Significance
Zebrafish Toll-like receptor (TLR) 9 (zebTLR9) and TLR21 (zebTLR21) have distinct CpG-oligodeoxynucleotide (CpG-ODN) sequence recognition profiles. The recognition profile of zebTLR9 is more like that of the TLR9s from mouse and rabbit, whereas the recognition profile of zebTLR21 is more similar to that of human TLR9 and TLR9s from domestic animals. These two zebTLRs are requlated by UNC93B1 and cooperatively mediate the immunologic and antimicrobial responses induced by CpG-ODN in zebrafish. Our findings address the molecular basis of CpG-ODN activities in zebrafish and provide information for the rational design of CpG-ODN for use as an antimicrobial agent in fishes.
Keywords: pattern recognition receptor, innate immunity, adjuvant
Abstract
CpG-oligodeoxynucleotides (CpG-ODNs) are potent immune stimuli currently under investigation as antimicrobial agents for different species. Toll-like receptor (TLR) 9 and TLR21 are the cellular receptors of CpG-ODN in mammals and chickens, respectively. The avian genomes lack TLR9, whereas mammalian genomes lack TLR21. Although fish contain both of these genes, the biological functions of fish TLR9 and TLR21 have not been investigated previously. In this study, we comparatively investigated zebrafish TLR9 (zebTLR9) and TLR21 (zebTLR21). The two TLRs have similar expression profiles in zebrafish. They are expressed during early development stages and are preferentially expressed in innate immune function-related organs in adult fish. Results from cell-based activation assays indicate that these two zebrafish TLRs are functional, responding to CpG-ODN but not to other TLR ligands. zebTLR9 broadly recognized CpG-ODN with different CpG motifs, but CpG-ODN with GACGTT or AACGTT had better activity to this TLR. In contrast, zebTLR21 responded preferentially to CpG-ODN with GTCGTT motifs. The distinctive ligand recognition profiles of these two TLRs were determined by their ectodomains. Activation of these two TLRs by CpG-ODN occurred inside the cells and was modulated by UNC93B1. The biological functions of these two TLRs were further investigated. The CpG-ODNs that activate both zebTLR9 and zebTLR21 were more potent than others that activate only zebTLR9 in the activation of cytokine productions and were more bactericidal in zebrafish. These results suggest that zebTLR9 and zebTLR21 cooperatively mediate the antimicrobial activities of CpG-ODN. Overall, this study provides a molecular basis for the activities of CpG-ODN in fish.
Bacterial and viral CpG-deoxynucleotides containing DNA (CpG-DNA) represent a type of pathogen-associated molecular pattern (PAMP) that activates immune cells and triggers host responses to microbial infections (1–3). Synthetic phosphorothioate-modified CpG-oligodeoxynucleotides (CpG-ODNs) mimic the functions of CpG-DNA and have been investigated as immune modulators for their adjuvant and antimicrobial activities in different species (4–7). In general, a CpG-ODN contains one or more copies of CpG-deoxynucleotides containing hexamer motifs (CpG motifs). A CpG-ODN’s immunostimulatory activities are dependent on its length, the number of CpG motifs, and the position, spacing, and surrounding bases of these CpG motifs.
A CpG-ODN can have varying immunostimulatory activity in different species. This species-specific property is determined by the nucleotide context of the CpG motifs within the CpG-ODN. For example, CpG-ODNs containing a purine-purine-CG-pyrimidine-pyrimidine motif, such as a GACGTT motif, are more potent in activating murine cells compared with those containing a GTCGTT motif. In contrast, the GTCGTT motif containing CpG-ODN generates stronger immune responses in humans and various domestic animals (8, 9).
Toll-like receptors (TLRs) are pattern recognition receptors that play crucial roles in the initiation of host defense against microbial invasion by binding to PAMPs from the invading microorganisms. Ten TLRs (TLR1–TLR10) have been identified in human cells, and 13 have been identified in mouse cells. These TLRs detect diverse structures of PAMP from lipids, lipoproteins, glycans, and proteins to nucleic acids (10, 11). Of these, TLR9, a member of a subfamily of intracellular TLRs comprising TLR3, TLR7, TLR8, and TLR9, is the cellular receptor that mediates the functions of CpG-ODN. The species-specific activity of a CpG-ODN is attributed to a species-specific ligand recognition of TLR9 (12–14). In mammals, cellular localization and activation of TLR9 are regulated by various accessory proteins, including UNC93 Caenorhabditis elegans homolog of B1 (UNC93B1) (15–17). Activation of TLR9 by CpG-ODN results in various immunologic effects, including up-regulation of MHC class I and II costimulatory molecules, activation of natural killer cells and B cells, and increased B-cell proliferation. In addition, TLR9 activation up-regulates T helper (Th) 1-polarized cytokine production, which promotes T-cell activation. Because of these potent immunostimulatory effects, CpG-ODNs are currently under investigation for various therapeutic applications, including antitumor and anti-infection therapies and as vaccine adjuvants (18–20).
Similar to their actions in mammalian species, in chickens CpG-ODNs activate marked immune responses and provide protection from microbial infections (4, 5, 21). Nevertheless, analysis of the chicken and zebra finch genomes found that the TLR9 gene is not present in avian genomes. Of the 10 avian TLRs, TLR1La, TLR1Lb, TLR2a, TLR2b, TLR3, TLR4, TLR5, and TLR7 are orthologs to mammalian TLRs, whereas TLR15 and TLR21 are not found in mammals (22). It was recently demonstrated that chicken TLR21 (chTLR21) is a functional homolog to mammalian TLR9 in terms of response to CpG-ODN stimulation (23, 24).
The immunostimulatory effects of CpG-ODNs have been investigated in numerous fish species as well. In these species, much like in mammalian and avian species, CpG-ODNs up-regulate the activation of macrophages, induce proliferation of leukocytes, and stimulate cytokine expression. In addition, CpG-ODNs have been shown to protect fish against bacterial and viral infections. The molecular bases for CpG-ODN activation in fish remain unclear, however (5, 6). The genomic DNA of zebrafish has been sequenced and annotated, leading to the discovery of at least 14 different types of TLR in fish, including TLR9 and TLR21 (25, 26); however, whether these two TLRs are functional has not been investigated previously. In the present study, we comparatively investigated the expression, structural relationship, CpG-ODN interaction, regulation by UNC93B1, and immunologic functions of zebrafish TLR9 (zebTLR9) and TLR21 (zebTLR21) to explore the molecular basis of the immunostimulatory activities of CpG-ODN in fish.
Results
Expression Profile of zebTLR9, zebTLR21, and zebUNC93B1.
The mRNA levels of zebTLR9, zebTLR21 and zebUNC93B1 were analyzed by RT-PCR. These three genes had parallel expression profiles at different stages of zebrafish embryonic development. Their transcripts were detected as early as 3 h after fertilization. The gene expressions reached plateau levels at 8–12 h and declined thereafter (Fig. S1A). In adult zebrafish, zebTLR9, zebTLR21, and zebUNC93B1 demonstrated parallel expression profiles in different tissues. They were highly expressed in organs involved in innate immunity, including the intestine, spleen, and kidney, compared with other organs, such as the liver, heart, and muscle (Fig. S1B).
Structural Relationship of zebTLR9 and zebTLR21.
ZebTLR9 contains 1,057 amino acid residues, and zebTLR21 contains 989 amino acid residues. Phylogenetic analysis revealed that these two TLRs are in different clades of the phylogenetic tree. Among the zebrafish TLR sequences analyzed, zebTLR9 is most closely related to zebTLR8a, whereas zebTLR21 is most closely related to zebTLR22, zebTLR5b, and zebTLR3. ZebTLR9 and zebTLR21 share a low homology of 21% identity at the protein level (Fig. S2). In general, a TLR contains an ectodomain with multiple leucine-rich repeats, a transmembrane domain, and a Toll/interleukin-1 receptor (TIR) cytosolic domain containing three regions (boxes 1, 2, and 3) for signaling transduction (27–29). Alignment of protein sequences of these two TLRs together with the sequence of human TLR (hTLR9) revealed that distinct from hTLR9 and zebTLR9, zebTLR21 does not contain an undefined region in its ectodomain. This undefined region is present in the TLR7, TLR8, and TLR9, which compose a subfamily of hTLRs (27). In addition, the proline 915 in box 2 of hTLR9 is conserved in zebTLR9, but not in zebTLR21 (Fig. S3). The amino acid residues in the box 2 region form a BB loop, which has been shown to be essential for binding downstream signaling molecules to initiate TLR signaling, and proline is conserved in box 2 of the TIR domain of all hTLRs except hTLR3 (27–29).
CpG-ODN Recognition Profile of zebTLR9 and zebTLR21.
To evaluate whether zebTLR9 and zebTLR21 are as functional as their orthologs in mammalian species and avian species, we established zebTLR9 and zebTLR21 cell-based activation assays with HEK293 cells. The zebTLR9- and zebTLR21-expressing cells were treated with a panel of phosphorothioate-modified CpG-ODNs with different sequences (Table 1). Both zebTLR9 and zebTLR21 responded to CpG-ODN stimulation, but displayed different recognition profiles. Although zebTLR9 had a broad ligand recognition profile for activation by CpG-ODN with GTCGTT, GACGTT, and AACGTT motifs (e.g., CpG-2006, -2007, -1681, -202, -1826, -2000, -2002, -1670, -HC4040, and -201), the GACGTT motif containing CpG-2000 and the AACGTT-containing CpG-HC4040 exhibited better activity to this TLR. In contrast, zebTLR21 was more strongly activated by CpG-2006 and –2007, two CpG-ODNs each with three copies of GTCGTT motifs and different spacing between the motifs (Fig. 1A). The same ligand recognition profiles were seen for zebTLR9 and zebTLR21 when the cells were stimulated by different CpG-ODNs with concentrations ranging from 0.3 to 10 μM (Fig. 1B) and when the cell-based activation assays were established with zebrafish ZF4 cells (Fig. 1C).
Table 1.
Sequences of phosphorothioate-modified CpG-ODN used in this study
| CpG-ODN | Sequence 5′-3′ |
| CpG-2006 | tcgtcgttttgtcgttttgtcgtt |
| CpG-2007 | tcgtcgttgtcgttttgtcgtt |
| CpG-2007-GC | tgctgcttgtgcttttgtgctt |
| CpG-1681 | accgatgtcgttgccggtgacg |
| CpG-202 | gatctcgctcgctcgctat |
| CpG-685 | tcgtcgacgtcgttcgttctc |
| CpG-684 | tcgacgttcgtcgttcgtcgttc |
| CpG-1826 | tccatgacgttcctgacgtt |
| CpG-1826-GC | tccatgagcttcctgagctt |
| CpG-2000 | tccatgacgttcctgcagttcctgacgtt |
| CpG-2002 | tccacgacgttttcgacgtt |
| CpG-1670 | accgataacgttgccggtgacg |
| CpG-HC4040 | tgactgtgaacgttcgagatga |
| CpG-201 | gatcacgtacgtacgtctat |
Fig. 1.
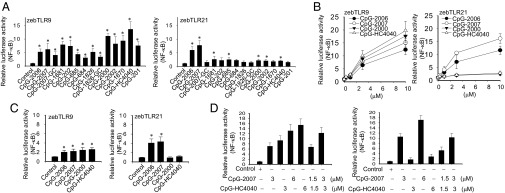
Activation of zebTLR9 and zebTLR21 by different CpG-ODNs. HEK293 cells (A, B, and D) and ZF4 cells (C) were transfected with expression vectors for zebTLR9 and for zebTLR21, plus an NF-κB luciferase reporter gene, and then treated with 3 μM or different concentrations of different CpG-ODNs as indicated. Relative luciferase activity was measured. Data are mean ± SD (n = 3). *P < 0.05 vs. control cells.
We further investigated the effect of using a mixture of two different types of CpG-ODN to activate these two TLRs. A of 1× plus 1× mixture of CpG-2007 and CpG-HC4040 did not generate stronger activation of zebTLR9 compared with the activation generated by a 2× concentration of CpG-2007 or CpG-HC4040 alone. Moreover, the addition of CpG-HC4040 did not enhance the activity of CpG-2007 in zebTLR21 activation (Fig. 1D). These results suggest that of the tested CpG-ODNs alone and in combination, CpG-2007 used alone would induce the maximal immune response through both zebTLR9 and zebTLR21.
To further investigate whether these activities of CpG-ODNs are CpG motif-dependent, we measured the activity of several CpG-inverted GpC-ODNs. Although our results indicate that the GpC-ODNs were able to activate both TLRs, the activities were relatively low compared with those of the parental CpG-ODNs (Fig. S4 and Fig. 1).
Activation of zebTLR9 and zebTLR21 by TLR Ligands Other Than CpG-ODNs.
Given that zebTLR21 is more closely related to zebTLR22, zebTLR5b, and zebTLR3 in the phylogenetic tree (Fig. S2), we further investigated whether zebTLR9 and zebTLR21 recognize any TLR ligands other than CpG-ODNs. PolyI:C, the TLR3 and TLR22 ligand, and flagellin, the TLR5 ligand, activated the HEK293 cells transfected with control vector, and this activation was not further enhanced when the cells were transfected with zebTLR9 or zebTLR21. In addition, in contrast to CpG-2006 and -2007, Pam3Cysk4 (TLR2 ligand), LPS (TLR4 ligand), CL075 and R848 (TLR7/8 ligands), and imiquimod (TLR7 ligand) did not activate cells transfected with vector for control, zebTLR9, and zebTLR21 (Fig. S5A). The same results were seen in the control vector, with the zebTLR9 and zebTLR21 expression vectors transfecting ZF4 cells (Fig. S5B). These results indicate that these two zebrafish TLRs are functional, have different CpG-ODN recognition profiles, and do not respond to ligands for other TLRs.
Molecular Determinants for Activation of zebTLR9 and zebTLR21.
To investigate the molecular determinants underlying the activation of zebTLR9 and zebTLR21, we generated ectodomain and cytoplasmic TIR domain-swapped chimeras and point mutants of these two TLRs and evaluated their activation. Two chimeras were generated, zebTLR9/21 (containing a zebTLR9 ectodomain and a zebTLR21 cytoplasmic TIR domain) and zebTLR21/9 (containing a zebTLR21 ectodomain and a zebTLR9 cytoplasmic TIR domain). Our results indicate that zebTLR9/21 had the same ligand recognition as zebTLR9, and that zebTLR21/9 had the same ligand recognition as zebTLR21 (Fig. S6). Thus, the ectodomains of these two TLRs determine their ligand recognition.
The point mutants generated were zebTLR9(C931S), zebTLR9(R935E), zebTLR9(P939H), zebTLR21(C827S), zebTLR21(R831E), and zebTLR21(L835H). In these mutant, three residues in box 2 of the zebTLR9 and zebTLR21 TIR domains were mutated. Mutation of these residues abrogated activation of these two TLRs by CpG-ODNs (Fig. S6). These results suggest that consistent with hTLRs, box 2 in the TIR domain plays an important role in initiating signal transduction on ligand ligation. In addition, although proline 915 of hTLR9 is not conserved in box 2 of zebTLR21, the replacement leucine 835 also plays a critical role in signal transduction (Figs. S3 and S6).
Regulation of zebTLR9 and zebTLR21 by UNC93B1.
Human and mouse TLR9s have been shown to reside in intracellular vesicles, where UNC93B1 regulates their cellular localization for activation (15–17, 30). Chloroquine, an agent that blocks endosomal acidification and thus prevents endosome maturation, has been shown to inhibit TLR9 activation (14, 31). This agent effectively blocked the activation of zebTLR9 by CpG-HC4040 and CpG-2007, and also blocked the activation of zebTLR21 by CpG-2006 and CpG-2007 in cell-based activation assays (Fig. S7A), suggesting that endosomal maturation is required for intracellular activation of these two TLRs. The cellular localization of zebTLR9 and zebTLR21 was further visualized by confocal microscopy after immunofluorescence staining, revealing an intracellular localization of these two TLRs. The majority of that zebTLR9 and zebTLR21 was localized in the endoplasmic reticulum (Fig. S7B).
To further investigate whether the cellular functions of these two TLRs are modulated by UNC93B1, we coexpressed zebTLR9 and zebTLR21 with this protein in cells. Immunofluorescence staining revealed colocalization of both TLRs with UNC93B1 (Fig. 2A). It was recently reported that acidic amino acid residues in the juxtamembrane region of human and mouse intracellular TLRs are required for the function and interactions of these TLRs with UNC93B1 (32). Evaluation of the zebTLR9 and zebTLR21 protein sequences also revealed the presence of acidic amino acid residues in the juxtamembrane regions of both TLRs. The residues were identified as a glutamic acid residue at position of 838 of zebTLR9 and an aspartic and glutamic acid residue at positions 740 and 742 of zebTLR21 (Fig. 2B). In consistent with these results, both zebTLR9 and zebTLR21 bound with UNC93B1 in immunoprecipitation reactions, and mutagenesis of these acidic amino acid residues reduced the binding (Fig. 2C). UNC93B1 rendered both zebTLR9 and zebTLR21 more responsive to CpG-ODN stimulation in cells. In contrast, mutagenesis of the acidic amino acid residues in both TLRs abrogated their function in mediating CpG-ODN activation in the absence or presence of UNC93B1 (Fig. 2D).
Fig. 2.
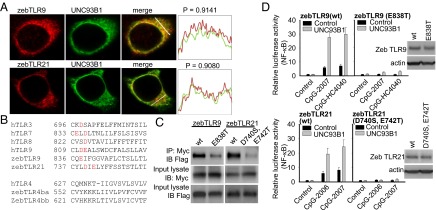
Regulation of zebTLR9 and zebTLR21 activation by UNC93B1. HEK293 cells were cotransfected with expression vectors for FLAG-tagged WT and mutant zebTLR9 and zebTLR21, Myc-tagged UNC93B1, and NF-κB luciferase reporter gene as indicated. (A) Cellular localizations of proteins were visualized by immunofluorescence staining. Colocalizations of UNC93B1 with zebTLR9 and zebTLR21 were quantified by calculating the Pearson correlation coefficient of the signaling intensities of the two proteins. (C) Protein binding was measured by immunoprecipitation followed by immunoblotting. (D) Cells were treated with 3 μM CpG-ODN, after which relative luciferase activity was measured. Data are mean ± SD (n = 3). Immunoblots shown represent expression levels of WT and acidic amino acid residue-mutated zebTLR9 and zebTLR21. (B) Sequence alignment of the juxtamembrane regions of different human and zebrafish TLRs. The acidic amino acid residues in intracellular TLR regions are shown in red.
To further investigate this regulation, we cloned the cDNA of zebUNC93B1. zebUNC93B1 shares 59.6% of protein identity with human UNC93B1. Coexpression of zebUNC93B1 in ZF4 cells rendered both zebTLR9 and zebTLR21 more responsive to CpG-ODN stimulation as well (Fig. S8). These findings suggest the function of UNC93B1 in the regulation of both zebrafish TLRs.
Function of zebTLR9 and zebTLR21 in Mediating CpG-ODN–Induced Cytokine Production in Zebrafish.
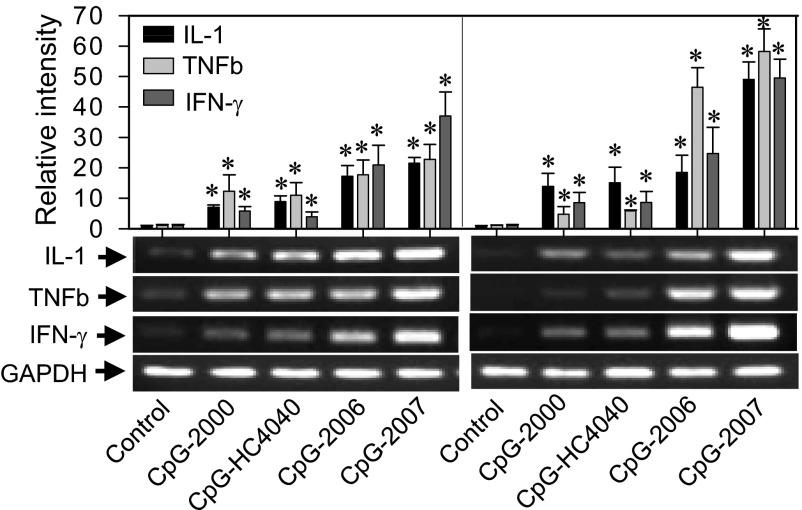
To evaluate the role of zebTLR9 and zebTLR21 in mediating the immunostimulatory activity of CpG-ODNs in zebrafish, we i.p. injected CpG-HC4040, CpG-2007, and the GpC variations into zebrafish, then isolated the zebrafish kidneys for RT-PCR analysis of IL-1, TNFb, and IFN-γ induction. The results showed more potent immunostimulatory activity for CpG-2007 than for CpG-HC4040. Moreover, coinciding with their activity in the cell-based activation assays, the activity of these CpG-ODNs in induced cytokine productions was CpG motif-dependent (Fig. S4 A and B). Four CpG-ODNs—CpG-2000, -HC4040, -2006, and -2007—were chosen for additional studies. Kidneys and intestines were then isolated from the fish for RT-PCR analysis of cytokine induction, followed by i.p. injection of these CpG-ODNs. All four CpG-ODNs displayed activity in the induction of cytokine production in zebrafish, but CpG-2006 and -2007 were more potent than -2000 and -HC4040 (Fig. 3).
Fig. 3.
Cytokine induction by different CpG-ODNs in zebrafish. Zebrafish were injected i.p. with 1 μg of CpG-ODN as indicated. Kidney (Left) and intestine (Right) were harvested and analyzed for cytokine induction by RT-PCR. (Upper) Relative intensity of different cytokines, normalized to GAPDH. Data are mean ± SD (n = 3). *P < 0.05 vs. control fish. (Lower) Representative blots from three independent RT-PCR analyses.
This profile of in vivo immunostimulatory activities of these four CpG-ODNs differs from their profile in the activation of zebTLR9 and zebTLR21. In the cell-based activation assays, CpG-2000 and -HC4040 demonstrated more activity than CpG-2006 and -2007 in the activation of zebTLR9, and zebTLR21 was activated by CpG-2006 and -2007, but was barely activated by -2000 and -HC4040 (Fig. 1A). These results suggest that both zebTLR9 and zebTLR21 are functional and that they cooperatively mediate the immunostimulatory activities of these CpG-ODNs in zebrafish.
Function of zebTLR9 and zebTLR21 in Mediating CpG-ODN–Induced Antimicrobial Response in Zebrafish.
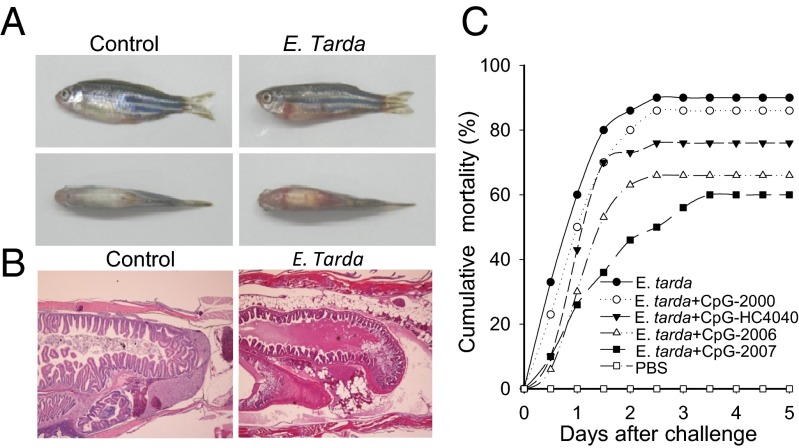
Edwardsiella tarda is a significant pathogen in various fish species. Signs of E. tarda infection may include skin lesions, gill pallor, eye swelling, excessive mucus secretion, scale erosions and ulcers, anal swelling, spiraling movements, and death. In acute cases, other signs may include ventral skin hyperemia and accumulation of red-colored ascitic fluid in the peritoneal cavity (33). E. tarda infection has been established as a model for studying microbial infection in zebrafish (34, 35). We adopted this model to further investigate the function of zebTLR9 and zebTLR21 in mediating the antimicrobial activity of CpG-ODNs. E. tarda generated a fast response in zebrafish. On the first day, a large percentage of the fish injected with bacteria alone were found to be moribund. The primary sign of this infection was a red abdomen, a result of hyperemia (Fig. 4A). Histopathological analysis by H&E staining of sections from these infected fish revealed several signs of E. tarda infection, including a relatively high number of leukocytes in tissues, accumulation of red-colored ascitic fluid in the peritoneal cavity, and desquamative catarrh with necrosis of epithelium in the intestines (Fig. 4B). The infection caused death within 4 d after bacterial injection, with no more deaths thereafter.
Fig. 4.
Antimicrobial activities of different CpG-ODNs in zebrafish. Thirty zebrafish in each group were injected i.p. with 5 × 105 cfu of E. tarda with or without 1 μg of CpG-ODN as indicated. (A and B) Skin hyperemia (A) and H&E staining (B) of sections from fish on day 2 after injection with/without E. tarda. (C) Cumulative percent mortality of fish after injection with E. tarda and CpG-ODN.
The protective activity of the CpG-ODNs was quantified based on the mortality rate of the zebrafish. Zebrafish injected with PBS and bacteria without CpG-ODN had cumulative mortality rates of 0% and 90%, respectively. The cumulative mortality rates of the zebrafish treated with CpG-2000, -HC4040, -HC2006, and -2007 were 86%, 76%, 66%, and 60%, respectively (Fig. 4C). A separate experiment with GpC variations also demonstrated the need for the CpG motif for the protective effect of CpG-ODNs (Fig. S4C). The antimicrobial activities of these CpG-ODNs were parallel to their in vivo immunostimulatory activities in the induction of cytokine production (Fig. 3). This finding further confirms that zebTLR9 and zebTLR21 cooperatively mediate the function of CpG-ODN in zebrafish.
Discussion
In the present study, we comparatively investigated the expression, structural relationship, regulation by UNC93B1, and activation of TLR9 and TLR21 in zebrafish in an attempt to address the molecular basis of the immunostimulatory activities of CpG-ODN in fish. Zebrafish have emerged as an excellent model system for the study of vertebrate innate immunity and infectious diseases (36, 37). The zebrafish genomes contain orthologs of mammalian TLRs, as well as some fish-specific TLRs (38, 39). In mammalian cells, TLR signaling is mediated by members of the MyD88 adaptor protein family, including MyD88, Mal, TRIF, TRAM, and SARM. On activation, TLR interacts with the TIR domain of adaptor molecules in this family through a BB loop in the box 2 region of its cytolic TIR domain. This loop recruits downstream IRAK and TRAF6 to the TLR signalosome for the activation of transcription factors, including NF-κB, AP-1, and IRF (27–29, 40–42). Orthologs of these signaling molecules and transcription factors have been identified in zebrafish. Zebrafish MyD88 and TRAF6 have been shown to play essential roles in microbe-induced immune responses (38, 39). In line with these findings, structural and mutagenesis analysis of amino acid residues, including leucine 835 in box 2 of zebTLR21, showed that box 2 is conserved and essential for zebTLR9 and zebTLR21 activation. These results that zebrafish and mammals have similar effector mechanisms for innate immunity to generate immune and antimicrobial responses.
In human and mouse cells, the trafficking and activation of intracellular TLRs are controlled by accessory proteins such as adaptor proteins, AP-1 to AP-4, and UNC93B1 (15–17). UNC93B1 was originally demonstrated to control TLR3, TLR7, and TLR9 activation in a 3d mutant mouse in a forward genetic screening study (43). The function of zebUNC93B1 has not yet been investigated. In the present study, UNC93B1 gene expression in zebrafish was detected, and cDNA of zebUNC93B1 was cloned. zebUNC93B1 shares 59.6% protein identity with human UNC93B1, which is relatively highly conserved through evolution, compared with the 38.2% shared protein identity between hTLR9 and zebTLR9 and the 41.8% between zebTLR21 and chTLR21. Acidic amino acid residues in the juxtamembrane region of mammalian intracellular TLRs for regulation of these TLRs by UNC93B1 were identified in both zebTLR9 and zebTLR21. In addition, UNC93B1 was found to colocalize and regulate the activation of zebTLR9 and zebTLR21. These results suggest that zebrafish and mammalian species may share a conserved mechanism involving UNC93B1 to control the localization and activation of intracellular TLRs.
Despite these similarities, however, there is a major difference in the innate immunity of zebrafish and mammals. In zebrafish, the innate immune system plays a major role in host defenses against microbial infections in the early stages of life. In their first 4 d of life, zebrafish exhibit no adaptive immune markers; full adaptive immunity does not develop until age 4–6 wk (36, 37). Expression of all major components of innate immune system at the very start of development is required for full innate immune function in the early stages of life. Consistent with this concept, in the present study, zebTLR9, zebTLR21 and zebUNC93B1 were expressed as early as 3 h after fertilization, and expression levels plateaued at 8–12 h and declined thereafter. In adult fish, these three genes were preferentially expressed in intestine, spleen, and kidney. This tissue-specific expression characteristic of both zebTLRs and zebUNC93B1 may explain why transcript levels of these genes started to decline at 12 h after fertilization in the RT-PCR analysis, because at this time, the body mass, such as muscle, which contains lower levels of these two zebTLRs and zebUNC93B1, was increased, resulting in reduced levels of zebTLR9, zebTLR21, and zebUNC93B1 transcripts in the fish.
To date, 14 different types of TLRs have been identified in fish (25, 26). Of these, TLR1, 2, 3, 4, 5, 7, 8, and 9 are commonly retained in mammalian species. An ortholog of TLR21 is found in avian species, whereas TLR14, 19, 20, 22, and 23 are fish-specific. Our phylogenetic analysis revealed that zebTLR9 is most closely related to zebTLR8a, and that zebTLR21 is most closely related to zebTLR22, zebTLR5b, and zebTLR3. This evolutional relationship is consistent with the results of the structural analysis of these two zebTLRs, which revealed that the undefined region conserved in the subfamily of TLR7, TLR8, andTLR9 is present in zebTLR9, but not in zebTLR21.
Little is known about the ligand recognition of fish TLRs. The ligand recognition of TLR2, TLR3, and TLR5 is conserved between fish and mammals, with recognition of lipoproteins, dsRNA, and flagellin, respectively (25, 26). In addition, a cell-based assay with HEK293 cells has show that fish TLR22 recognizes dsRNA (44). This list has been expanded in the present study by the demonstration that both zebTLR9 and zebTLR21 are functional and recognize CpG-ODNs. Furthermore, species-specific recognition of TLR9s in mammals is well known. CpG-ODNs containing CACGTT motifs have better activity to murine TLR9, whereas CpG-ODNs containing GTCGTT motifs are more potent in the activation of hTLR9 and of immune responses in cells isolated from a variety of domestic animals, including sheep, goat, horse, pig, and dog (4, 8, 9, 45). In this study, zebTLR9 was broadly activated by CpG-ODNs containing GTCGTT motifs and by CpG-ODNs containing GACGTT and AACGTT motifs, although the latter had better activity to this TLR. In this regard, zebTLR9 is more like the TLR9s from mouse and rabbit. The rabbit TLR9 has been shown to have a broader ligand recognition profile than hTLR9 and mouse TLR9 for recognizing different CpG-ODNs with GTCGTT or GACGTT motifs (46). In contrast, zebTLR21 was preferentially activated by GTCGTT motifs containing CpG-ODN. This findings demonstrates that the ligand recognition profile of zebTLR21 is more similar to that of hTLR9 and other TLR9s from domestic animals. Mammals do not contain TLR21. The interactions between chTLR21 and CpG-ODN have been characterized. CpG-ODNs containing GTCGTT motifs has been shown to have better activity to chTLR9 compared with CpG-ODNs with GACGTT motifs, whereas the GACGTT motifs containing CpG-ODN also activate chTLR21 (23, 24). In this aspect, the ligand recognition specificity of TLR21 is somewhat conserved between chicken and zebrafish.
Because of their potent immunostimulatory activities, CpG-ODNs are studied for their applications as antimicrobial agents and vaccine adjuvants in fish, in addition to their therapeutic applications in humans and domestic animals (4–7, 18–20). We further investigated the molecular basis for the activity of CpG-ODNs in zebrafish. A panel of four CpG-ODNs, all of which activate zebTLR9 (CpG-2000, -HC4040, -2006, and -2007) and two of which activate zebTLR21 (CpG-2006 and -2007) in cell-based activation assays were investigated for their in vivo activity in the induction of cytokine production and in the protection against the lethal effects of E. tarda infection. Our results show that all four CpG-ODNs are active in zebrafish in the activation of cytokine production and induction of antimicrobial responses, despite the fact that CpG-2000 and CpG-HC4040 were barely active in zebTLR21 in the cell-based assay. In addition, the CpG-ODNs that activate both zebTLRs (CpG-2006 and -2007) were more potent in the induction of immunologic responses in zebrafish compared with those that activate only zebTLR9 (CpG-2000 and -HC4040), despite the fact that these two CpG-ODNs showed better activity to zebTLR9. This finding reflects an outcome generated by activation of both TLR9 and TLR21 together in zebrafish. Thus, we conclude that these two zebTLRs are functional and cooperatively mediate the function of CpG-ODNs in zebrafish, and that CpG-ODNs with activity to both zebTLRs can generate the strongest immune responses in fish.
Materials and Methods
Zebrafish.
AB strain zebrafish were obtained from TaiKong or the Taiwan Zebrafish Core Facility, and were maintained at 28 °C with a 14-h light/10-h dark cycle.
Bacterial Strain, Media, and Infection.
An E. tarda strain isolated from eel was obtained from the Bioresource Collection and Research Center, Taiwan. To prepare bacteria for infection, the bacteria were grown in tryptic soy broth overnight at 37 °C with shaking. The overnight cultures were 1–10 diluted and shaken for another 3 h at 37 °C to obtain bacteria in logarithmic growing phase. Colony-forming units of the bacteria were quantitated by spectrophotometry and serial plating dilutions of the culture on tryptic soy agar. For infection, adult zebrafish were anesthetized in 160 μg/mL tricaine and injected i.p. with 5 × 105 cfu of E tarda with or without 1 μg of CpG-ODN. These fish were observed twice a day for signs of disease and mortality.
Statistical Analysis.
Groups of data are expressed as mean ± SD. Statistical analyses were performed using the Student t test. All groups were from three or more independent experiments. P < 0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank the Zebrafish International Resource Center and the Taiwan Zebrafish Core facility at National Tsing Hua University (NTHU) and the National Health Research Institutes (NHRI) (supported by Grant 101-2321-B-400-014 from the National Science Council of Taiwan) for providing the zebrafish used in these experiments. This research was conducted under the Graduate Program of Biotechnology in Medicine sponsored by NTHU and NHRI. This work was supported in part by grants from the NHRI (02A1-IMPP02-014, to T.-H.C.), the National Science Council of Taiwan (NSC102-2320-B-400-009-MY3, to T.-H.C.), and the Major State Basic Research Development Program of China (973 Program 2013CB967201, to X.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.J.I. is a guest editor invited by the Editorial Board.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. KF697668).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305273110/-/DCSupplemental.
References
- 1.Tokunaga T, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG, I: Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72(4):955–962. [PubMed] [Google Scholar]
- 2.Krieg AM, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 3.Sato Y, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273(5273):352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 4.Mutwiri G, et al. Biological activity of immunostimulatory CpG DNA motifs in domestic animals. Vet Immunol Immunopathol. 2003;91(2):89–103. doi: 10.1016/s0165-2427(02)00246-5. [DOI] [PubMed] [Google Scholar]
- 5.Chaung HC. CpG oligodeoxynucleotides as DNA adjuvants in vertebrates and their applications in immunotherapy. Int Immunopharmacol. 2006;6(10):1586–1596. doi: 10.1016/j.intimp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Carrington AC, Secombes CJ. A review of CpGs and their relevance to aquaculture. Vet Immunol Immunopathol. 2006;112(3-4):87–101. doi: 10.1016/j.vetimm.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 9.Pisetsky DS. Mechanisms of immune stimulation by bacterial DNA. Springer Semin Immunopathol. 2000;22(1-2):21–33. doi: 10.1007/s002810000021. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 11.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21(13):R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 13.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98(16):9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang TH, Lee J, Kline L, Mathison JC, Ulevitch RJ. Toll-like receptor 9 mediates CpG-DNA signaling. J Leukoc Biol. 2002;71(3):538–544. [PubMed] [Google Scholar]
- 15.Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20(4):420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12(3):168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BL, et al. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 19.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13(5):552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 20.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60(7):795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Haygreen L, Davison F, Kaiser P. DNA vaccines for poultry: The jump from theory to practice. Expert Rev Vaccines. 2005;4(1):51–62. doi: 10.1586/14760584.4.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Brownlie R, Allan B. Avian toll-like receptors. Cell Tissue Res. 2011;343(1):121–130. doi: 10.1007/s00441-010-1026-0. [DOI] [PubMed] [Google Scholar]
- 23.Brownlie R, et al. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol Immunol. 2009;46(15):3163–3170. doi: 10.1016/j.molimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Keestra AM, de Zoete MR, Bouwman LI, van Putten JP. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. J Immunol. 2010;185(1):460–467. doi: 10.4049/jimmunol.0901921. [DOI] [PubMed] [Google Scholar]
- 25.Rebl A, Goldammer T, Seyfert HM. Toll-like receptor signaling in bony fish. Vet Immunol Immunopathol. 2010;134(3-4):139–150. doi: 10.1016/j.vetimm.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Palti Y. Toll-like receptors in bony fish: From genomics to function. Dev Comp Immunol. 2011;35(12):1263–1272. doi: 10.1016/j.dci.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Bell JK, et al. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24(10):528–533. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter S, O’Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J. 2009;422(1):1–10. doi: 10.1042/BJ20090616. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408(6808):111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- 30.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane DE, Manzel L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol. 1998;160(3):1122–1131. [PubMed] [Google Scholar]
- 32.Kim J, et al. Acidic amino acid residues in the juxtamembrane region of the nucleotide-sensing TLRs are important for UNC93B1 binding and signaling. J Immunol. 2013;190(10):5287–5295. doi: 10.4049/jimmunol.1202767. [DOI] [PubMed] [Google Scholar]
- 33.Park SB, Aoki T, Jung TS. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res. 2012;43(1):67. doi: 10.1186/1297-9716-43-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pressley ME, Phelan PE, 3rd, Witten PE, Mellon MT, Kim CH. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev Comp Immunol. 2005;29(6):501–513. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 35.van der Vaart M, Spaink HP, Meijer AH. Pathogen recognition and activation of the innate immune response in zebrafish. Adv Hematol. 2012;2012:159807. doi: 10.1155/2012/159807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan C, Kim CH. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008;25(4):341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Meijer AH, Spaink HP. Host–pathogen interactions made transparent with the zebrafish model. Curr Drug Targets. 2011;12(7):1000–1017. doi: 10.2174/138945011795677809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meijer AH, et al. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol Immunol. 2004;40(11):773–783. doi: 10.1016/j.molimm.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Phelan PE, Mellon MT, Kim CH. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio) Mol Immunol. 2005;42(9):1057–1071. doi: 10.1016/j.molimm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Beutler B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 41.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 42.O’Neill LA. When signaling pathways collide: Positive and negative regulation of Toll-like receptor signal transduction. Immunity. 2008;29(1):12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7(2):156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 44.Matsuo A, et al. Teleost TLR22 recognizes RNA duplex to induce IFN and protect cells from birnaviruses. J Immunol. 2008;181(5):3474–3485. doi: 10.4049/jimmunol.181.5.3474. [DOI] [PubMed] [Google Scholar]
- 45.Rankin R, et al. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 2001;11(5):333–340. doi: 10.1089/108729001753231713. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, et al. Activation of rabbit TLR9 by different CpG-ODN optimized for mouse and human TLR9. Comp Immunol Microbiol Infect Dis. 2012;35(5):443–451. doi: 10.1016/j.cimid.2012.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.