins.
A previous report showed that mutants containing partial deletions of the lipAB genes in N. meningitidis resulted in a reduction in PSA production (to ∼5% of wild type) and that this polymer was intracellular rather than being exported to the cell surface (30). However, in the absence of a complete lipAB deletion, it is possible that residual parts of the genes retained in the mutant contributed to the phenotype. A complete lipAB deletion was therefore constructed in N. meningitidis. No bacteriophage is available for PSA-producing N. meningitidis, so we analyzed PSA from both systems using immunoblots with αPSA monoclonal antibody. As expected, complementation of E. coli ΔkpsC and ΔkpsS mutants with the corresponding gene from either E. coli or N. meningitidis leads to PSA with a size distribution resembling the parent product (Fig. 1A and Fig. S2). The same is true for complementation of the corresponding N. meningitidis mutants (Fig. 1B). Cell-free lysate from the ∆lipAB mutant was examined by immunoblotting with α-PSA monoclonal antibody, and residual PSA was detected, consistent with previous reports (30). The PSA from ∆lipAB possessed a significantly higher average apparent molecular mass compared with the parent, suggesting a significantly longer average chain length. A similar effect is also seen in an E. coli ΔkpsE (PCP protein) mutant that cannot export CPS from the periplasm to the surface, so the altered chain lengths may simply reflect disruption of the biosynthesis complex (35). However, with the ΔkpsC, ΔkpsS, and ΔlipAB mutants, the absence of acylation could also influence PAGE migration. When the kpsCS genes from E. coli were inserted into the N. meningitidis chromosome (replacing lipAB), the wild-type size distribution of PSA was restored (Fig. 1B). The collective data therefore suggest that LipA and LipB serve as functional homologs of KpsC and KpsS, respectively. Given their sequence and functional identity, hereafter we will refer to the homologs as KpsC/S proteins.
KpsC Proteins Contain a Duplicated Domain.
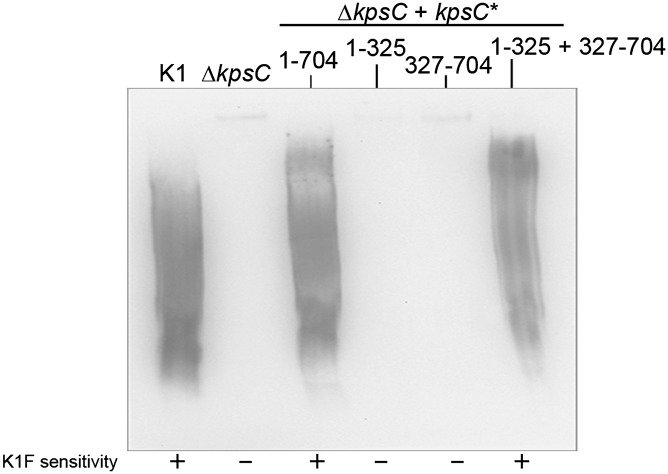
It has been reported that E. coli KpsC is composed of two domains that share similarity (9). Bioinformatic analysis revealed that the same is true of the N. meningitidis KpsC; the two domains share 45% similarity. However, both domains of KpsC also have predicted secondary structures similar to KpsS (Fig. S3). N. meningitidis KpsC comprises 704 residues, and the two predicted domains (residues 1–325 and 327–704) were coexpressed from compatible plasmids in the E. coli ΔkpsC mutant. Immunoblot analysis shows that neither domain is sufficient for CPS biosynthesis but that the two domains together are able to reconstitute CPS synthesis, indicating that they are independently folded (Fig. 2). Bacteriophage K1F sensitivity confirmed that the resulting CPS is exported to the cell surface. The observation that KpsC forms independently folding functional domains potentially complicates interpretation of mutant phenotypes arising from an insertionally inactivated gene (29, 30).
Fig. 2.
Restoration of surface PSA CPS in E. coli ΔkpsC cells transformed with plasmids encoding polypeptides encompassing predicted domains of N. meningitidis KpsC. Full-length KpsC contains 704 amino acids, and the numbers refer to the residues included in each construct. The concentrations of l-arabinose and anhydrotetracycline used to induce gene expression were 0.1% and 1 μg/mL, respectively. The numbers refer to residues from KpsC encompassed by each polypeptide. PSA production was examined on a PVDF immunoblot probed with mAb 2.2B, and surface PSA was detected by sensitivity to bacteriophage K1F. kpsC1-704 encodes the full-length N. meningitidis KpsC.
Construction of Functional Truncated Derivatives of KpsC and KpsS.
An in vitro assay using purified proteins is required to definitively test the hypothesis that KpsC and KpsS are the Kdo transferases involved in the synthesis of the poly-Kdo linker. Overexpression of KpsC and KpsS homologs from E. coli and N. meningitidis with various affinity tags yielded insoluble and/or unstable proteins, precluding their purification. To overcome this problem, N- and C-terminal truncated constructs were made for each of the N. meningitidis proteins, creating derivatives that could be expressed and purified. To determine the amount of protein that could be deleted without compromising function, the truncated KpsC and KpsS proteins were expressed without tags in the corresponding E. coli ∆kpsC or ∆kpsS backgrounds to ensure their ability to restore cell-surface PSA (Fig. S4 A and B). Surprisingly, as many as 110 amino acids could be deleted from the N-terminal end of KpsC before compromising its ability to restore CPS export in the kpsC mutant, at least when overexpressed. However, N-terminal truncations larger than 23 residues resulted in PSA chains with higher apparent average molecular masses than the wild-type product (Fig. S4A). A KpsC derivative lacking 32 C-terminal residues retained activity, but removal of 45 residues abrogated function. In contrast, deletion of any N-terminal residues abrogated function of KpsS, but a 10 amino acid C-terminal truncation derivative retained activity with no effect on the PAGE profile of the resulting CPS (Fig. S4B). Full-length KpsS comprised 419 residues. MalE fusions of the 70 amino acid N-terminal truncation of KpsC (MalE-KpsC70-704) and the 10 amino acid C-terminal truncation of KpsS (MalE-KpsS1-409) were purified (Fig. S4C) for in vitro activity assays.
KpsC and KpsS Are β-Kdo Transferases.
Activities of the MalE-fusion proteins were examined using an acceptor comprising a phosphatidylglycerol (PG) derivative possessing one C18:1 acyl chain and one C12:0 acyl chain containing a fluorescent tag (NBD-PG). CMP-β-Kdo is highly unstable (36, 37) so a coupled assay was used to make the donor in situ from CTP and Kdo using CMP-β-Kdo synthetase (KdsB). A small amount of degradation of the lipid substrate was seen on TLC in the absence of enzyme (Fig. 3), and analysis of the reaction by MS revealed small amounts of an m/z 377 ion, consistent with release of NBD-C12:0 (Fig. S5A). As expected, no additional (enzymatic) products were evident by TLC when the lipid substrate was incubated with KdsB, MalE-KpsC70-704, or MalE-KpsS1-409 in isolation (Fig. 3), and the LC-MS spectra of these samples contained no ions corresponding to Kdo-containing species. However, a unique product was observed by TLC in reactions containing KdsB and MalE-KpsS1-409, consistent with the addition of one or more Kdo residues (Fig. 3). LC-MS of this reaction mixture revealed unique ions corresponding to NBD-PG-Kdo (m/z 1089) (Table 1). Its identity was confirmed by MS/MS analysis, revealing both NBD-PG (m/z 869) and Kdo (m/z 219) (Fig. S5B). This data unequivocally identified KpsS as a Kdo-transferase. Also present in the reaction mixture was (C18:1)-PG-Kdo (m/z 729.6). Due to the inherent instability of the acceptor, it is impossible to know whether the NBD-C12:0 group was lost before or after Kdo addition. Interestingly, no ions were detected that would correspond to the (C18:1)-PG molecule lacking Kdo. It is possible that the small quantities of hydrolyzed acceptor do not ionize well in the mass spectrometer and are consequently undetectable, or that these molecules are efficiently modified with Kdo.
Fig. 3.
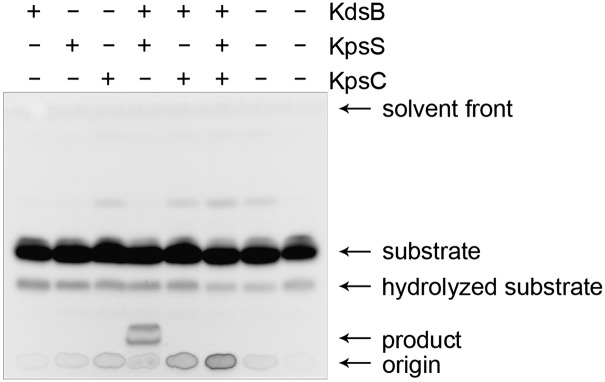
TLC separation of the reaction mixtures using NBD-PG acceptor. The enzymes included in each reaction are indicated.
Table 1.
LC-MS/MS of products from reactions with MalE-KpsS1-409 and MalE-KpsC71-704
| Reaction | Expected m/z | Observed m/z | Species |
| KpsS1-409 reaction | |||
| 870.0 1- | 869.8 1- | NBD-PG | |
| 1,090.2 1- | 1,089.8 1- | NBD-PG-Kdo | |
| 544.6 2- | 544.5 2- | ||
| 730.8 1- | 729.6 1- | (C18:1)-PG-Kdo | |
| MS/MS m/z 544.5 2- | 219.2 1- | 219.2 1- | Kdo |
| 870.0 1- | 869.6 1- | NBD-PG | |
| 434.5 2- | 434.4 2- | ||
| MS/MS of m/z 729.6 1- | 219.2 1- | 219.1 1- | Kdo |
| 509.6 1- | 509.4 1- | (C18:1)-PG | |
| KpsC71-704 reaction | |||
| 870.0 1- | 869.7 1- | NBD-PG | |
| KpsS1-409/KpsC71-704 reaction | |||
| 870.0 1- | 869.8 1- | NBD-PG | |
| 1,170.1 1- | 1,169.7 1- | (C18:1)-PG-Kdo3 | |
| 584.6 2- | 584.4 2- | ||
| 1,390.3 1- | 1,389.7 1- | (C18:1)-PG-Kdo4 | |
| 694.6 2- | 694.4 2- | ||
| 804.7 2- | 804.5 2- | (C18:1)-PG-Kdo5 | |
| MS/MS of m/z 584.4 2- | 219.2 1- | 219.1 1- | Kdo |
| 509.6 1- | 509.4 1- | (C18:1)-PG | |
| 729.8 1- | 729.5 1- | (C18:1)-PG-Kdo1 | |
| 950.0 1- | 949.5 1- | (C18:1)-PG-Kdo2 | |
| MS/MS of m/z 694.4 2- | 219.2 1- | 219.2 1- | Kdo |
| 439.5 1- | 439.2 1- | Kdo2 | |
| 729.8 1- | 729.5 1- | (C18:1)-PG-Kdo1 | |
| 950.0 1- | 949.5 1- | (C18:1)-PG-Kdo2 | |
| 1,170.2 1- | 1,169.6 1- | (C18:1)-PG-Kdo3 | |
| MS/MS of m/z 804.5 2- | 219.2 1- | 219.1 1- | Kdo |
| 439.5 1- | 439.3 1- | Kdo2 | |
| 729.8 1- | 729.5 1- | (C18:1)-PG-Kdo1 | |
| 1,170.2 1- | 1,169.6 1- | (C18:1)-PG-Kdo3 | |
NBD-PG is diacylated phosphatidylglycerol with C18:1 and nitrobenzoxadiazole (NBD)-C12:0 acyl chains.
The TLC profile of the reaction mixture containing all three enzymes contained no obvious new product(s) (Fig. 3A). However, LC-MS revealed products containing three, four, and five Kdo residues, and these were confirmed by MS/MS (Table 1 and Fig. S5C). All of these species contained monoacyl (C18:1)-PG, rather than the diacyl NBD-PG acceptor. The loss of the NBD-C12:0 chain explains the absence of detectable new products in TLC, which relies on fluorescence from the NBD moiety. The simplest interpretation of the collective data is that KpsS adds the first Kdo residue to the PG acceptor, creating a substrate for KpsC, which can then add several Kdo residues. However, the data do not preclude the possibility that KpsC has no Kdo transferase activity itself but instead has an activating effect on KpsS. To resolve this question, NBD-PG was preincubated with MalE-KpsS1-409. The substrate/product was purified by SepPak C18 and then reincubated with either MalE-KpsS1-409 or MalE-KpsC70-704 alone and in combination. TLC profiles suggested that the MalE-KpsS1-409 product was completely consumed in these reactions (Fig. S6A), but residual product was identified by LC-MS/MS (m/z 729.8). These reactions contained unique species comprising two and three Kdo residues (Table 2 and Fig. S6 B and C), confirming that KpsC is a Kdo transferase.
Table 2.
LC-MS/MS of Kdo-containing species from KpsC reactions
| Expected m/z | Observed m/z | Species | |
| 584.6 2- | 584.8 2- | (C18:1)-PG-Kdo3 | |
| 474.5 2- | 474.8 2- | (C18:1)-PG-Kdo2 | |
| 729.8 1- | 729.6 1- | (C18:1)-PG-Kdo | |
| MS/MS of m/z 584.8 2- | 219.2 1- | 219.1 1- | Kdo |
| 439.4 1- | 439.3 1- | Kdo2 | |
| 659.5 1- | 659.4 1- | Kdo3 | |
| 329.3 2- | 329.2 2- | ||
| 509.6 1- | 509.5 1- | (C18:1)-PG | |
| 729.8 1- | 729.6 1- | (C18:1)-PG-Kdo | |
| 950.0 1- | 949.7 1- | (C18:1)-PG-Kdo2 | |
| MS/MS of m/z 474.8 2- | 219.2 1- | 219.1 1- | Kdo |
| 439.4 1- | 439.2 1- | Kdo2 | |
| 509.6 1- | 509.5 1- | (C18:1)-PG | |
| 729.8 1- | 729.6 1- | (C18:1)-PG-Kdo | |
| MS/MS of m/z 729.6 1- | 219.2 1- | 219.1 1- | Kdo |
| 509.6 1- | 509.5 1- | (C18:1)-PG |
NBD-PG was pretreated with MalE-KpsS1-409, and hydrophobic moieties were separated from other reaction components using C18 resin and then used in reactions containing MalE-KpsC71-704. NBD-PG is diacylated phosphatidylglycerol with C18:1 and nitrobenzoxadiazole (NBD)-C12:0 acyl chains. Kdo-containing material was identified using an extracted ion chromatogram with m/z 219.
Discussion
Similarities in the protein sequences of KpsC and KpsS homologs in E. coli and N. meningitidis have been recognized, but differences in various mutant phenotypes led to the proposal that the CPS biosynthesis pathways in these bacteria were subtly different. This ambiguity resulted in various proposed functions for KpsS and KpsC; these included lipidation of CPS (26, 28), aiding in transport (31), linking synthesis and export (30), and protecting the polymer during synthesis by forming a physical barrier around the CPS (33). Here, we established that KpsS and KpsC are β-Kdo transferases responsible for the formation of the conserved poly-Kdo linker attaching (lyso-)PG to CPS. However, the data do not preclude the possibility that these enzymes also perform additional structural roles in the multienzyme complex.
E. coli EV36 ΔkpsC makes PSA that is not lipidated (11). We proposed that this CPS results from low levels of initiation on an aberrant acceptor lacking the hydrophobic character necessary for it to bind a PVDF membrane. This proposal is consistent with published experiments showing that KpsS and KpsC are part of the essential enzyme requirement for de novo PSA biosynthesis in membrane preparations (38), where aberrant initiation may not be possible. Like the corresponding E. coli mutant (11), intracellular PSA accumulates in N. meningitidis ΔlipAB (30). This material possesses an increased apparent size (compared with the wild type) compared with immunoblotting using nylon membranes, but it differs in its ability to bind PVDF. These differences are difficult to interpret without knowledge of the nature of the aberrant acceptor(s). However, because CPS from complemented E. coli and N. meningitidis ΔkpsCS mutants is exported to the cell surface and appears identical to the authentic product in immunoblots, we conclude that KpsC and KpsS are functionally identical in each species and that the essential features of the biosynthetic pathways for these CPSs are conserved.
KpsC tolerated extensive deletions at its N and C termini without compromising function. The CPS produced by mutants containing these truncated derivatives binds PVDF membrane and is properly exported, consistent with the authentic acceptor being used. How the truncations affect the size distribution of CPS is not clear, but variations in the PAGE profiles also occur when the level of KpsC expression is altered (Fig. S2), and in kpsE mutants (35), so the phenotype may just reflect altered stoichiometries in biosynthesis components. In ABC transporter-dependent LPS O antigen biosynthesis, glycan chain length is controlled by one of two strategies. E. coli O9a serves as the prototype for O antigens where “capping residues” are added to the nonreducing end to terminate polymerization (6, 39, 40). The nucleotide-binding polypeptide of the ABC transporter possesses a carbohydrate-binding module (CBM) that recognizes and exports only terminated polymer (6). In other O-antigen systems, represented by K. pneumoniae O2a, there is no capping residue or CBM, and the O-antigen chain length is instead controlled by the relative activities of the chain-extension enzymes and the ABC transporter (41). The K. pneumoniae O2a transporter shows no specificity for the structure of the exported glycan (41). The properties of the CPS ABC transporters (6, 12, 13, 42) suggest similarity to the latter model. KpsC has been shown to interact with the CPS polymerase and the export machinery (33), so it is possible that truncations of KpsC affect these interactions, and the proper coupling of synthesis and export, resulting in “unregulated” polymerase activity.
In the assembly of the poly-Kdo linker, KpsS adds the first Kdo residue to a (lyso-)PG acceptor using CMP-β-Kdo as the donor sugar. Following the activity of KpsS, KpsC is required to add multiple Kdo residues. Authentic poly-Kdo linker contains both β2,4- and β2,7-linked Kdo (11), and KpsC has two independently folding domains, so it is tempting to speculate that these domains possess different linkage specificities. Unfortunately, definitively establishing the linkage specificities will require more reaction product than is produced under the current conditions. Interestingly, all Kdo-containing products in reactions with both KpsS and KpsC possessed lyso-PG (lacking the NBD-C12:0 acyl chain). The in vitro substrate is susceptible to degradation in the absence of enzyme, and it is possible that KpsS may be highly selective for lyso-derivatives. In vivo, the generation of lyso-PG derivatives is mediated by phospholipase A (PLA). E. coli has two PLA activities; the well-characterized outer membrane PLA was previously shown not to be required for CPS biosynthesis or generation of the lyso-derivatives attached to CPS (11), and the identity of the inner membrane/cytosolic PLA remains unknown (43).
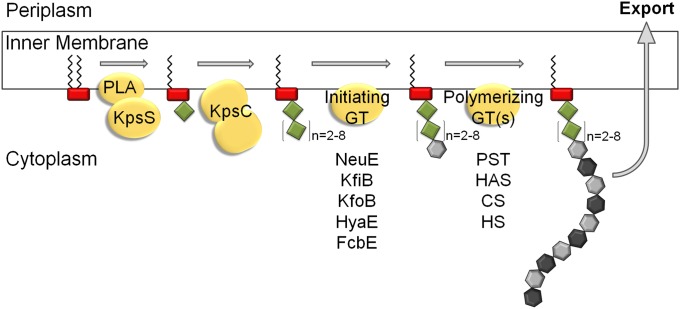
The observation that KpsS and KpsC transfer Kdo directly to the phospholipid acceptor has implications for the overall mechanism of synthesis, particularly as to the controversy in the older literature concerning the involvement of undecaprenol-linked intermediates (1, 2). It seems unlikely that the repeating unit domain of CPS would be assembled independently and then transferred to the poly-Kdo linker, and there is no candidate enzyme for such an activity encoded within the CPS biosynthesis locus. A model for the assembly scheme is proposed in Fig. 4. The Kdo-linker oligosaccharides synthesized by KpsS and KpsC in vitro contained both even and odd numbers of Kdo residues. In contrast, the authentic CPSs contain only odd or even numbers, depending on the serotype (11). We propose that the precise in vivo product depends on constraints imposed by other components in the assembly system. We have proposed that a priming enzyme adds the first residue(s) of the CPS repeat unit to the poly-Kdo linker to create an acceptor appropriate for CPS polymerization (2). Notably, each CPS biosynthesis locus encodes a serotype-specific protein of unknown function, but sharing some similarity with known glycosyltransferases. NeuE is found in E. coli K1 and K92 and N. meningitidis groups B, C, W-135, and Y, which all produce PSA. NeuE is required for de novo synthesis of PSA in vitro (38). KfiB and KfoB are found in E. coli K5 and K4, respectively, whereas the candidates in P. multocida types A, D, and F are HyaE, DcbE, and FcbE, respectively (reviewed in ref. 2). Because the poly-Kdo linker contains an alternating oligomer of β2,4 and β2,7 linkages, an initiating enzyme with specificity for one particular linkage type would result in a linker with either odd or even numbers of Kdo residues (Fig. 4).
Fig. 4.
Proposed model for CPS biosynthesis. The headgroup of (lyso)phosphatidylglycerol is represented by a red rectangle, Kdo residues as green diamonds, and CPS repeat unit sugars as gray hexagons. KpsC is depicted as a single protein with two domains. CS, chondroitin synthase; GT, glycosyltransferase; HAS, hyaluronan synthase; HS, heparosan synthase; PLA, phospholipase A; PST, polysialyltransferase. Candidate initiating GTs and known polymerizing GTs from different capsule-assembly systems are identified.
KpsS and KpsC are examples of previously undescribed β-Kdo transferases. Using the sequence of KpsS as a probe, we found genes encoding putative Kdo transferases in genetic loci responsible for production of CPS or O antigens known to contain β-Kdo in their glycostructures. Sequence alignment shows minimal conservation, which is not surprising given the variation in linkages and acceptors for these enzymes (Fig. S7). However, they all contain a conserved HP motif similar to one reported previously in some sialyltransferases (44). The function of the HP motif is unknown, but sialic acid, like Kdo, contains a carboxylate at the anomeric center. Because the sialyltransferases use an inverting mechanism, the HP motif is not likely to be in the catalytic center, and determining its role will require further investigation. Well-developed bioinformatic tools have identified more than 100,000 glycosyltransferase enzymes in 94 current families in the database (32). KpsC and KpsS were not identified as members of any family, and they potentially represent a unique fold. Consequently, it may be feasible to design inhibitors that target only retaining β-Kdo transferases, leaving unaffected the more prevalent inverting Kdo transferases involved in LPS biosynthesis. Any antiinfective resulting from this approach would specifically target bacteria with CPS involving a poly-Kdo linker. Database searches suggest that KpsC and KpsS are not widely distributed outside pathogens so target selectivity may minimize unintended effects on commensal bacteria.
Materials and Methods
Bacterial Strains and Plasmids.
E. coli K1 (EV36) and N. meningitidis 992B (NRCC4726) were gifts from E. Vimr (University of Champaign-Urbana, Urbana, IL) and W. Wakarchuk (Ryerson University, Toronto, ON, Canada), respectively (Table S1). E. coli isolates were grown in Luria broth (LB) at 37 °C overnight. N. meningitidis was grown on gonococcus (GC) plates supplemented with 1% glucose at 37 °C in 5% CO2 for 18 h. The E. coli ΔkpsC and ΔkpsS mutants were described previously (11). N. meningitidis mutants were made by allelic replacement (See SI Materials and Methods and Fig. S8 for details). Genetic complementation of E. coli mutants was performed with genes expressed from a modified pBAD vectors with either arabinose- or anhydrotetracycline-inducible promoters (Table S1) (45).
Detection of Polysialic Acid.
Bacteriophage K1F-sensitivity assays and immunoblotting were performed as previously described (2, 35). The PSA-specific monoclonal antibody mAb 2–2B used in immunoblotting was a gift from M Apicella (University of Iowa, Iowa City, IA).
Expression and Purification of Enzymes.
MalE-KpsC71-704 and its truncated derivatives were expressed in E. coli TOP10. Overnight cultures were used at 1/100 dilution to inoculate LB media supplemented with 150 μg/mL ampicillin. Cultures were grown at 37 °C for 2 h with shaking before inducing with IPTG to a final concentration of 0.5 mM at 20 °C overnight. Cells were harvested by centrifugation, resuspended in 50 mM NaHEPES, 200 mM NaCl, pH 8, and lysed using an Emulsiflex C5 homogenizer (Avestin). Protease inhibitor mixture (Sigma-Aldrich) was added, and the suspension was cleared by successive centrifugation steps at 25 000 × g for 30 min and 100,000 × g for 1 h at 4 °C. MalE-KpsC was purified from the supernatant over 2 mL amylose resin (New England Biolabs). The column was washed with 20 mL of buffer and eluted with 20 mL of buffer containing 10 mM maltose with collection of 1-mL fractions. Protein purity was assessed by SDS/PAGE analysis, and the concentration was measured using A280.
MalE-KpsS1-409 was expressed in E. coli ΔkpsS. Conditions used for protein expression and preparation of cell-free lysates were the same as those used for MalE-KpsC. However, MalE-KpsS was confined to the membrane fraction (100,000 × g pellet). Membranes were solubilized with 20 mL of cell-lysis buffer containing 2% n-dodecyl-β-d-maltoside (DDM) (Sigma-Aldrich) at 4 °C for 1–2 h. An additional centrifugation step at 100,000 × g for 30 min removed any remaining insoluble material. The supernatant was then loaded onto 2 mL of amylose resin and washed extensively with buffer containing 0.005% DDM. MalE-KpsS was eluted with 20 mL of buffer containing 0.005% DDM and 10 mM maltose and collected in 1-mL fractions. Protein purity was assessed by SDS/PAGE analysis, and concentration was measured using A280. These proteins were stable at 4 °C for several days.
The CMP-β-Kdo synthetase, KdsB, was expressed and purified as previously described (46).
Kdo Transferase Assays.
A coupled reaction was developed using the CMP-β-Kdo synthetase, KdsB, and the substrate (acceptor) 1-oleoyl-2-{12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-[phospho-rac-(1-glycerol)] (NBD-PG) (Avanti Polar Lipids). Optimized reactions (SI Materials and Methods) contained 2 mM Kdo, 5 mM CTP, 0.5 μg of KdsB, 0.25 mM NBD-PG, 50 mM NaHEPES, pH 8, 10 mM MgCl2, 0.01% DDM, and 4 μg of KpsC70-704/KpsS1-409. The amount of MalE-KpsC70-704 and MalE-KpsS1-409 added was calculated such that the KpsC70-704/KpsS1-409 portions of the fusion proteins were equivalent to 4 μg. Reaction mixtures were incubated at 25 °C, then stopped with the addition of an equal amount of 50% acetonitrile, 1% SDS, and 10 mM EDTA. Aliquots of the reaction mixtures (1 μL) were spotted on Silica gel TLC plates and developed with 25:15:4:2 chloroform:methanol:water:acetic acid. Substrate and products were detected using a hand-held UV lamp.
Mass Spectrometry.
Reactions were partially purified using C18 SepPak cartridge using 70% acetonitrile to elute. The samples were dried using a SpeedVac and analyzed by mass spectrometry as previously described (SI Materials and Methods) (11).
Supplementary Material
Acknowledgments
We thank Dr. Warren Wakarchuk for helpful discussions, Drs. Dyanne Brewer and Armen Charchoglyan for mass spectrometry analysis, and Dianne Moyles for electron microscopy. This work was supported by funding from the Canadian Institutes of Health Research and the National Sciences and Engineering Research Council (awarded to C.W.). C.W. holds a Canada Research Chair, and L.M.W. gratefully acknowledges a Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312637110/-/DCSupplemental.
References
- 1.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 2.Willis LM, Whitfield C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res. 2013;378:35–44. doi: 10.1016/j.carres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Orskov I, Orskov F, Jann B, Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuire EJ, Binkley SB. The structure and chemistry of colominic acid. Biochemistry. 1964;3:247–251. doi: 10.1021/bi00890a017. [DOI] [PubMed] [Google Scholar]
- 5.Silver RP, et al. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981;289(5799):696–698. doi: 10.1038/289696b0. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev. 2010;74(3):341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuthbertson L, Mainprize IL, Naismith JH, Whitfield C. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol Mol Biol Rev. 2009;73(1):155–177. doi: 10.1128/MMBR.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vimr ER, Steenbergen SM. Early molecular-recognition events in the synthesis and export of group 2 capsular polysaccharides. Microbiology. 2009;155(Pt 1):9–15. doi: 10.1099/mic.0.023564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigg GP, Barrett B, Roberts IS. The localization of KpsC, S and T, and KfiA, C and D proteins involved in the biosynthesis of the Escherichia coli K5 capsular polysaccharide: evidence for a membrane-bound complex. Microbiology. 1998;144(Pt 10):2905–2914. doi: 10.1099/00221287-144-10-2905. [DOI] [PubMed] [Google Scholar]
- 10.McNulty C, et al. The cell surface expression of group 2 capsular polysaccharides in Escherichia coli: the role of KpsD, RhsA and a multi-protein complex at the pole of the cell. Mol Microbiol. 2006;59(3):907–922. doi: 10.1111/j.1365-2958.2005.05010.x. [DOI] [PubMed] [Google Scholar]
- 11.Willis LM, et al. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci USA. 2013;110(19):7868–7873. doi: 10.1073/pnas.1222317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver RP, Prior K, Nsahlai C, Wright LF. ABC transporters and the export of capsular polysaccharides from gram-negative bacteria. Res Microbiol. 2001;152(3-4):357–364. doi: 10.1016/s0923-2508(01)01207-4. [DOI] [PubMed] [Google Scholar]
- 13.Lo TM, Ward CK, Inzana TJ. Detection and identification of Actinobacillus pleuropneumoniae serotype 5 by multiplex PCR. J Clin Microbiol. 1998;36(6):1704–1710. doi: 10.1128/jcm.36.6.1704-1710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohlbrenner WE, Fesik SW. Determination of the anomeric specificity of the Escherichia coli CTP:CMP-3-deoxy-D-manno-octulosonate cytidylyltransferase by 13C NMR spectroscopy. J Biol Chem. 1985;260(27):14695–14700. [PubMed] [Google Scholar]
- 15.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt H, et al. Structural and mechanistic analysis of the membrane-embedded glycosyltransferase WaaA required for lipopolysaccharide synthesis. Proc Natl Acad Sci USA. 2012;109(16):6253–6258. doi: 10.1073/pnas.1119894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinogradov E, et al. Structures of lipopolysaccharides from Klebsiella pneumoniae: Eluicidation of the structure of the linkage region between core and polysaccharide O chain and identification of the residues at the non-reducing termini of the O chains. J Biol Chem. 2002;277(28):25070–25081. doi: 10.1074/jbc.M202683200. [DOI] [PubMed] [Google Scholar]
- 18.Ovchinnikova OG, et al. Localization and molecular characterization of putative O antigen gene clusters of Providencia species. Microbiology. 2012;158(Pt 4):1024–1036. doi: 10.1099/mic.0.055210-0. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt MA, Jann K. Structure of the 2-keto-3-deoxy-D-manno-octonic-acid-containing capsular polysaccharide (K12 antigen) of the urinary-tract-infective Escherichia coli O4:K12:H- Eur J Biochem. 1983;131(3):509–517. doi: 10.1111/j.1432-1033.1983.tb07291.x. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharjee AK, Jennings HJ, Kenny CP. Structural elucidation of the 3-deoxy-D-manno-octulosonic acid containing meningococcal 29-e capsular polysaccharide antigen using carbon-13 nuclear magnetic resonance. Biochemistry. 1978;17(4):645–651. doi: 10.1021/bi00597a013. [DOI] [PubMed] [Google Scholar]
- 21.Altman E, Brisson JR, Gagné SM, Perry MB. Structure of the capsular polysaccharide of Actinobacillus pleuropneumoniae serotype 5b. Eur J Biochem. 1992;204(1):225–230. doi: 10.1111/j.1432-1033.1992.tb16628.x. [DOI] [PubMed] [Google Scholar]
- 22.Altman E, Brisson JR, Perry MB. Structure of the capsular polysaccharide of Haemophilus pleuropneumoniae serotype 5. Eur J Biochem. 1987;170(1-2):185–192. doi: 10.1111/j.1432-1033.1987.tb13685.x. [DOI] [PubMed] [Google Scholar]
- 23.Simpson DA, Hammarton TC, Roberts IS. Transcriptional organization and regulation of expression of region 1 of the Escherichia coli K5 capsule gene cluster. J Bacteriol. 1996;178(22):6466–6474. doi: 10.1128/jb.178.22.6466-6474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol. 2000;35(3):529–541. doi: 10.1046/j.1365-2958.2000.01717.x. [DOI] [PubMed] [Google Scholar]
- 25.Satola SW, Schirmer PL, Farley MM. Complete sequence of the cap locus of Haemophilus influenzae serotype b and nonencapsulated b capsule-negative variants. Infect Immun. 2003;71(6):3639–3644. doi: 10.1128/IAI.71.6.3639-3644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frosch M, Müller A. Phospholipid substitution of capsular polysaccharides and mechanisms of capsule formation in Neisseria meningitidis. Mol Microbiol. 1993;8(3):483–493. doi: 10.1111/j.1365-2958.1993.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 27.Boyce JD, Chung JY, Adler B. Pasteurella multocida capsule: Composition, function and genetics. J Biotechnol. 2000;83(1-2):153–160. doi: 10.1016/s0168-1656(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 28.Bronner D, et al. Expression of the capsular K5 polysaccharide of Escherichia coli: biochemical and electron microscopic analyses of mutants with defects in region 1 of the K5 gene cluster. J Bacteriol. 1993;175(18):5984–5992. doi: 10.1128/jb.175.18.5984-5992.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cieslewicz M, Vimr E. Thermoregulation of kpsF, the first region 1 gene in the kps locus for polysialic acid biosynthesis in Escherichia coli K1. J Bacteriol. 1996;178(11):3212–3220. doi: 10.1128/jb.178.11.3212-3220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzeng YL, et al. Translocation and surface expression of lipidated serogroup B capsular Polysaccharide in Neisseria meningitidis. Infect Immun. 2005;73(3):1491–1505. doi: 10.1128/IAI.73.3.1491-1505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukupolvi-Petty S, Grass S, St Geme JW., 3rd The Haemophilus influenzae Type b hcsA and hcsB gene products facilitate transport of capsular polysaccharide across the outer membrane and are essential for virulence. J Bacteriol. 2006;188(11):3870–3877. doi: 10.1128/JB.01968-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coutinho PM, Deleury E, Davies GJ, Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328(2):307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 33.Steenbergen SM, Vimr ER. Biosynthesis of the Escherichia coli K1 group 2 polysialic acid capsule occurs within a protected cytoplasmic compartment. Mol Microbiol. 2008;68(5):1252–1267. doi: 10.1111/j.1365-2958.2008.06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross RJ, Cheasty T, Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larue K, Ford RC, Willis LM, Whitfield C. Functional and structural characterization of polysaccharide co-polymerase proteins required for polymer export in ATP-binding cassette transporter-dependent capsule biosynthesis pathways. J Biol Chem. 2011;286(19):16658–16668. doi: 10.1074/jbc.M111.228221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugai T, Lin CH, Shen GJ, Wong CH. CMP-KDO synthetase: Overproduction and application to the synthesis of CMP-KDO and analogs. Bioorg Med Chem. 1995;3(3):313–320. doi: 10.1016/0968-0896(95)00023-a. [DOI] [PubMed] [Google Scholar]
- 37.Lin CH, Murray BW, Ollmann IR, Wong CH. Why is CMP-ketodeoxyoctonate highly unstable? Biochemistry. 1997;36(4):780–785. doi: 10.1021/bi962055c. [DOI] [PubMed] [Google Scholar]
- 38.Andreishcheva EN, Vann WF. Gene products required for de novo synthesis of polysialic acid in Escherichia coli K1. J Bacteriol. 2006;188(5):1786–1797. doi: 10.1128/JB.188.5.1786-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke BR, et al. In vitro reconstruction of the chain termination reaction in biosynthesis of the Escherichia coli O9a O-polysaccharide: The chain-length regulator, WbdD, catalyzes the addition of methyl phosphate to the non-reducing terminus of the growing glycan. J Biol Chem. 2011;286(48):41391–41401. doi: 10.1074/jbc.M111.295857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke BR, Cuthbertson L, Whitfield C. Nonreducing terminal modifications determine the chain length of polymannose O antigens of Escherichia coli and couple chain termination to polymer export via an ATP-binding cassette transporter. J Biol Chem. 2004;279(34):35709–35718. doi: 10.1074/jbc.M404738200. [DOI] [PubMed] [Google Scholar]
- 41.Kos V, Cuthbertson L, Whitfield C. The Klebsiella pneumoniae O2a antigen defines a second mechanism for O antigen ATP-binding cassette transporters. J Biol Chem. 2009;284(5):2947–2956. doi: 10.1074/jbc.M807213200. [DOI] [PubMed] [Google Scholar]
- 42.Roberts I, et al. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raetz CR. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freiberger F, et al. Biochemical characterization of a Neisseria meningitidis polysialyltransferase reveals novel functional motifs in bacterial sialyltransferases. Mol Microbiol. 2007;65(5):1258–1275. doi: 10.1111/j.1365-2958.2007.05862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frirdich E, Vinogradov E, Whitfield C. Biosynthesis of a novel 3-deoxy-D-manno-oct-2-ulosonic acid-containing outer core oligosaccharide in the lipopolysaccharide of Klebsiella pneumoniae. J Biol Chem. 2004;279(27):27928–27940. doi: 10.1074/jbc.M402549200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.