Significance
We show that the selective overactivation of autophagy can cause cell death with unique morphological features distinct from apoptosis or necrosis. This unique type of autophagic cell death, termed “autosis,” occurs not only in vitro but also in vivo in cerebral hypoxia–ischemia. Moreover, autosis is inhibited both in vitro and in vivo by cardiac glycosides, which are Na+,K+-ATPase antagonists used in clinical medicine. Our findings contribute to the basic understanding of cell-death mechanisms and suggest strategies for protecting cells against stresses such as hypoxia–ischemia.
Abstract
A long-standing controversy is whether autophagy is a bona fide cause of mammalian cell death. We used a cell-penetrating autophagy-inducing peptide, Tat-Beclin 1, derived from the autophagy protein Beclin 1, to investigate whether high levels of autophagy result in cell death by autophagy. Here we show that Tat-Beclin 1 induces dose-dependent death that is blocked by pharmacological or genetic inhibition of autophagy, but not of apoptosis or necroptosis. This death, termed “autosis,” has unique morphological features, including increased autophagosomes/autolysosomes and nuclear convolution at early stages, and focal swelling of the perinuclear space at late stages. We also observed autotic death in cells during stress conditions, including in a subpopulation of nutrient-starved cells in vitro and in hippocampal neurons of neonatal rats subjected to cerebral hypoxia–ischemia in vivo. A chemical screen of ∼5,000 known bioactive compounds revealed that cardiac glycosides, antagonists of Na+,K+-ATPase, inhibit autotic cell death in vitro and in vivo. Furthermore, genetic knockdown of the Na+,K+-ATPase α1 subunit blocks peptide and starvation-induced autosis in vitro. Thus, we have identified a unique form of autophagy-dependent cell death, a Food and Drug Administration-approved class of compounds that inhibit such death, and a crucial role for Na+,K+-ATPase in its regulation. These findings have implications for understanding how cells die during certain stress conditions and how such cell death might be prevented.
The lysosomal degradation pathway of autophagy plays a crucial role in enabling eukaryotic cells to adapt to environmental stress, especially nutrient deprivation (1). The core autophagy machinery was discovered in a genetic screen in yeast for genes essential for survival during starvation, and gene knockout or knockdown studies in diverse model organisms provide strong evidence for a conserved prosurvival function of autophagy during starvation (1). This prosurvival function of autophagy results from its ability to mobilize intracellular energy resources to meet the demand for metabolic substrates when external nutrient supplies are limited.
In contrast to this well-accepted, prosurvival function of autophagy, there has been much debate as to whether autophagy—especially at high levels—also functions as a mode of cell death (2). Historically, based on morphological criteria, three types of programmed cell death have been defined: type I apoptotic cell death; type II autophagic cell death; and type III, which includes necrosis and cytoplasmic cell death (3). Autophagic cell death was originally defined as a type of cell death that occurs without chromatin condensation and is accompanied by large-scale autophagic vacuolization of the cytoplasm. This form of cell death, first described in the 1960s, has been observed ultrastructurally in tissues where developmental programs (e.g., insect metamorphosis) or homeostatic processes in adulthood (e.g., mammary involution following lactation or prostate involution following castration) require massive cell elimination (4–6). Autophagic cell death has also been described in diseased tissues and in cultured mammalian cells treated with chemotherapeutic agents or other toxic compounds (4–6).
The term “autophagic cell death” has been controversial, because it has been applied to scenarios where evidence is lacking for a causative role of autophagy in cell death (i.e., there is cell death with autophagy but not by autophagy). However, using more stringent criteria to define autophagic cell death, several studies in the past decade have shown that autophagy genes are essential for cell death in certain contexts. This includes cases of tissue involution in invertebrate development as well as in cultured mammalian cells lacking intact apoptosis pathways (6, 7). In apoptosis-competent cells, high levels of autophagy can also lead to autophagy gene-dependent, caspase-independent cell death (8–10). In neonatal mice, neuron-specific deletion of Atg7 protects against cerebral hypoxia–ischemia-induced hippocampal neuron death (11), and in adult rats, shRNA targeting beclin 1 decreases neuronal death in the thalamus that occurs secondary to cortical infarction (12).
Although such studies provide genetic support for autophagy as a bona fide mode of cell death, the nature of autophagic cell death that occurs in mammalian cells and tissues in response to physiological/pathophysiological stimuli remains poorly defined. It is unclear whether cells that die by autophagy have unique morphological features or a unique death machinery. The only morphological feature that has been linked to autophagic cell death—autophagic vacuolization—may be observed in cells undergoing apoptotic or necrotic cell death, and no proteins, aside from the core autophagy proteins, have been shown to be required for autophagic cell death.
Here we identify a form of autophagic cell death, autosis, which has unique morphological features; depends on the cellular Na+,K+-ATPase; and occurs during treatment with autophagy-inducing peptides, starvation, and cerebral hypoxia–ischemia.
Results
Autophagy-Inducing Peptides Trigger Autophagy-Dependent Cell Death.
Previously, we discovered a potent autophagy-inducing cell permeable peptide (13), Tat-Beclin 1, composed of 11 amino acids of the HIV Tat protein transduction domain, a diglycine linker, and 18 amino acids (267–284 aa) derived from the autophagy protein, Beclin 1. This peptide induced autophagy without cytotoxicity at low doses, but caused cell death at higher doses (13). This finding suggested that the Tat-Beclin 1 peptide might induce a form of autophagy-dependent cell death and serve as a model for defining characteristics of autophagy-dependent cell death that occurs in pathophysiological settings.
We examined the relationship between Tat-Beclin 1-induced autophagy and cell death. In HeLa cells, Tat-Beclin 1 led to dose-dependent induction of autophagy, as measured by ratios of light chain 3 (LC3)-II/I and degradation of the autophagy substrate, p62 (Fig. 1A), as well as cell death, as measured by trypan blue exclusion (Fig. 1B). Increasing durations of exposure to a fixed concentration of Tat-Beclin 1 resulted in a time-dependent increase in autophagy and cell death (Fig. S1 A and B). No autophagy induction or cell death was observed after treatment with a control peptide, Tat-Scrambled (13). Thus, Tat-Beclin 1 induces cell death in parallel with its ability to induce autophagy in a dose- and time-dependent manner.
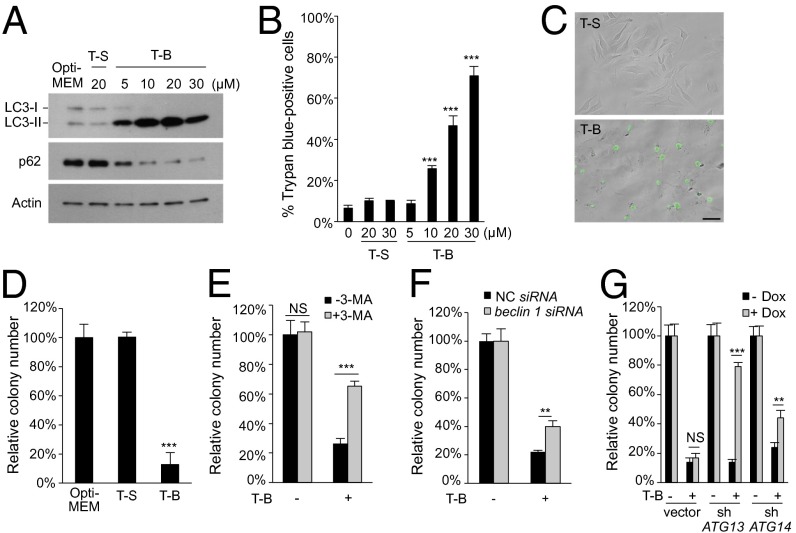
Fig. 1.
Tat-Beclin 1 induces autophagy-dependent cell death. (A) Western blot of LC3 and p62 in HeLa cells treated with Tat-Scrambled (T-S) or Tat-Beclin 1 (T-B) peptides for 5 h. (B) Cell death of HeLa cells treated T-S or T-B for 5 h. (C) Representative images of Sytox Green staining of HeLa cells treated with T-S or T-B (20 µM, 5 h). (Scale bar, 50 µm.) (D) Clonogenic cell survival of HeLa cells treated with T-S or T-B (20 µM, 5 h). (E) Clonogenic survival of HeLa cells treated with T-B (20 µM, 4 h) ± 10 mM 3-MA. (F) Clonogenic survival of siRNA-transfected HeLa cells treated with T-B (20 µM, 3h). (G) Clonogenic survival of doxycycline (Dox)-inducible U2OS/TR cells stably transfected with empty vector, shATG13, or shATG14 ± Dox (1 µg/mL) for 5 d before treatment with T-B (25 µM, 5 h). For B and D–G, error bars represent mean ± SEM and similar results were observed in three independent experiments. For D–G, the number of colonies in untreated controls was standardized as 100%. NS, not significant; **P < 0.01; ***P < 0.001; t test. See also Fig. S1.
We confirmed that Tat-Beclin 1 induced HeLa cell death by detection of cells positive for Sytox Green (a nucleic dye excluded by live cells) (Fig. 1C), an increase of propidium iodide (PI)-positive cells (Fig. S1 C and D), and a decline of cellular ATP levels (Fig. S1E). In addition, Tat-Beclin 1, but not Tat-Scrambled, significantly reduced clonogenic survival (Fig. 1D). Tat-Beclin 1 also induced cell death in a variety of additional tumor cell lines, in human and rat fibroblasts, and in primary and E1A/Ras-transformed murine embryonic fibroblasts (MEFs) (Fig. S1F).
We investigated whether Tat-Beclin 1-induced autophagy is mechanistically related to Tat-Beclin 1-induced cell death by using pharmacological and genetic approaches to inhibit autophagy. Treatment with 3-methyladenine (3-MA), an inhibitor of class III PI3K activity and autophagosome formation, partially blocked Tat-Beclin 1-induced HeLa cell death, as measured increased cellular levels of ATP (Fig. S1G), a decreased percentage of trypan blue-positive cells (Fig. S1H), and increased clonogenic survival (Fig. 1E). siRNA knockdown of the essential autophagy gene, beclin 1, decreased autophagy in HeLa cells (Fig. S1I), decreased Tat-Beclin 1-induced cell death (Fig. S1J), and increased clonogenic survival (Fig. 1F). Furthermore, doxycycline-inducible shRNA knockdown of ATG13 or ATG14 in U2OS cells decreased Tat-Beclin 1-induced autophagy (Fig. S1K), protected against Tat-Beclin 1-induced cell death (Fig. S1L), and increased clonogenic survival (Fig. 1G). Blockade of autophagosomal/lysosomal fusion by bafilomycin A1, a vacuolar proton ATPase inhibitor, did not reduce Tat-Beclin 1-induced cell death (Fig. S1M), suggesting that this form of cell death does not require late stages of autophagy. In addition, another autophagy-inducing peptide, Tat-vFLIP α2 (which acts by releasing ATG3 from cellular FLIP) (14), also induced autophagy (Fig. S1N) which was associated with dose- and time-dependent cell death (Fig. S1 O and P) and reduced by ATG14 knockdown in U2OS cells (Fig. S1Q). Thus, autophagy-inducing peptides trigger cell death that requires the autophagy machinery.
Autophagy Peptide-Induced Death Does Not Require Apoptotic or Necropoptotic Machinery.
We next asked whether the apoptosis and/or necroptosis death machinery is involved in Tat-Beclin 1-induced cell death. We found that neither z-VAD, an inhibitor of caspases and apoptosis, nor necrostatin-1 (Nec-1), an inhibitor of RIPK1 kinase-mediated necroptosis, rescued Tat-Beclin 1-induced cell death as measured by levels of cellular (Fig. S1G), trypan blue exclusion (Fig. S1H), or clonogenic survival (Fig. S2A). Adenovirus E1A/Ras-transformed MEFs with null mutations in the two proapoptotic genes, Bax and Bak, were susceptible to Tat-Beclin 1-induced cell death (Fig. 2A and Fig. S2B) but were resistant to death induced by the apoptosis-inducing agents, staurosporine and etoposide (Fig. S2C). Genetic deletion of two key regulators of necroptosis, Ripk1 and Ripk3, failed to protect primary MEFs from Tat-Beclin 1-induced cell death (Fig. 2B and Fig. S2D). Thus, neither the apoptotic nor necroptotic death machinery is required for Tat-Beclin 1-induced cell death.
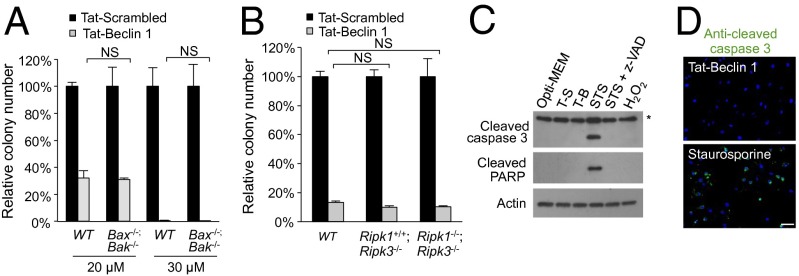
Fig. 2.
Tat-Beclin 1-induced cell death does not require the apoptotic or necroptotic machinery. (A) Clonogenic survival of wild-type and Bax−/−;Bak−/− MEFs treated with peptide (5 h). (B) Clonogenic survival of wild-type, Ripk1+/+;Ripk3−/−, and Ripk1−/−;Ripk3−/− MEFs treated with peptide (20 µM, 5 h). In A and B, the number of colonies of Tat-Scrambled-treated cells was standardized as 100%. (C) Western blot of cleaved caspase 3 and cleaved PARP in HeLa cells treated with 20 µM Tat-Scrambled (T-S), 20 µM Tat-Beclin 1 (T-B), 1 µM staurosporine (STS) ± 100 µM Z-VAD-FMK (z-VAD), or 32 mM H2O2 for 5 h. The asterisk denotes a cross-reacting band. (D) Representative images of cleaved caspase 3 staining in HeLa cells treated with 20 µM Tat-Beclin 1 or 1 µM staurosporine for 5 h. Scale bar, 50 µm. For A and B, error bars represent mean ± SEM of triplicate samples and similar results were observed in three independent experiments. NS, not significant; t test. See also Fig. S2.
Additional assays confirmed the lack of apoptosis in Tat-Beclin 1-induced death. In contrast to staurosporine, Tat-Beclin 1 did not activate caspase 3, as shown biochemically by the lack of cleavage of caspase 3 or its substrate PARP (Fig. 2C) and by the lack of immunofluorescence staining for active caspase 3 (Fig. 2D). In addition, minimal pancaspase activity was detected in Tat-Beclin 1-treated cells by flow cytometry (Fig. S2 E and F). Consistent with nonapoptotic cell death, no TUNEL staining (Fig. S2G) or DNA ladder formation (Fig. S2H) was detected in Tat-Beclin 1-treated cells. We also confirmed that Tat-Beclin 1 [derived from a structurally flexible region in the Beclin 1 evolutionarily conserved domain (15)] did not exhibit a pore-forming ability to release cytochrome c from mitochondria (Fig. S2I), as do certain other amphipathic α-helical peptides (16). Furthermore, antioxidants that block reactive oxygen species-mediated cell death failed to rescue Tat-Beclin 1-induced cell death (Fig. S2J). Similar to Tat-Beclin 1, Tat-vFLIP α2 also failed to induce caspase 3 or PARP cleavage (Fig. S2K). Thus, taken together, our data indicate that autophagy-inducing peptide-triggered cell death is genetically and biochemically distinct from apoptosis or necroptosis.
Autophagy Peptide-Induced Cell Death Has Unique Morphological Features.
To characterize the nature of this autophagy-dependent, nonapoptotic, and nonnecrotic cell death, we performed live-cell imaging of Tat-Beclin 1-treated cells (Fig. 3A and Movie S1). During the initial phase, cells exhibit relatively normal morphology with increased vacuolar dynamics and perinuclear accumulation of numerous vacuoles. After a few hours, cells undergo an abrupt demise (lasting ∼15–20 min) characterized by the rapid shrinkage of the nucleus with a portion of its surface developing a concave appearance corresponding to a round, vacuole-like entity, reflecting (based on our EM data; see below) a local separation of the inner and outer nuclear membranes. This is followed by focal plasma membrane rupture and extracellular extrusion of cytoplasmic contents. Cells treated with Tat-Beclin 1 display increased substrate adherence that persists until their final demise (unlike apoptotic or necrotic cells, which generally float).
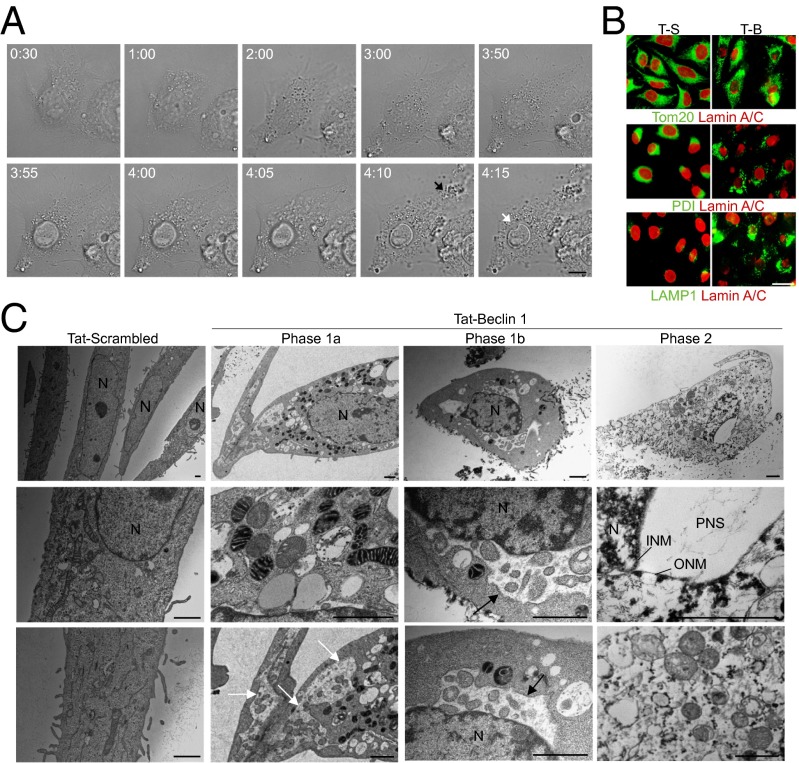
Fig. 3.
Morphological features of Tat-Beclin 1-induced autosis. (A) Representative live-cell imaging of HeLa cells treated with 25 µM Tat-Beclin 1 for 5 h (Movie S1; times shown as hh:mm). The black arrow denotes released intracellular components from a ruptured cell membrane and the white arrow denotes perinuclear space between the inner nuclear membrane and cytoplasm at a region of nuclear concavity. (Scale bar, 10 µm.) (B) Representative images of mitochondrial (Tom20), ER (PDI), late endosome/lysosome (LAMP1), and nuclear lamin-A/C staining in HeLa cells treated with Tat-Scrambled (T-S) or Tat-Beclin 1 (T-B) (20 µM, 5 h). (Scale bar, 20 µm.) (C) EM analysis of HeLa cells treated with peptide (20 µM, 5 h). White arrows show dilated and fragmented ER; black arrows show regions where the perinuclear space has swollen and contains clumps of cytoplasmic material. (Scale bars, 1 µm.) See also Fig. S3.
The concave nuclear appearance observed in Tat-Beclin 1-induced cell death is associated with abnormalities in nuclear lamin-A/C staining (lack of a uniform circular appearance and focal regions of dense staining) (Fig. 3B and Fig. S3A). Tat-Beclin 1-treated dying cells also exhibit an abnormal fragmented pattern of Tom20 (mitochondrial marker) and PDI [endoplasmic reticulum (ER) marker] staining, and a striking increase in expression of LAMP1, a marker of late endosomes/autolyosomes (which would be expected in the setting of a robust autophagy response) (Fig. 3B). Tat-vFLIP α2-treated dying cells have a similar concave nuclear appearance and similar abnormalities in lamin-A/C, Tom20, PDI, and LAMP1 staining (Fig. S3B).
We performed ultrastructural analyses to further characterize the morphology of Tat-Beclin 1-induced death (Fig. 3C and Fig. S3C). As apparent from live-cell imaging, there were two phases of the death process; phase 1 is characterized by a slow phase of gradual change and phase 2 is characterized by an abrupt phase of final collapse and cell death. Morphologically, phase 1 can be divided into two stages. In phase 1a, the nucleus becomes convoluted (but the perinuclear space is normal); chromatin is moderately condensed, forming darker regions in the nucleus with borders that are fuzzy (in contrast to clumps of chromatin that typically have sharp borders in apoptosis); many of the mitochondria are electron dense and some have an abnormal internal structure (clumps instead of bands); the ER is dilated and fragmented; and numerous autophagosomes, autolysosomes, and empty vacuoles are present. In phase 1b, the perinuclear space becomes swollen at discrete regions surrounding the inner nuclear membrane, and these swollen areas contain membrane-bound regions with a density and granularity resembling the cytosol. In some cases, the perinuclear space extends through substantial distances in the cytoplasm. In phase 2, there is focal ballooning of the perinuclear space (which appears empty), often associated with a concavity of the nuclear surface. At this late stage, the morphology appears necrotic; mitochondria and other organelles are swollen; and autophagosomes, autolysosomes, and ER are rare. There appears to be lysis of the plasma membrane (which is difficult to discern from EM analyses but is substantiated by PI staining, Sytox Green staining, and live-cell imaging of Tat-Beclin 1-treated cells).
These ultrastructural changes are distinct from previous classifications of cell death (3), including type-3B cytoplasmic death (also called “paraptosis”), which also has perinuclear swelling. In type-3B cell death, perinuclear swelling is moderate and uniform around the entire nuclear perimeter, whereas in death of autophagy-inducing peptide-treated cells, there is a pronounced ballooning in a focal region of the perinuclear space. To avoid confusion with terms such as “autophagic cell death” (which is sometimes applied to states in which it is not clear that autophagy is required for cell death and/or in which autophagic features coexist with apoptosis or necrosis), we coined the term “autosis” to define cell death mediated by autophagy genes and characterized by focal perinuclear swelling. Although several studies have described cell death that is blocked by genetic inhibition of autophagy (6), such studies have not described increased substrate adherence and focal perinuclear swelling of dying cells. Thus, autosis represents a previously undescribed form of cell death by autophagy.
Starvation Induces Autosis.
We next investigated whether autosis occurs during physiological stress conditions associated with high levels of autophagy. Nutrient starvation is a potent physiological inducer of autophagy in eukaryotic cells, and previous studies have shown that autophagy delays apoptosis in cells subjected to starvation, including HeLa cells (1). However, upon subjecting HeLa cells to amino-acid and serum starvation, we found that, unlike the vast majority of cells which detach from their substrate and undergo apoptosis (as evidenced by active caspase 3 staining), a small subpopulation (∼1%) of cells become more substrate-adherent and lack evidence of caspase 3 activation (Fig. 4A). This population of substrate-adherent, caspase 3-negative cells undergoes plasma-membrane rupture and cell death, as identified by Sytox-Green staining (Fig. S4A), and also displays a marked increase (approximately threefold) in numbers of autophagosomes (GFP-LC3 puncta) compared with numbers in the majority population of starved cells that float and undergo apoptosis (Fig. 4 B and C). These substrate-adherent cells have nuclei with concave regions and focal swelling of the perinuclear space (Fig. 4 A, B, and D and Fig. S4A) and display similar abnormalities in lamin-A/C staining as observed in Tat-Beclin 1 and Tat-vFLIP α2 peptide-treated cells (Fig. S4A). Similar to Tat-Beclin 1 peptide treatment, we observed phase-1 starved cells with increased autophagosomes/autolysosomes, and regions of perinuclear swelling containing organelles and phase-2 cells with rare autophagosomes/autolysosomes and dilated empty regions in the perinuclear space (Fig. 4D). We also observed features of autosis in starved adherent murine bone marrow-derived macrophages (BMDMs) and primary MEFs (Fig. 4E), suggesting that starvation-induced autosis occurs in primary cells and is not merely a consequence of mutations that confer resistance to apoptosis in continuous cell lines. Moreover, the frequency of autotic cell death was higher (∼5%) in primary cells than in HeLa cells.
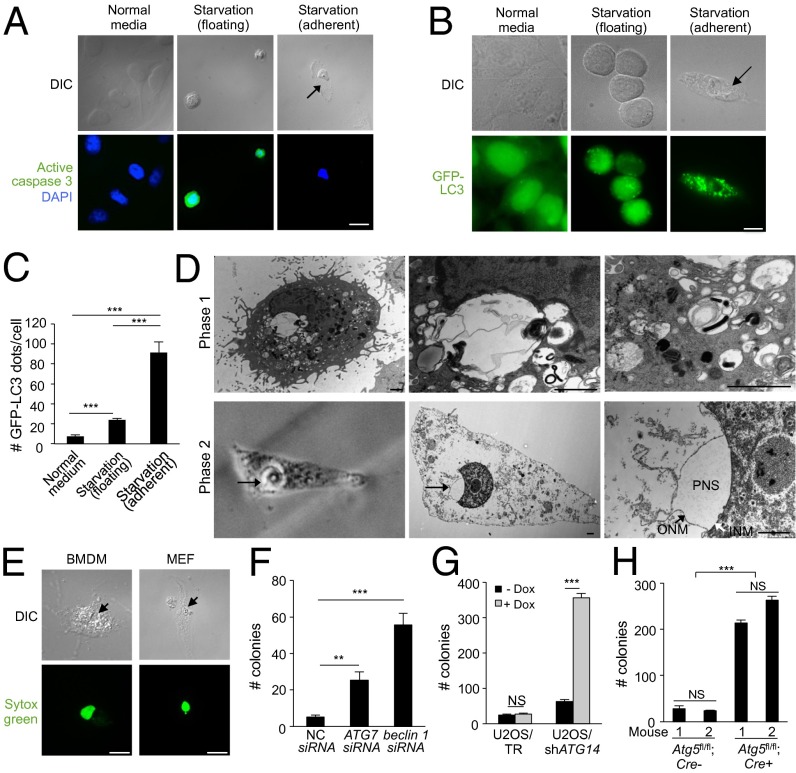
Fig. 4.
Starvation induces autosis. (A) Representative images of active caspase-3 staining in HeLa cells 48 h after starvation (HBSS). (Center) Active caspase 3-positive floating cells with rounded nuclei. (Right) Active caspase 3-negative adherent cell with concave nucleus and swollen perinuclear space. (Scale bar, 20 µm.) (B and C) Representative images (B) and quantitation (C) of GPF-LC3 dots (autophagosomes) in HeLa/GFP-LC3 cells (>50 cells analyzed per sample) grown in normal medium or in floating and adherent HeLa/GFL-LC3 cells 6 h after starvation. (Scale bar, 10 µm.) (D) (Upper) EM images of phase-1 substrate-adherent HeLa cell 6 h after starvation. (Lower) CLEM images of phase-2 substrate-adherent HeLa cell with concave nucleus and swollen perinuclear space (PNS) (arrow) 8 h after starvation. (Lower Left) Phase contrast microscopy; (Lower Center and Lower Right) EM of same cell. The black arrow in Right Lower shows outer nuclear membrane (ONM) and the white arrow shows inner nuclear membrane (INM). (Scale bars, 1 µm.) (E) Representative images of a Sytox Green-positive adherent primary murine BMDM and MEF 48 h after starvation. (Scale bar, 10 µm.) (F) Clonogenic survival of siRNA-transfected adherent HeLa cells starved for 48 h. NC, nontargeting control siRNA. (G) Clonogenic survival of doxycycline (Dox)-inducible adherent U2OS/TR and U2OS/shATG14 cells ± Dox treatment (1 µg/mL) for 5 d before starvation for 72 h. (H) Clonogenic survival of adherent BMDMs (two mice per genotype; Atg5fl/fl;Lyz-Cre− and Atg5fl/fl;Lyz-Cre+ littermates) starved for 72 h. For C and F–H, error bars represent mean ± SEM of triplicate samples and similar results were observed in three independent experiments. For A, B and E, arrows denote concave nucleus and swollen perinuclear space. NS, not significant; **P < 0.01; ***P < 0.001; t test. See also Fig. S4.
To confirm that autophagy is required for starvation-induced death in cells with autotic morphology, we assessed the effects of autophagy gene knockdown on the clonogenic survival of substrate-adherent starved cells. In this assay, floating (apoptotic and necrotic) cells were washed away after 48 h (HeLa cells) or 72 h (U2OS cells and BMDMs) starvation, and the clonogenic potential of the remaining adherent cells was assessed (Fig. S4B). Both ATG7 and beclin 1 siRNA treatment (Fig. S4C) increased colony numbers formed by starved adherent HeLa cells (Fig. 4F), and ATG14 shRNA expression (Fig. S4D) increased colony numbers formed by starved adherent U2OS cells (Fig. 4G). Lysozyme:Cre-mediated deletion of Atg5 (Fig. S4E) also increased colony numbers formed by starved adherent Atg5flox/flox BMDMs (Fig. 4H). [ATG7 siRNA, beclin 1 siRNA, ATG14 shRNA and Atg5 deletion had minimal effect on the clonogenic survival of cells cultured in normal media (Fig. S4 C–E)]. Thus, autophagy genes are required for starvation-induced autosis.
Autosis Occurs During Rat Cerebral Hypoxic–Ischemic Injury.
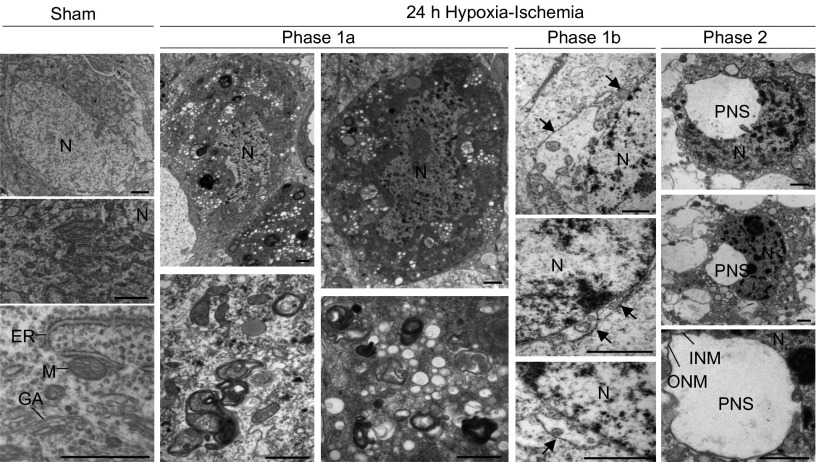
After establishing ultrastructural criteria for autosis in cultured cells, we evaluated whether autosis occurs in vivo. We performed EM analysis of neuronal death following cerebral hypoxia–ischemia in the brains of neonatal rats (Fig. 5); we focused on dying neurons in the hippocampus CA3 region because we had previously shown that most of these neurons degenerate with autophagic features (from 6 h after hypoxia–ischemia) without signs of apoptosis or necrosis (17). At 24 h after cerebral hypoxia–ischemia, most of the dying neurons displayed prominent autophagic features, such as numerous autophagosomes and autolysosomes, and empty vacuoles (phase 1a). Strikingly, some dying neurons displayed characteristics of phase-1b or the full phase-2 features of autosis—focal ballooning of the perinuclear space associated with nuclear concavity. Thus, autotic cell death occurs in certain pathophysiological settings in vivo.
Fig. 5.
Morphological features of cerebral hypoxia–ischemia-induced autosis. EM analysis of dying neurons in hippocampal region CA3 in brains of 7-d-old rats 24 h after exposure to cerebral hypoxia–ischemia. Arrows show regions where the perinuclear space is swollen and contains clumps of cytoplasmic material. (Scale bars, 1 µm.) GA, Golgi apparatus; INM, inner nuclear membrane; M, mitochondrion; N, nucleus; ONM, outer nuclear membrane; PNS, perinuclear space.
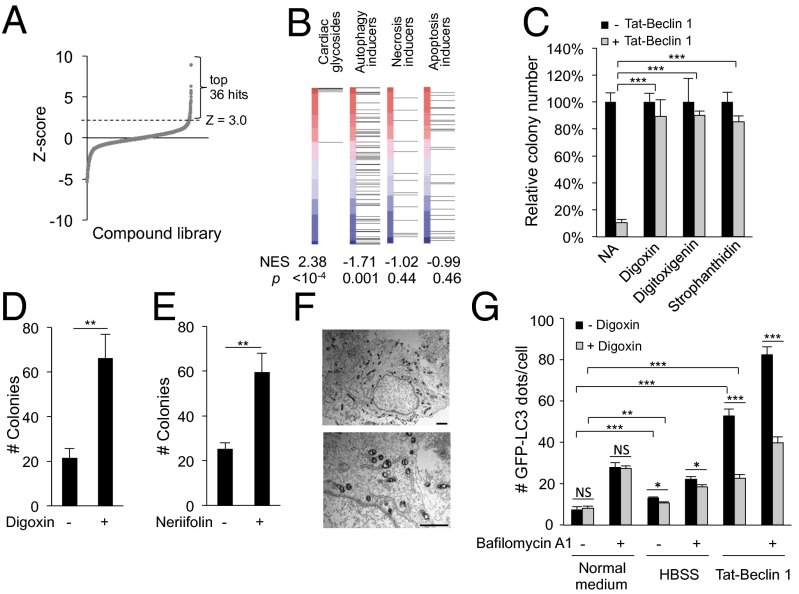
A High-Throughput Chemical Screen Identifies Cardiac Glycosides as Potent Inhibitors of Autosis.
To gain insight into the regulation of autosis, we performed high-throughput compound screening to identify inhibitors of Tat-Beclin 1-induced cell death, focusing on compound libraries consisting of bioactive agents with known targets. We measured levels of cellular ATP (as a proxy of cellular viability) 5 h after Tat-Beclin 1 treatment of HeLa cells in the presence of ∼5,000 Food and Drug Administration (FDA)-approved drugs and bioactive compounds with characterized mechanisms of action (Fig. S5A). We chose for further analysis the 36 top hits that had z scores ≥3.0 in the primary screen (Fig. 6A and Dataset S1). These 36 hits were classified into nine families based on their chemical structures and/or biological functions (Dataset S2). Of these 36 hits, eight compounds demonstrated >40% rescue of autosis in a repeat ATP assay and were chosen for further analysis (Dataset S3). Of these eight compounds, only five, including three cardiac glycosides (digoxin, digitoxigenin, and strophanthidin) and two purinergic receptor antagonists (suramin and NF 023) demonstrated more than 80% rescue of Tat-Beclin 1 peptide-induced cell death as measured by Sytox Green staining (Fig. S5B). The purinergic receptor antagonists, but not the cardiac glycosides, blocked cellular peptide entry (Fig. S5 C and D) and were not studied further. Thus, our chemical screen identified cardiac glycosides as the only class of agents that inhibited the Tat-Beclin 1-induced cell death without blocking cellular peptide entry.
Fig. 6.
Cardiac glycosides rescue autosis. (A) Ranked distribution of z scores for each compound in primary chemical screen (Dataset S1) for inhibitors of Tat-Beclin 1-induced cell death. Thirty-six top hits with z ≥ 3.0 (Dataset S2) were selected for a secondary screen (Dataset S3). (B) Comparison of CSEA of cardiac glycosides in the primary autosis screening with compound sets of specific autophagy, necrosis, or apoptosis inducers. P, permutation P value for the NES compared with a null distribution. Red–blue vertical bars represent list of screened compounds, ranked according to z score (greatest rescue of autosis at top). Each horizontal line indicates where a specific compound falls within ranked compound list. (C) Clonogenic survival of HeLa cells treated with Tat-Beclin 1 (20 µM, 5 h) + 5 µM digoxin, digitoxigenin, or strophanthidin. The number of colonies of untreated cells was standardized as 100%. (D and E) Clonogenic survival of adherent HeLa cells starved for 48 h ± 10 nM digoxin (D) or 1 nM neriifolin (E). (Nanomolar concentrations were used as toxicity of digoxin and neriifolin was observed during starvation with micromolar concentrations.) (F) Representative EM images of a HeLa cell treated with 20 µM Tat-Beclin 1 + 5 µM digoxin (5 h). (Scale bar, 1 µm.) (G) Quantitation of GFP-LC3 dots (>100 cells analyzed per sample) in HeLa/GFP-LC3 cells treated with 20 µM Tat-Beclin 1 or starved in HBSS for 2 h ± 0.1 µM digoxin and/or 20 nM bafilomycin A1. For C–E and G, error bars represent mean ± SEM of triplicate samples and similar results were observed in three independent experiments. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001; t test. See also Figs. S5 and S6 and Datasets S1–S4.
To assess the significance of the effects of cardiac glycosides on autosis in an unbiased manner, we tested whether the nine cardiac glycosides present in our library were statistically enriched among top-scoring compounds. We calculated a weighted Kolmogorov–Smirnov-like statistic, the normalized enrichment score (NES), using compound set enrichment analysis (CSEA). CSEA demonstrated strong, highly significant enrichment for cardiac glycosides (P < 10−4) (Fig. 6B). In addition to the three cardiac glycosides with z scores ≥3.0 in the primary screen, we confirmed that other cardiac glycosides in the compound libraries also exhibited a significant rescue effect (Dataset S4), as did a cardiac glycoside, neriifolin, which was not in the compound libraries and is known to exert neuroprotective actions (18) (Fig. S5E). We also performed CSEA using other previously identified compound sets for autophagy inducers, specific necrosis inducers, and specific apoptosis inducers (19); none of these sets were enriched among our top-scoring compounds (Fig. 6B). Although there are previous reports that cardiac glycosides may increase basal autophagy (20, 21), an extensive set of autophagy inducers drawn from the literature were overrepresented among compounds that enhanced, rather than rescued, Tat-Beclin 1-induced cell death. The lack of concordance between compounds that inhibited autophagy, apoptosis, or necrosis with the rescue of Tat-Beclin 1-induced autosis is consistent with the latter representing a distinct death process.
Consistent with these bioinformatics analyses, the cardiac glycoside, digoxin, had no effect on apoptotic death induced by staurosporine or necrotic death induced by H2O2 (Fig. S5 F and G), whereas digoxin rescued Tat-Beclin 1–induced cell death with IC50 values below 0.1 µM (Fig. S5H). Digoxin also rescued Tat-vFLIP α2-induced death in HeLa cells (Fig. S5H) and Tat-Beclin 1-induced cell death in U2OS cells (Fig. S5I). We also found that clonogenic survival was rescued in Tat-Beclin 1-treated HeLa cells by digoxin, digitoxigenin, and strophanthidin (Fig. 6C); in Tat-vFLIP α2-treated HeLa cells by digoxin (Fig. S6J); and in the adherent subpopulation of HeLa cells subjected to prolonged starvation by digoxin (Fig. 6D) and neriifolin (Fig. 6E). Thus, cardiac glycosides rescue cell death triggered by multiple inducers of autosis.
Digoxin partially reversed the majority of morphological abnormalities in cells undergoing Tat-Beclin 1-induced autosis. Upon light microscopy analysis, cells treated with Tat-Beclin 1 and digoxin displayed minimal nuclear abnormalities and lacked abnormal patterns of mitochondrial (Tom20), ER (PDI), late endososome/lysosome (LAMP1), and nuclear lamin-A/C staining (Fig. S6A). Ultrastructurally, the majority of digoxin-rescued cells had a normal-shaped nuclear membrane without any focal swelling of the perinuclear space, intact ER structure, and the absence of increased numbers of autophagosomes and autolysosomes (Fig. 6F). The only morphological abnormality of autosis not reversed by digoxin in Tat-Beclin 1-treated cells was the presence of electron-dense mitochondria; however, digoxin alone (in the absence of Tat-Beclin 1) resulted in electron-dense mitochondria (Fig. S6B). Together, these data indicate that digoxin reverses the morphological changes of autosis except for mitochondrial abnormalities, but these do not appear to be related to cell death.
We performed Western blot analyses of LC3 and p62 and quantitation of GFP-LC3 puncta to further evaluate the effects of digoxin on autophagy. Under basal conditions, consistent with prior reports (20, 21), we observed a dose-dependent increase in LC3-II conversion and mild reduction in p62 levels (although we did not detect an increase in GFP-LC3 puncta) (Fig. 6G and Fig. S6C). In contrast, doses as low as 100 nM resulted in a mild decrease in starvation-induced autophagy and a more dramatic decrease in Tat-Beclin 1-induced autophagy (Fig. 6G and Fig. S6C). The increased p62 accumulation was not due to changes in p62 mRNA expression (Fig. S6D). Although cardiac glycosides have been reported to induce apoptosis (22, 23), we did not observe caspase activation in Tat-Beclin 1-treated cells in the presence of digoxin (Fig. S6E).
Na+,K+-ATPase Regulates Autosis.
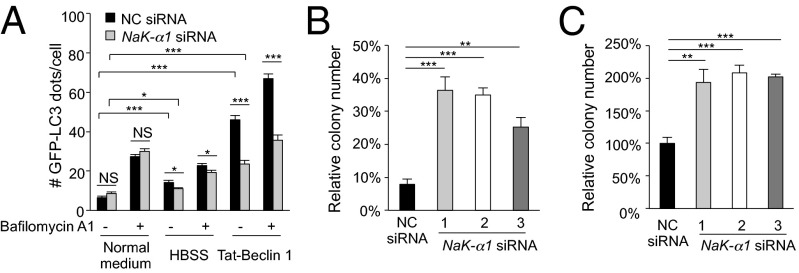
Cardiac glycosides are inhibitors of Na+,K+-ATPase, a plasma membrane pump that generates Na+ and K+ gradients across the membrane and acts as a versatile signal transducer (22). We therefore examined whether Na+,K+-ATPase regulates autosis using siRNA knockdown of the α1 subunit of Na+,K+-ATPase. Similar to digoxin treatment, Na,K-α1–subunit knockdown (Fig. S7 A and B) resulted in a mild decrease in starvation-induced autophagy and a more dramatic decrease in Tat-Beclin 1-induced autophagy (Fig. 7A and Fig. S7A). The observed increase in p62 accumulation was not due to changes in p62 mRNA expression (Fig. S7C) or to a block in peptide delivery into cells (Fig. S7D). In parallel with inhibition of autophagy, three different siRNAs against Na,K-α1 inhibited Tat-Beclin 1- and Tat-vFLIP α2-induced death (Fig. S7E). They also increased clonogenic survival of Tat-Beclin 1-treated cells (Fig. 7B) and adherent cells subjected to starvation (Fig. 7C). Na,K-α1 siRNA also exerted a protective effect against autosis in human U2OS (Fig. S7 F and G) and in mouse NIH 3T3 (Fig. S7 H and I) cells. Digoxin did not enhance Na,K-α1 siRNA-mediated protection against autosis triggered by autophagy-inducing peptides (Fig. S7J), suggesting that digoxin and Na+,K+-ATPase inhibition block autosis through the same mechanism.
Fig. 7.
Na+,K+-ATPase regulates autosis. (A) Quantitation of GFP-LC3 dots (>100 cells analyzed per sample) in HeLa/GFP-LC3 cells 72 h after transfection with indicated siRNA and treatment with Tat-Beclin 1 (20 µM, 2 h) or starvation (HBSS, 2 h) ± 20 nM bafilomycin A1. (B) Clonogenic survival of HeLa cells transfected with indicated siRNA for 72 h and then treated with Tat-Scrambled or Tat-Beclin 1 (20 µM, 5 h). Shown are the percentage of colonies in Tat-Beclin 1– vs. Tat-Scrambled–treated cells for each siRNA. (C) Clonogenic survival of adherent HeLa cells transfected with indicated siRNA for 72 h, and then starved for 48h. Clonogenic survival of control siRNA transfected cells in starvation conditions relative to normal medium standardized as 100%. For A–C, error bars represent mean ± SEM of triplicate samples and similar results were observed in three independent experiments. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001; t test. See also Fig. S7.
Cardiac Glycoside-Mediated Protection Against Neuronal Autosis in Rat Cerebral Hypoxia–Ischemia.
A previous chemical screen to identify compounds that provide neuroprotection in a mouse brain slice-based model for ischemic stroke revealed neriifolin as a strong hit, and whole-animal studies have shown that neriifolin and other cardiac glycosides provide neuroprotection in neonatal models of cerebral hypoxia–ischemia (18, 24, 25). Given these observations, coupled with our findings described above that rat hippocampal CA3 region neurons die by autosis following hypoxia–ischemia, we evaluated whether neriifolin could protect neonatal rats against cerebral hypoxia–ischemia and reduce autosis in the hippocampal region CA3.
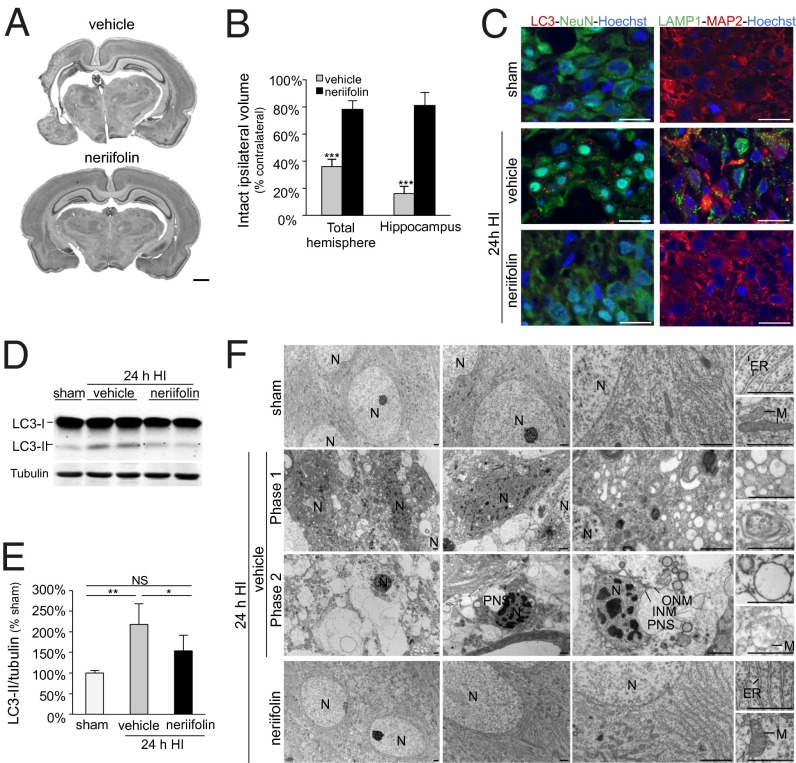
In agreement with results in mice (18), we found that neriifolin was highly neuroprotective in rats; it dramatically increased the volume of intact tissue in the ipsilateral hemisphere of neonatal animals 1 wk after cerebral hypoxia–ischemia (Fig. 8 A and B). This effect was particularly notable in the hippocampus (Fig. 8B and Fig. S8A), where significant neuronal pathology, especially in the CA3 region, was detected as early as 24 h after hypoxia–ischemia injury (Fig. S8B). The CA3 region of the hippocampus was protected at 24 h and 7 d after neonatal hypoxia–ischemia by neriifolin treatment compared with vehicle-treated pups (Fig. S8 A and B). In parallel with this neuroprotection, neriifolin prevented the increase in autophagy in the CA3 region of the hippocampus that occurred after hypoxia–ischemia injury, as measured by detection of decreased numbers of endogenous LC3 puncta and LAMP1 puncta by immunofluorescence and immunoperoxidase staining (Fig. 8C and Fig. S8C) and decreased levels of LC3-II (Fig. 8 D and E). Strikingly, in contrast to the characteristic features of autosis (numerous autophagosomes, autolysosomes, and empty vacuoles; abnormal mitochondria and ER; and focal separation of the inner and outer nuclear membrane) observed in the CA3 region of vehicle-treated pups 24 h after cerebral hypoxia–ischemia, the CA3-region neurons of neriifolin-treated animals displayed no ultrastructural features associated with autosis (Fig. 8F). Thus, cardiac glycosides block the increase in autophagy and protect hippocampal neurons against cerebral hypoxia–ischemia-induced autosis in vivo.
Fig. 8.
Neonatal hypoxic–ischemic brain damage and hippocampal CA3 region autophagy and autosis are reduced by treatment with the cardiac glycoside, neriifolin. (A) Representative Nissl-stained coronal sections through the brain showing the neuroprotective effect of neriifolin (Lower) compared with vehicle (Upper) 1 wk after hypoxia–ischemia (HI). (Scale bar, 1 mm.) (B) Volumes of intact tissue ipsilaterally compared with contralaterally 1 wk after neonatal cerebral hypoxia–ischemia and indicated treatment. Values are mean ± SD (n = 6 for neriifolin and n = 9 for vehicle). ***P < 0.001; Welch’s ANOVA test. (C) Representative confocal microscopy images of LC3 dots (red) and LAMP1 dots (green) in CA3 hippocampal neurons after 24h hypoxia–ischemia and indicated treatment or sham operation. NeuN (green) and MAP2 (red) are neuronal markers. Hoechst staining (blue) shows cell nuclei. (Scale bars, 20 µm.) (D and E) Representative LC3 immunoblots (D) and quantification of LC3-II/tubulin levels (E) from immunoblots of hippocampi of rats subjected to hypoxia–ischemia. Values are mean ± SD (n = 6 for neriifolin and n = 9 for vehicle). NS, not significant; *P < 0.05; **P < 0.001; Kruskal–Wallis test. (F) EM analysis of neriifolin effects in hippocampal region CA3 of 7-d-old rats 24 h after hypoxia–ischemia. INM, inner nuclear membrane; M, mitochondrion; N, nucleus; ONM, outer nuclear membrane; PNS, perinuclear space. (Scale bars, 1 µm.)
Discussion
Our findings identify a unique form of autophagic cell death—autosis—that meets two essential criteria put forth by the Nomenclature Committee on Cell Death (2): suppression by inhibition of the autophagic pathway, and lack of features of apoptosis and necrosis. The form of death that we observed in adherent cells subjected to starvation and in cells treated with autophagy-inducing peptides not only meets these criteria for autophagic cell death, but also has a distinctive morphological and chemical inhibition signature. In addition to the classical morphological criteria of autophagic cell death (increased autolysosomes in dying cells lacking features of apoptosis and necrosis), death induced in starved adherent cells and by autophagy-inducing peptides is accompanied by ER dilation and stereotypic nuclear changes, involving an early convoluted appearance, the formation of focal concave regions of the nucleus with surrounding focal swelling of the perinuclear space, and the accumulation of structures within this space at early stages of the process. This form of cell death, but not apoptosis or necrosis, is also selectively blocked by pharmacological and genetic inhibition of Na+,K+-ATPase.
Although the underlying mechanisms of the morphological changes of autosis and the pathway by which Na+,K+-ATPase mediates autosis remain to be determined, the discovery of this unique morphological and chemical inhibition signature has important biological implications. Our findings pave the road to the discovery of physiological and pathophysiological conditions in which autophagy functions as a death mechanism, and may provide a candidate treatment for diseases in which such death contributes to pathogenesis. For example, using the morphological criteria we established for autosis in cells subjected to starvation or treatment with autophagy-inducing peptides, we identified the presence of autotic death in rat hippocampal neurons subjected to hypoxic–ischemic injury. Moreover, we showed that a class of FDA-approved chemical compounds—cardiac glycosides—that inhibited autosis in an in vitro chemical compound screen also reduced hippocampal neuronal autosis and conferred neuroprotection in vivo in neonatal rats subjected to cerebral hypoxia–ischemia. Thus, by defining a unique form of autophagic cell death and by performing an in vitro chemical screen that identified a specific class of inhibitors of this form of cell death (e.g., cardiac glycosides), we have been able to establish a scientific rationale for the use of cardiac glycosides in the treatment of a clinically important disease, neonatal cerebral hypoxia–ischemia. Based on our identification of specific morphological criteria for autosis, it should be possible to determine additional pathophysiological settings in which autosis plays a role and which may be ameliorated by cardiac glycosides. Conversely, mediators in the regulatory network of autosis may serve as candidate targets in cancer chemotherapy or other settings where pharmacological induction of cell death may be beneficial.
We found that autosis occurs in at least two distinct physiological/pathophysiological conditions: starvation and cerebral hypoxia–ischemia. At present, it is not yet known which, if any, previously reported instances of autophagic cell death involve autosis (except for hypoxia–ischemia-induced hippocampal-region-CA3 death evaluated in this study). It is possible that the unique morphological changes we describe for autosis are present but have been missed in observations of autophagic cell death in other settings, especially those that lack concurrent features of apoptosis or necrosis and/or in tissues (e.g., heart and kidneys) where high levels of autophagy are postulated to play a role in ischemia–reperfusion injury (26, 27). While the future identification of specific biochemical markers of autosis will facilitate such investigations, it should be possible to determine whether cell death occurs via autosis using the morphological criteria we describe, as well as studies examining the inhibitory effect of cardiac glycosides. One cautionary note is that certain isoforms of the Na+,K+-ATPase in the rodent (but not human) are resistant to cardiac glycosides; for example, the rodent-α3 subunit expressed predominantly in brain is sensitive, whereas the rodent-α1 subunit expressed in many peripheral tissues is resistant (28).
Although we are not aware of previous reports of similar nuclear morphological abnormalities in autophagic cell death, the expression of sterol reductases that are localized to the ER and outer nuclear membrane, TM7SF2 and DHCR1, results in massive ER and perinuclear space expansion resembling that observed in autotic cells (29). These observations suggest that disruption of ER/outer nuclear membrane cholesterol metabolism may produce the phenotype of ER and focal perinuclear space expansion. This phenotype is possibly caused by alterations of ER-membrane properties, including transport or channel conductance, which would result in osmotic changes and disruption of signaling through the nuclear envelope. Given the crucial role of the ER in autophagosomal biogenesis (30), we speculate that stimulation of very high levels of autophagy may perturb normal ER membrane biogenesis/homeostatic mechanisms, leading to similar expansions of the ER lumen and perinuclear space. Further studies are needed to investigate the underlying mechanisms of the morphological abnormalities observed in autosis.
Cardiac glycosides, a large family of naturally derived steroidal compounds, were first described for the treatment of heart diseases in 1785 (22). Approximately 50 y ago, Na+,K+-ATPase was identified as the cellular target of cardiac glycosides. This membrane protein uses energy from ATP hydrolysis to facilitate the transport of potassium ions into cells and sodium ions out of cells; inhibition of Na+,K+-ATPase results in an increase in intracellular sodium and calcium ions. Cardiac glycosides also have diverse effects on cellular signaling, proliferation, metabolism, survival, gene expression, attachment, and protein trafficking. Our chemical screen revealed cardiac glycosides as the most potent inhibitors of autotic cell death, and we found that they inhibited both autophagy and autotic cell death in the setting of starvation, autophagy-inducing peptide treatment, and neonatal hippocampal hypoxic–ischemic injury. The mechanism of action appears to be inhibition of the target of cardiac glycosides, Na+,K+-ATPase, as we observed similar effects with Na+,K+-ATPase α1 subunit siRNA knockdown in human and mouse cells. We speculate that the effects of the Na+,K+-ATPase on increasing cell attachment (31) may contribute to the increased substrate adherence of cells undergoing autotic death. In addition, it is possible that nuclear envelope-associated Na+,K+-ATPase activity (32) may alter membrane ionic transport and osmolarity and thereby contribute to the ER and perinuclear space expansion observed in autotic cells.
Previous studies have shown that neriifolin and other cardiac glycosides reduce cerebral infarct size in rodent cerebral hypoxia–ischemia models (18, 24, 25); however, their mechanism of neuroprotection has been unknown. Our observations suggest that inhibition of autophagy and autophagy-dependent death pathways may be a central mechanism of cardiac glycoside-mediated neuroprotection. Several cell-death morphologies have been identified in different regions of the brain after neonatal hypoxic–ischemic injury, but neuron-specific deletion of Atg7 or intracerebroventricular treatment with the autophagy inhibitor, 3-MA, is sufficient to reduce infarct lesion volume, indicating that autophagy may be upstream of multiple death pathways. This supports our previous recommendation that postischemic treatment of neonatal cerebral hypoxia–ischemia should target autophagy (17, 33). In the present study, we observed inhibition of autophagy and autotic cell death in hippocampal-CA3-region neurons of rats treated with neriifolin, but the inhibition of autotic cell death in this region of the hippocampus is not sufficient to explain the dramatic reduction in overall ipsilateral infarct size following hypoxia–ischemia. Taken together with previous studies on autophagy, cell death, and neonatal cerebral hypoxia–ischemia, the most likely explanation for the overall neuroprotection in neriifolin-treated rats is the blockade of both autophagy-dependent autotic death, as well as other death pathways triggered by high levels of autophagy. Thus, cardiac glycosides and/or other agents targeting Na+,K+-ATPase may not only ameliorate diseases associated with autotic cell death, but also diseases in which autophagy is upstream of other death execution pathways. We cannot definitively rule out indirect effects of neriifolin on neuroprotection, but these seem unlikely in view of our in vitro observations that cardiac glycosides inhibit stress-induced autophagy and autosis in a cell-autonomous manner.
It is noteworthy that—during cerebral hypoxia or ischemia—the brain releases an endogenous form of cardiac glycoside (ouabain or endobain) that inhibits Na+,K+-ATPase (34). Thus, by releasing its own inhibitor of Na+,K+-ATPase in response to hypoxia–ischemia, the neonatal brain may have developed an important mechanism to reduce autophagy and cell death by autosis. A broader question is whether basal levels of endogenous cardiac glycosides may serve as a naturally occurring “brake” which functions in multiple mammalian tissues to maintain autophagy at physiological levels that promote cell survival, rather than at pathological levels that promote cell death.
Materials and Methods
Cell Culture.
HeLa cells were obtained from American Type Culture Collection. Information on the source of wild-type, Ripk3−/−, Ripk1−/−;Ripk3−/−, and Bax−/−;Bak−/− MEFs, Atg5flox/flox and Atg5flox/flox-LysM-Cre BMDMs, and U2OSTetR, U2OSTetR/shATG14, and U2OSTetR/shATG13, and other cells used in this study and culture conditions is provided in SI Materials and Methods.
Autophagy-Inducing Peptides.
Tat-Scrambled, Tat-Beclin 1, and Tat-vFLIP α2 (14) were synthesized and administered to cells as described (13).
Antibodies and siRNAs.
See SI Materials and Methods for details of antibodies and siRNAs used in this study.
Cell Death Assays.
See SI Materials and Methods for details of trypan-blue staining, Sytox Green staining, CellTiter-Glo assays, PI staining, active caspase-3 detection, TUNEL staining, DNA fragmentation assays, and clonogenic survival assays.
Microscopy Studies.
See SI Materials and Methods for details.
High-Throughput Chemical Screening.
Chemical screening and CSEA were performed as previously described (35). Details of screening methodology and analyses are provided in SI Materials and Methods.
Autophagy Analyses.
See SI Materials and Methods for details.
Rat Model of Neonatal Cerebral Hypoxia–Ischemia.
Neonatal rat cerebral hypoxia–ischemia experiments were performed as described (17). Immediately after carotid artery occlusion, rat pups were injected intraperitoneally with either neriifolin (0.25 mg/kg diluted in 0.5% ethanol/PBS) (Sigma, S961825) or vehicle (0.5%ethanol/PBS). See SI Materials and Methods for details. All experiments were performed in accordance with Swiss laws for the protection of animals and were approved by the Vaud Cantonal Veterinary Office (authorization no. 1745.2).
Supplementary Material
Acknowledgments
We thank Noboru Mizushima, Qiong Shi, Herbert Virgin, Xiaodong Wang, Qing Zhong, and Sandra Zinkel for providing critical reagents; Shuguang Wei for assistance with high-throughput screening; Beatriz Fontoura for helpful discussions; Zhongju Zou for technical support; Haley Harrington for assistance with manuscript preparation; the University of Texas (UT) Southwestern Live Cell Imaging Facility; and the Electron Microscopy Facility of the University of Lausanne. This work was supported by National Institutes of Health Grants U54 AI057156 and RO1 CA109618 (to B.L.), RO1 A140646 (to D.R.G.); Contract HHSN268201000044C (to S.Y.S.), PO1 CA95471 (to Dr. Steven McKnight, UT Southwestern Medical Center), and P30 CA142543 (to the UT Southwestern Simmons Cancer Center); and by the Swiss National Science Foundation Grant 310030-130769 (to J.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319661110/-/DCSupplemental.
References
- 1.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L, et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke PGH. Developmental cell death: Morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181(3):195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 4.Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol. 2004;36(12):2405–2419. doi: 10.1016/j.biocel.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Yuan J. Autophagy in cell death: An innocent convict? J Clin Invest. 2005;115(10):2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das G, Shravage BV, Baehrecke EH. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol. 2012;4(6):a008813. doi: 10.1101/cshperspect.a008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamy L, et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell. 2013;23(4):435–449. doi: 10.1016/j.ccr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42(1):23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Reef S, et al. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell. 2006;22(4):463–475. doi: 10.1016/j.molcel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Wu WK, et al. The autophagic paradox in cancer therapy. Oncogene. 2012;31(8):939–953. doi: 10.1038/onc.2011.295. [DOI] [PubMed] [Google Scholar]
- 11.Koike M, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172(2):454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing S, et al. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy. 2012;8(1):63–76. doi: 10.4161/auto.8.1.18217. [DOI] [PubMed] [Google Scholar]
- 13.Shoji-Kawata S, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494(7436):201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS, et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11(11):1355–1362. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W, et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 2012;22(3):473–489. doi: 10.1038/cr.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer DR, Gudz TI, Novgorodov SA, Erdahl WL. The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J Biol Chem. 1995;270(9):4923–4932. doi: 10.1074/jbc.270.9.4923. [DOI] [PubMed] [Google Scholar]
- 17.Ginet V, Puyal J, Clarke PGH, Truttmann AC. Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am J Pathol. 2009;175(5):1962–1974. doi: 10.2353/ajpath.2009.090463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JK, et al. Cardiac glycosides provide neuroprotection against ischemic stroke: Discovery by a brain slice-based compound screening platform. Proc Natl Acad Sci USA. 2006;103(27):10461–10466. doi: 10.1073/pnas.0600930103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen S, et al. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene. 2011;30(45):4544–4556. doi: 10.1038/onc.2011.168. [DOI] [PubMed] [Google Scholar]
- 20.Hundeshagen P, Hamacher-Brady A, Eils R, Brady NR. Concurrent detection of autolysosome formation and lysosomal degradation by flow cytometry in a high-content screen for inducers of autophagy. BMC Biol. 2011;9:38. doi: 10.1186/1741-7007-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, et al. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int J Biochem Cell Biol. 2012;44(11):1813–1824. doi: 10.1016/j.biocel.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7(11):926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 23.Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8(1):36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Kaur S, Rehni AK, Singh N, Jaggi AS. Studies on cerebral protection of digoxin against ischemia/reperfusion injury in mice. Yakugaku Zasshi. 2009;129(4):435–443. doi: 10.1248/yakushi.129.435. [DOI] [PubMed] [Google Scholar]
- 25.Dunn DE, et al. In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models. J Neurochem. 2011;119(4):805–814. doi: 10.1111/j.1471-4159.2011.07439.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki C, et al. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368(1):100–106. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 27.Matsui Y, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 28.Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwerger M, Kolb T, Richter K, Karakesisoglou I, Herrmann H. Induction of a massive endoplasmic reticulum and perinuclear space expansion by expression of lamin B receptor mutants and the related sterol reductases TM7SF2 and DHCR7. Mol Biol Cell. 2010;21(2):354–368. doi: 10.1091/mbc.E09-08-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22(1):R29–R34. doi: 10.1016/j.cub.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Contreras RG, Shoshani L, Flores-Maldonado C, Lázaro A, Cereijido M. Relationship between Na(+),K(+)-ATPase and cell attachment. J Cell Sci. 1999;112(Pt 23):4223–4232. doi: 10.1242/jcs.112.23.4223. [DOI] [PubMed] [Google Scholar]
- 32.Galva C, Artigas P, Gatto C. Nuclear Na+/K+-ATPase plays an active role in nucleoplasmic Ca2+ homeostasis. J Cell Sci. 2012;125(Pt 24):6137–6147. doi: 10.1242/jcs.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puyal J, Ginet V, Clarke PGH. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: A challenge for neuroprotection. Prog Neurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 34.De Angelis C, Haupert GT., Jr Hypoxia triggers release of an endogenous inhibitor of Na(+)-K(+)-ATPase from midbrain and adrenal. Am J Physiol. 1998;274(1 Pt 2):F182–F188. doi: 10.1152/ajprenal.1998.274.1.F182. [DOI] [PubMed] [Google Scholar]
- 35.Shaw SY, et al. Disease allele-dependent small-molecule sensitivities in blood cells from monogenic diabetes. Proc Natl Acad Sci USA. 2011;108(2):492–497. doi: 10.1073/pnas.1016789108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.