Significance
The primary question that we address in this study is what happens when a carbonate-bearing crust is subducted to depths where the Earth’s mantle is metal saturated. Subduction plays an important role in the evolution of the Earth’s interiors, but the mechanism of the interaction between the oxidized slab and reduced mantle remains unclear. Here we report the results of high-pressure redox-gradient experiments on the interaction between Mg-Ca-carbonate and metallic iron, modeling the processes at the mantle–slab boundary, and present mechanisms of diamond formation ahead of and behind the redox front. We demonstrate that the redox mechanism revealed in this study can explain the contrasting heterogeneity of natural diamonds on the composition of inclusions, carbon isotopic composition, and nitrogen impurity content.
Keywords: carbonate–iron interaction, high-pressure experiment, mantle mineralogy, deep carbon cycle
Abstract
Subduction tectonics imposes an important role in the evolution of the interior of the Earth and its global carbon cycle; however, the mechanism of the mantle–slab interaction remains unclear. Here, we demonstrate the results of high-pressure redox-gradient experiments on the interactions between Mg-Ca-carbonate and metallic iron, modeling the processes at the mantle–slab boundary; thereby, we present mechanisms of diamond formation both ahead of and behind the redox front. It is determined that, at oxidized conditions, a low-temperature Ca-rich carbonate melt is generated. This melt acts as both the carbon source and crystallization medium for diamond, whereas at reduced conditions, diamond crystallizes only from the Fe-C melt. The redox mechanism revealed in this study is used to explain the contrasting heterogeneity of natural diamonds, as seen in the composition of inclusions, carbon isotopic composition, and nitrogen impurity content.
Subduction of crustal material plays an important role in the global carbon cycle (1–6). Depending on oxygen fugacity and pressure-temperature (P-T) conditions, carbon exists in the Earth's interior in the form of carbides, diamond, graphite, hydrocarbons, carbonates, and CO2 (7–11). In the upper mantle, the oxygen fugacity (fO2) varies from one to five log units below the fayalite-magnetite-quartz (FMQ) buffer, with a trend of a decrease with depth (6, 12–15). At a depth of ∼250 km, mantle is reported to become metal saturated (16, 17), which holds true for all mantle regions below, including the transition zone and lower mantle. The subduction of the oxidized crustal material occurs to depths greater than 600 km (4–6). The main carbon-bearing minerals of the subducted materials are carbonates, which are thermodynamically stable up to P-T conditions of the lower mantle (10, 11, 18). As evidenced by the compositions of inclusions in diamond, which vary from strongly reduced, e.g., metallic iron and carbides (19–23), to oxidized, e.g., carbonates and CO2 (6, 20, 24–28), carbonates may be involved in the reactions with reduced deep-seated rocks, including Fe0-bearing species (29–31). A scale of these reactions is determined mainly by the capacity of subducted carbonate-bearing domains. An important consequence of such an interaction is that it can produce diamond. However, studies on diamond synthesis via the reactions between oxidized and reduced phases are limited (32–35).
To understand the mechanisms of the interaction of carbon-bearing oxidized- and reduced-mineral assemblages, we performed high-pressure experiments with an iron-carbonate system; an approach was used that enabled the creation of an oxygen fugacity gradient in the capsules (Materials and Methods and SI Materials and Methods).
Results and Discussion
The experimental results and the phase compositions are given in Table 1 and Table S1, respectively. At temperatures of 1,000 and 1,100 °C, the iron–carbonate interaction can be described, in general, by the reaction
Table 1.
Experimental conditions and results
| Run no. | P, GPa | T, °C | t, h | Initial composition, mg |
Phase association of zones* (from center to periphery of samples) | |
| Mg0,9Ca0,1CO3 | Fe0 | |||||
| 1567 | 6.5 | 1,350 | 20 | 440 | 507 | [Сoh, (Fe-C)L] → [Coh, Mws, Gr] → [Mws, (Carb+Mws)L, Gr] → [Fms, (Carb+Mws)L, Dm, Dm*] |
| 1250 | 6.5 | 1,450 | 20 | 340 | 700 | [Сoh, (Fe-C)L] → [Coh, Mws, Gr, Dm] → [Mws, (Carb+Mws)L, Gr, Dm] →[(Carb+Mws)L, Mws, Gr, Dm] |
| 1566 | 6.5 | 1,550 | 10 | 340 | 535 | [Сoh, (Fe-C)L, Dm] → [Coh, Mws, Dm] → [Mws, (Carb+Mws)L, Gr, Dm] → [(Carb+Mws)L, Gr, Dm] |
| 1571 | 6.5 | 1,600 | 8 | 440 | 494 | [Coh, (Fe-C)L, Dm] → [Coh, Mws, Dm] → [Mws, (Carb+Mws)L, Gr,Dm] → [(Carb+Mws)L, Gr, Dm] |
| 1541 | 7.5 | 1,000 | 60 | 110 | 55 | Coh → [Coh, Mws, Gr] → [Mws, Marg, Gr] → [Fms, Marg] |
| 1540 | 7.5 | 1,100 | 60 | 110 | 53.9 | [Mws, Marg, Gr] → [Fms, Marg, Gr] |
| 1532 | 7.5 | 1,200 | 60 | 110 | 58 | [Mws, (Carb+Mws)L, Gr] → [Fms, (Carb+Mws)L, Mws, Gr, Dm**] |
| 1528 | 7.5 | 1,300 | 60 | 130 | 80 | [Mws, (Carb+Mws)L, Gr, Dm] → [Fms, (Carb+Mws)L, Mws, Gr, Dm, Dm**] |
| 1525 | 7.5 | 1,400 | 60 | 130 | 78 | [Mws, (Carb+Mws)L, Gr, Dm] → [Fms, (Carb+Mws)L, Mws, Gr, Dm, Dm**] |
| 1517 | 7.5 | 1,450 | 30 | 140.1 | 59.1 | [Mws, (Carb+Mws)L, Gr, Dm] → [(Carb+Mws)L, Mws, Fms, Gr, Dm] |
| 1515 | 7.5 | 1,550 | 20 | 127.2 | 56.1 | [Mws, (Carb+Mws)L, Gr, Dm] → [(Carb+Mws)L, Mws, Fms, Gr, Dm] |
| 1521 | 7.5 | 1,650 | 8 | 140 | 75.8 | [Mws, (Carb+Mws)L, Dm] → [(Carb+Mws)L, Fms, Dm] |
| Mg0,9Ca0,1CO3 | Fe3С | |||||
| 1561 | 7.5 | 1,000 | 60 | 110 | 63 | Coh → [Coh, Mws] → [Coh, Mws, Gr] → [Mws, Arg, Gr] → [Fms, Arg] |
| 1560 | 7.5 | 1,100 | 60 | 110 | 57 | Coh → [Coh, Mws] → [Coh, Mws, Gr] → [Mws, Arg, Gr] → [Fms, Arg] |
| 1552 | 7.5 | 1,200 | 60 | 110 | 67 | Coh → [Coh, Mws, Gr] → [Mws, (Carb+Mws)L, Gr] → [Fms, (Carb+Mws)L, Mws, Dm**] |
| 1548 | 7.5 | 1,300 | 60 | 120 | 107 | Coh → [Coh, Gr, Mws] → [Mws, (Carb+Mws)L, Gr] → [(Carb+Mws)L, Mws, Fms, Gr, Dm**] |
| 1545 | 7.5 | 1,400 | 60 | 120 | 110 | Coh → [Coh, Gr, Mws] → [Mws, (Carb+Mws)L, Gr] → [Fms, (Carb+Mws)L, Mws, Gr, Dm, Dm**] |
Compositions of phases from different zones are given in Table S1. Arg, aragonite; (Carb+Mws)L, carbonate melt with dissolved magnesiowustite; Coh, cohenite (Fe3C); Dm, diamond; Dm**, diamond growth on seeds; (Fe-C)L, iron-carbon melt; Fms, ferromagnesite; Gr, graphite; Marg, Mg-aragonite; Mws, magnesiowustite.
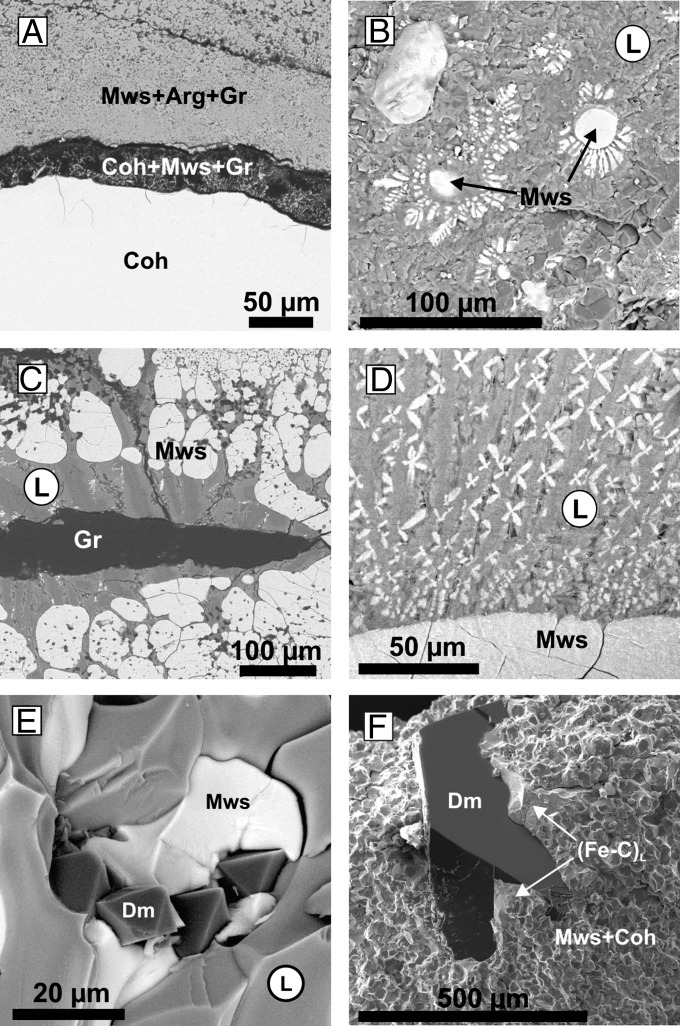
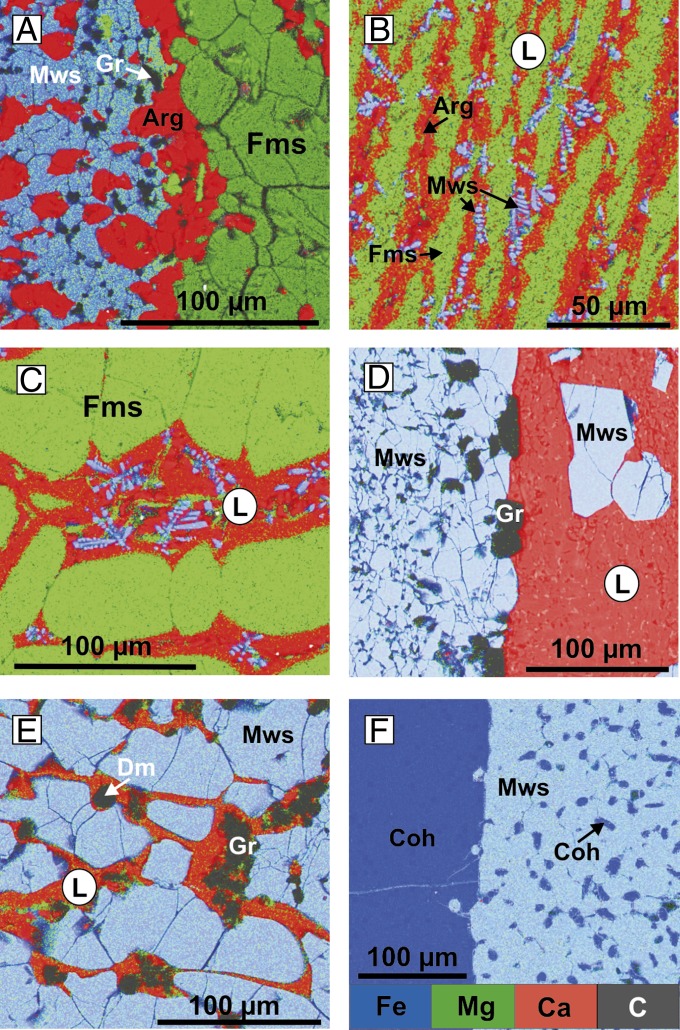
Iron carbide (Fe3C, cohenite) in the above reaction is formed on saturation of the iron with reduced carbon. This iron carbide further reacts with the initial carbonate to produce an association of magnesiowustite + metastable graphite + Ca-rich carbonate. Finally, in the central (reduced) part of the capsules, Fe3C is produced, which is surrounded by a reaction zone (Fig. 1A), consisting of carbide, graphite, and magnesiowustite. The magnesiowustite exhibits an increase in the Mg number (Mg#) from 0.13 at the contact with carbide to 0.37 at the periphery of the zone. In the outer (oxidized) part of the capsules, an assembly of magnesiowustite (Mg# = 0.38), aragonite (Ca# = 0.89), and graphite (Fig. 2A) is formed. When cohenite was used as the starting material instead of iron, the character of the interaction did not change, but the amount of graphite significantly increased.
Fig. 1.
SEM micrographs: (A) reaction zone (cohenite + magnesiowustite + graphite) (N 1541); (B) magnesiowustite crystals in a dendritic aggregate of quenched carbonate and magnesiowustite (N 1521); (C) lens of metastable graphite in a dendritic aggregate of carbonate and magnesiowustite (N 1517); (D) dendritic aggregate of carbonate and magnesiowustite (N 1532); (E) diamond and magnesiowustite in quenched carbonate melt (N 1515); (F) diamond in polycrystalline aggregates of magnesiowustite, cohenite, and quenched iron-carbon melt (N 1566). Coh, cohenite (Fe3C); Mws, magnesiowustite; L, carbonate melt with dissolved magnesiowustite; (Fe-C)L, iron-carbon melt; Arg, aragonite; Gr, graphite; Dm, diamond.
Fig. 2.
SEM micrographs of representative phase assemblages, combined with compositional maps: (A) magnesiowustite + aragonite + graphite at the contact with ferromagnesite (N 1541); (B) dendritic aggregate of carbonates and magnesiowustite (N 1515); (C) ferromagnesite in carbonate melt (N 1517); (D) magnesiowustite and metastable graphite in carbonate melt (N 1250); (E) magnesiowustite, metastable graphite, and diamond in carbonate melt (N 1525); (F) reaction zone (cohenite + magnesiowustite) (N 1250). Fms, ferromagnesite.
At 1,200 °C and higher temperatures, a Ca-rich carbonate melt coexisting with almost Ca-free magnesiowustite and ferromagnesite was present in the system. On quenching, this melt crystallized to dendritic aggregates of Ca,Mg,Fe carbonates and magnesiowustite (Figs. 1 B and D and 2 B–E). A notation [CaCO3 + (Fe,Mg)O]L will further be used to designate the Ca-rich carbonate melt with dissolved magnesiowustite. As a result of the redox reaction, metallic iron extracts carbon from carbonate to form carbide. A reaction zone, consisting of high-Fe magnesiowustite, Fe3C, and graphite is formed around the carbide (Fig. 2F). Cohenite reduces some carbon from the carbonate melt, according to the reaction
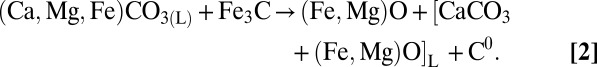 |
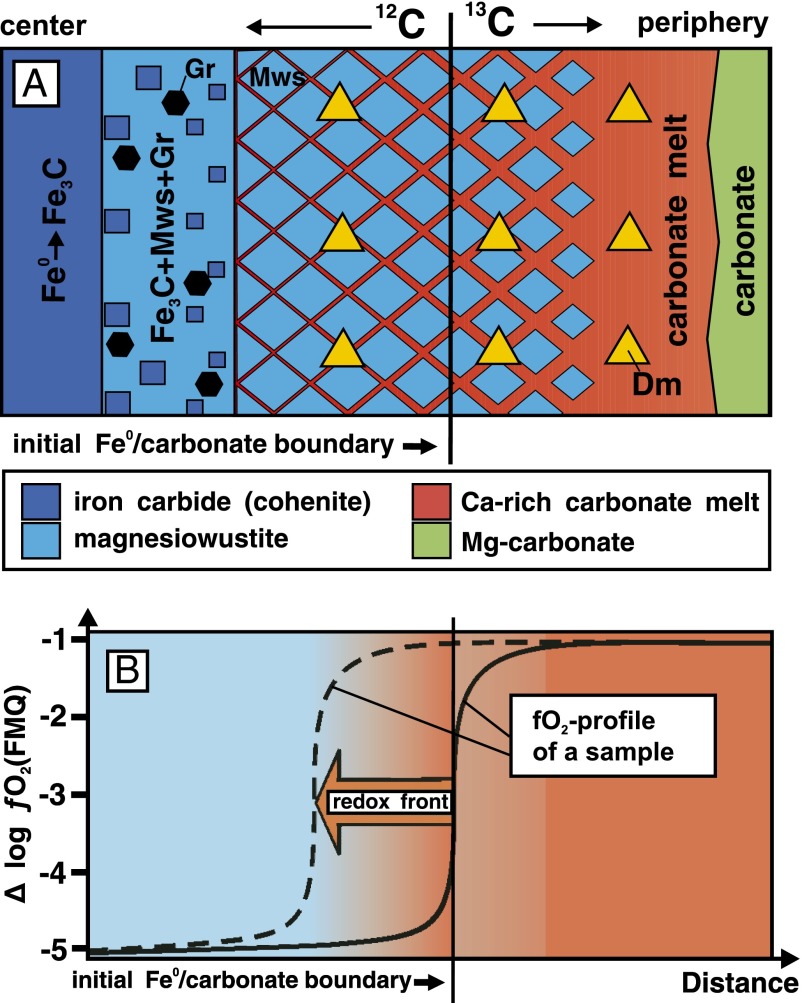
Thus, in the center, where carbide and the reaction zone (Mws + Coh + Gr) are present, the fO2 values correspond approximately to that of the iron-wustite (IW) buffer (about FMQ-5 log units). In the peripheral part of the capsules, where the magnesiowustite + carbonate melt + C0 association is formed, fO2 corresponds to values characteristic of magnesiowustite-poor carbonate melt in equilibrium with graphite or diamond (about FMQ-1 log unit) (14). Therefore, at the initial stages of the interaction, the oxygen fugacity gradient over the capsules is nearly 4 log units. As a consequence, a redox front arises at the iron/carbonate boundary. As the interaction progresses, the fO2 gradient in the capsules gradually decreases. A reconstructed scheme of the iron-carbonate interaction is shown in Fig. 3. The exchange between the oxidized periphery and reduced center involves a fluid and Ca-rich carbonate melt, capable of dissolving and transporting a significant amount of magnesiowustite. In this process, the melt is partly consumed to form magnesiowustite and elemental carbon. The rate of the iron–carbonate interaction can be estimated by the width of the reaction zone containing magnesiowustite. It was determined that the fronts of the iron–carbonate and carbonate–cohenite reactions propagate at similar rates, which increase from 6.7 to 14.8 µm/h as the temperature increases from 1,000 °C to 1,400 °C, respectively, at 7.5 GPa. The redox interaction ceases when Fe3C is completely consumed.
Fig. 3.
A scheme of the iron–carbonate interaction, illustrating the mechanism of redox front formation via fO2 gradient: (A) distribution of phases in a sample; (B) fO2 profiles in a sample at the initial stage (solid line) and at a certain moment of the redox interaction (dashed line). Arrow denotes direction of the redox front propagation.
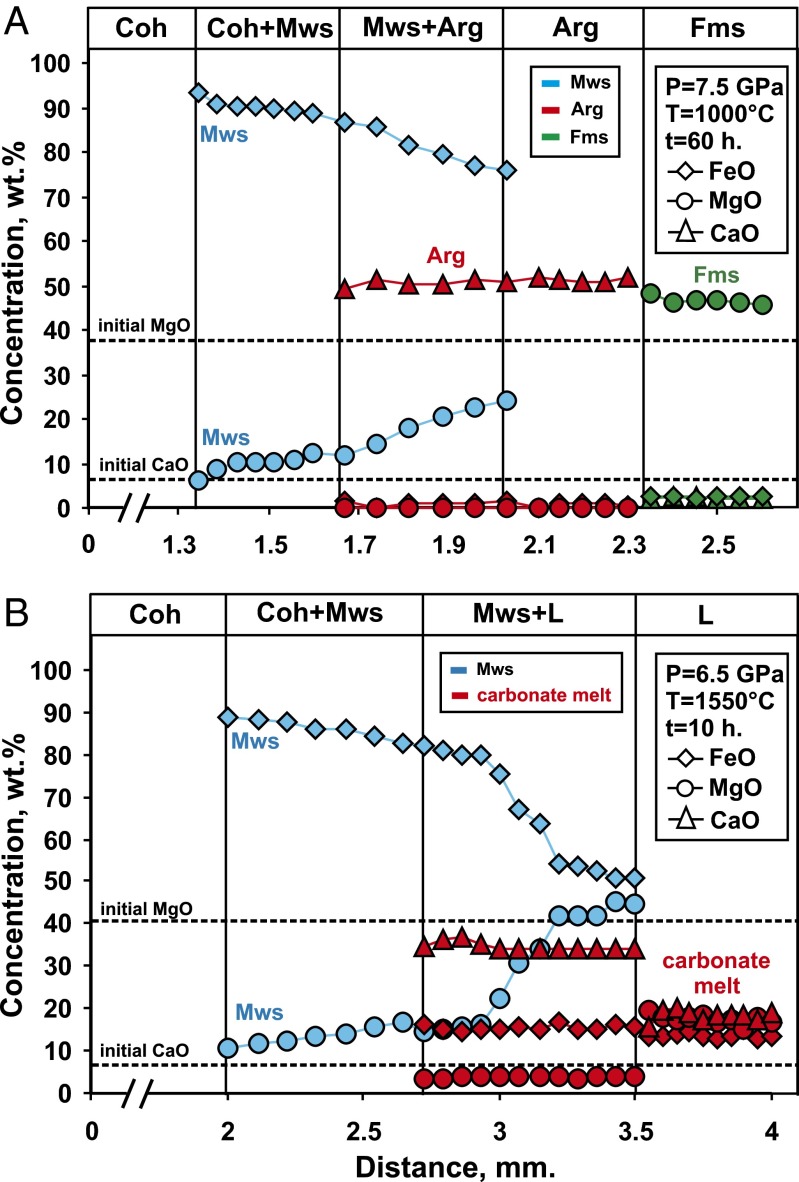
The compositions of the final phases and their trends are shown in Table S1 and Fig. 4. The Mg# of magnesiowustite and ferromagnesite, coexisting with the carbonate melt, increases with temperature. An increase in temperature, and thus the degree of partial melting, leads to a decrease in Ca# of the carbonate melt. Analyses of the quench aggregate show that the carbonate melt can dissolve a significant amount of magnesiowustite, as high as 15 wt.% at 1,200 °C and up to 22 wt.% at 1,650 °C.
Fig. 4.
Concentration profiles of FeO, MgO, and CaO across the redox front from the center to periphery of samples: (A) N 1541 (1,000 °C, 7.5 GPa); (B) N 1566 (1,550 °C, 6.5 GPa).
The formation of elemental carbon (graphite and diamond) through the iron–carbonate redox interaction deserves special consideration. In most experiments, as a result of the oxidation of iron carbide, metastable graphite in association with magnesiowustite is formed in the central zone (Fig. 2 D and E). Crystallization of graphite rather than diamond can be accounted for by the absence of the solvent, as well as by the P-T conditions insufficient for the direct graphite-to-diamond transformation. Finally, in most experiments after complete exhaustion of cohenite, a graphite lens (Fig. 1C) with inclusions of carbide in association with magnesiowustite remains in the center of the samples. However, in some experiments at temperatures ≥1,450 °C (Table 1), a quenched metal melt with relatively large (up to 1 mm) diamond crystals (Fig. 5) occurred. The number of diamond nucleation centers is rather small (10−1/mm3), and the growth rate is ∼40 µm/h. Crystallization of these diamonds is associated with the appearance of a metal-carbon melt, which arises due to the existence of the metastable Fe-graphite eutectic (8, 36). At the initial stages of the interaction, the iron is gradually saturated with carbon to form metal-carbon melt and carbide. If graphite is in contact with the metal melt, nucleation of diamond occurs. As iron reacts with carbonate, the metal melt saturates with carbon, and the iron is gradually consumed by oxidation. Thereby, carbon supersaturation is established in the residual melt, which facilitates further growth of the diamond. A similar mechanism possibly operates in the micropools of metal melt, located in the reaction zone containing magnesiowustite. In this case, diamond microcrystals (3–5 μm in size) and their aggregates are formed. Finally, the metal-carbon melt is consumed completely and replaced with diamonds, in association with magnesiowustite. It is important to note that in experiments where Fe3C was used instead of Fe0 as the starting material, no metal-carbon melt was generated, and consequently, no diamonds were seen to form ahead of the redox front.
Fig. 5.
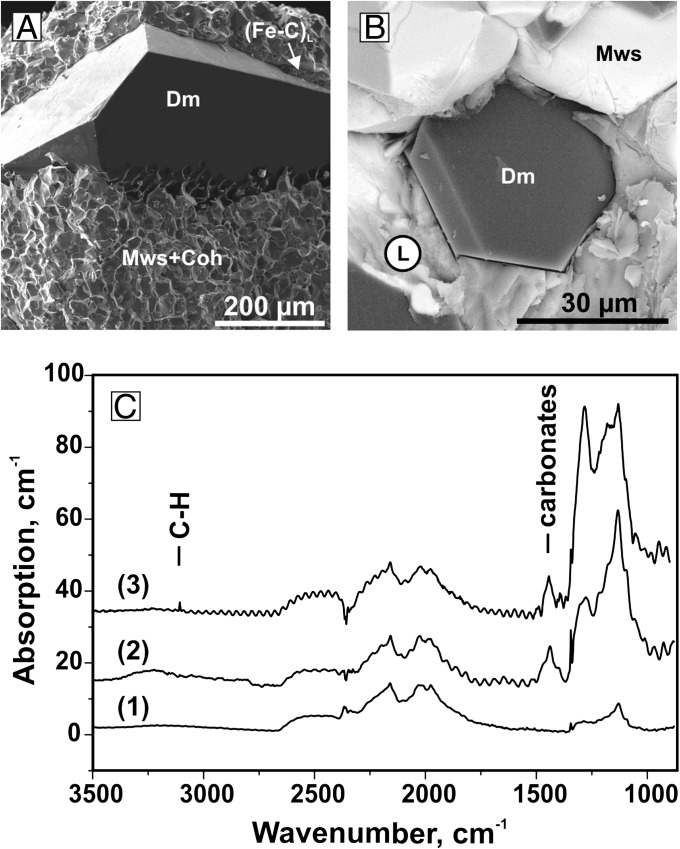
SEM micrographs of crystallized diamonds and their typical infrared spectra: (A) diamond crystal from the metal-carbon melt (N 1566, 6.5 GPa, 1,550 °C); (B) diamond crystal from carbonate melt (N 1521, 7.5 GPa, 1,650 °C); (C) typical infrared absorption spectra of diamond crystals from (1) metal-carbon melt and (2 and 3) carbonate melt. The spectra have been vertically displaced for clarify.
Behind the redox front, in the oxidized conditions, diamond growth on the seeds in the carbonate melt is established at temperature as low as 1,200 °C. At 1,300 °C, spontaneous growth of diamond crystals occurs on the seeds, which demonstrates heterogeneous nucleation. At temperatures ≥1,400 °C, homogeneous nucleation of diamond takes place (Fig. 5). With increasing temperature from 1,200 °C to 1,650 °C, the average diamond growth rate increases from 0.04 to 2 μm/h, respectively. The number of diamond nuclei in the carbonate melt increases from tens to hundreds per cubic millimeter as the temperature increases from 1,400 °C to 1,650 °C. The formation of diamond from the carbonate melt can generally be described by reaction 2. These diamonds grew through the carbon reduction from the carbonate melt, as long as the Fe3C was present in the capsule. New diamonds nucleate near the interface between the interacting Fe3C and carbonate melt. When the Fe3C is completely consumed, redox reaction 2 ceases, but a significant amount of carbon in the form of metastable graphite remains in the central part of the capsule. Therefore, in prolonged experiments, diamond growth continues owing to the transport of carbon dissolved in the carbonate melt, along with the recrystallization of magnesiowustite. Thus, we can conclude that the carbonate melt, generated in the course of the carbonate–iron interaction at temperatures ≥1,200 °C, plays a key role in the formation of diamond, being both a crystallization medium and the source of carbon.
Infrared absorption measurements show that diamond crystals synthesized in the metal-carbon melt contain 100–200 atomic ppm of nitrogen impurity in the form of single substitutional atoms (C-centers; Fig. 5). Inclusions of the quenched Fe-C melt are typical of these crystals. Diamonds, formed in the carbonate melt, exhibit significantly higher nitrogen concentrations ranging from 1,000 to 1,500 ppm. Nitrogen in this case is present mainly as C-centers, but for diamonds crystallized at higher temperatures (>1,500 °C) with considerable run duration (20 h), a significant portion of nitrogen (30–40%) occurs in the aggregated form, as nitrogen pairs or the so-called A-centers. In addition, absorption spectra frequently showed an absorption peak at ∼1,440–1,450 cm−1, which is associated with carbonate inclusions, and a weak peak at 3,107 cm−1, which is due to hydrogen-related defects (Fig. 5). It is interesting to note that, whereas the nitrogen content of ∼200 ppm is typical for most synthetic diamonds produced from metal–carbon systems, concentrations of around 1,000 ppm is normal for natural type Ia diamonds.
Carbon isotope analysis reveals that the starting carbonate has δ13C = +0.2 ‰, relative to the Pee Dee Belemnite (PDB) standard. The iron carbide formed in the reduced part of the capsules at 6.5 GPa and temperatures of 1,350 °C, 1,450 °C, and 1,550 °C has δ13C of −3.7‰, −5.9‰, and −5.0‰, respectively. The carbonate melt, which is present in the oxidized part of the capsules, is enriched in the heavy carbon isotope and has δ13C = +2.1‰ (1,350 °C), +1.7‰ (1,450 °C), and +1.4 ‰ (1,550 °C). Thus, the isotope fractionation, accompanying the formation of iron carbide from the carbon of carbonate, has an average magnitude of 6.5‰.
The interaction of carbonate with Fe0 is considered a simplified model of the processes attending the interaction of Fe0-saturated peridotites with oxidized subducted crustal material in the deep zones of the Earth. Therefore, we believe that the basic regularities, found in this study, may reasonably be applied to natural, more complex systems. Taking into account the results of previous studies on diamond crystallization in different systems modeling natural media, we can discuss the possible effects of the individual components on the diamond-forming processes. First, it should be noted that the presence of alkalies will significantly decrease the temperature of generation of carbonate melts (37–39). A similar effect will be produced by H2O-CO2 fluids (40–42). It was experimentally shown that the addition of these components in the system significantly increases the diamond-forming ability of mantle fluids/melts (43, 44), acting in the oxidized conditions behind the redox front. Under reducing conditions, ahead of the redox front, where the diamond crystallized from Fe-C melt only, the presence of H2O will lead to the inhibitory effect on the diamond-forming process (45). The addition of sulfur to the Fe-C system significantly decreases the melting temperature (46, 47). The produced melts are two immiscible liquids, with compositions similar to the Fe3C and FeS. Sulfide melts are the least efficient diamond-forming media compared with carbonate, fluid, and carbonate-silicate-fluid environments (48, 49). The role of sulfides as reducing agents in the interaction with carbonates or CO2 fluid, leading to the formation of elemental carbon (graphite or diamond), was experimentally demonstrated (35, 50).
The applicability of the redox mechanism is to the existence of deep zones in the Earth, containing native iron or iron carbides, and the possibility of subduction of carbonate minerals to these depths. In fact, there has been a growing body of evidence for the ultradeep subduction of carbonates and their participation in the formation of diamonds. This evidence involves the discovery of inclusions in diamonds, consisting of carbonates in association with the superdeep phases CaSiO3 or MgO + MgSiO3, as well as some experimental and geochemical observations (51–54). Our results suggest that the Ca-rich carbonate melt, which forms through the carbonate–iron interaction and can be generated even in the absence of alkalis and H2O, can be considered as a transmantle interstitial melt. High solubility of magnesiowustite in this carbonate melt produces a relatively low-melting temperature. The minimum temperature of nucleation and growth of diamonds in the carbonate melt, established in this study, is lower than that previously found for dry alkaline-carbonate melts (55).
Another important implication of the proposed redox front model is that it can explain the occurrence of inclusions in natural diamonds, with contrasting fO2, which are traditionally considered as indicators of differing redox conditions (6, 21). The specifics of nucleation and growth of diamond under the carbonate–Fe0 interaction may result in diamond formation at reduced conditions. Findings of central inclusions in diamond, represented by Fe0 + Fe3C + graphite and FeO + graphite assemblages (21), indicate possible relevance of the studied interaction to the natural processes. On the other hand, diamonds, which are thought to originate from the lower part of the transition zone, contain primary alkali-earth carbonate inclusions (e.g., CaCO3) (52). These diamonds could have formed from a Ca-rich carbonate melt, produced as a result of the subduction of oxidized material. We believe this to be a possible situation in nature, whereby diamonds nucleate in the metal melt and subsequently crystallize in the carbonate melt. In addition, it was determined that magnesiowustite formed in our experiments both at reduced and oxidized conditions, and its composition changes significantly as the redox front propagates. Therefore, we propose that the variations in the Mg/Fe ratio found for magnesiowustite inclusions in diamonds (53, 56) are directly related to the processes investigated in our experimental study.
A noteworthy finding in our study is highly contrasting concentrations of nitrogen impurities, exhibited by diamonds, crystallized at reduced and oxidized conditions in the course of a single redox interaction. This observation demonstrates that the partition coefficients of nitrogen are different for diamond crystallization in metal and carbonate melts and will require additional investigation. Nevertheless, even with the present results, it becomes clear that the changes in the crystallization conditions, brought about by the redox front propagation, can give rise to contrasting zonation in nitrogen and related defects distribution within a single diamond crystal. In general, the nitrogen content increases as the conditions evolve from reducing to oxidizing, with variations being on the order of ∼1,000 ppm.
Our results show that the carbide, formed via the interaction of carbonate with carbon-free iron, is significantly depleted in the heavy-carbon isotope relative to the carbonate source. It is logical that the residual carbonate is enriched with 13C. Thus, experimental data showed that nitrogen-poor diamonds grew in the reduced 13C-depleted part of capsule, whereas nitrogen-rich diamonds occurred in the oxidized 13C-enriched part of capsule. This general tendency of a simultaneous decrease of 13C value and N abundance in diamonds fits well the “limit sector” correlation of N abundance and 13C values in natural diamonds worldwide (57). One of the possible reasons of such observed interrelations of nitrogen and 13C concentrations in natural diamonds can be a segregation of carbonate melt to form a diaper and its subsequent migration (38, 58) that can lead to the formation of contrasting domains of carbon isotopes in the Earth’s low mantle. Taking into account the average magnitude of the fractionation of 6.5‰ and the nitrogen behavior (see above), the redox interaction can be considered as one of the mechanisms responsible for the complex compositional heterogeneity of natural diamonds (59–64).
Most of subducted carbonates have compositions of the MgCO3-CaCO3 series. Based on our results with such carbonate systems, we conclude that inclusions of Ca-rich carbonates, especially in association with magnesiowustite and other mantle minerals, are likely the products of the mechanism deduced from our experimentation.
Materials and Methods
Experiments were performed at pressures of 7.5 and 6.5 GPa, at temperatures of 1,000–1,650 °C and 1,350–1,600 °C, respectively, and with durations from 8 to 60 h, using a split-sphere multianvil apparatus (65). Details on the P-T calibration and accuracy of the measurements are given in ref. 66. Pt capsules of relatively large volume (6 and 10 mm in diameter at 7.5 and 6.5 GPa, respectively, 3.5–4.0 mm long) were used to enable a detailed study of the effects associated with the redox front propagation. The starting materials were mixtures of natural magnesite and dolomite, with bulk composition of (Mg0.9Ca0.1)CO3, powdered Fe0 (99.999%), and presynthesized Fe3C. A pellet of pressed iron or cohenite was placed into a carbonate container, which was then loaded into Pt capsules. This sandwich-type assembly of the reagents provided an fO2 gradient over the samples and prevented the reaction between the metallic iron and Pt (Fig. S1). After runs, samples from different parts of capsules were studied using X-ray and electron microprobe analyses, optical and scanning electron microscopy, and Raman and infrared spectroscopy. Phase identification of run products was performed by X-ray diffraction (a DRON-3 diffractometer) and Raman spectroscopy. Raman spectra were measured using a Horiba J.Y. LabRAM HR800 spectrometer, with an Ar-ion laser (514 nm). An investigation of phase relations and measurements of energy dispersive spectra (EDS) of various phases were performed using a Tescan MIRA3 LMU SEM. The morphology of microdiamond crystals was studied using a Tescan MIRA3 LMU SEM and an Olympus BX51 optical microscope. Infrared absorption spectra of diamonds were measured using a Bruker Vertex 70 FTIR spectrometer fitted with a Hyperion 2000 IR microscope. The isotopic analysis of carbon was performed using an isotope-ratio MAT-Delta mass spectrometer. Chemical compositions of synthetic phases were investigated using a Cameca Camebax-Micro microprobe. For electron microprobe analysis, polished sections of the samples were prepared. Mineral phases were analyzed with a focused electron beam of 1 μm diameter. Compositions of the quenched melt were defined using a defocused beam of 20–30 μm in diameter.
Supplementary Material
Acknowledgments
We thank L. A. Taylor for useful comments and suggestions that helped to improve the manuscript. Insightful and constructive reviews by E. Ohtani and J. G. Liou are appreciated. Financial support from the Russian Foundation for Basic Research Grant 12-05-00740 and Siberian Branch of the Russian Academy of Sciences Integration Grant 31, and Deep Carbon Observatory is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313340110/-/DCSupplemental.
References
- 1.Sobolev VS, Sobolev NV. New evidence of the sinking to great depths of the eclogitized rocks of Earth crust. Dokl Akad Nauk SSSR. 1980;250(3):683–685. [Google Scholar]
- 2.Green DH, Eggins SM, Yaxley G. Mantle dynamics: The other carbon-cycle. Nature. 1993;365(6443):210–211. [Google Scholar]
- 3.Dasgupta R, Hirschmann MM. The deep carbon cycle and melting in Earth's interior. Earth Planet Sci Lett. 2010;298(1-2):1–13. [Google Scholar]
- 4.Harte B, Richardson S. Mineral inclusions in diamonds track the evolution of a Mesozoic subducted slab beneath West Gondwanaland. Gondwana Res. 2012;21(1):236–245. [Google Scholar]
- 5.Walter MJ, et al. Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions. Science. 2011;334(6052):54–57. doi: 10.1126/science.1209300. [DOI] [PubMed] [Google Scholar]
- 6.Shirey SB, et al. Diamonds and the geology of mantle carbon. Rev Mineral Geochem. 2013;75(1):355–421. [Google Scholar]
- 7.Luth RW. In: Mantle Petrology. Fei Y, Bertka CM, Mysen BO, editors. Geochemical Society, St. Louis; 1999. pp. 297–316. [Google Scholar]
- 8.Lord OT, Walter MJ, Dasgupta R, Walker D, Clark SM. Melting in the Fe-C system to 70 GPa. Earth Planet Sci Lett. 2009;284(1-2):157–167. [Google Scholar]
- 9.Ryabchikov ID. Mechanisms of diamond formation: Reduction of carbonates or partial oxidation of hydrocarbons. Dokl Earth Sci. 2009;429(1):1346–1349. [Google Scholar]
- 10.Boulard E, et al. New host for carbon in the deep Earth. Proc Natl Acad Sci USA. 2011;108(13):5184–5187. doi: 10.1073/pnas.1016934108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oganov AR, Hemley RJ, Hazen RM, Jones AP. Structure, bonding and mineralogy of carbon at extreme conditions. Rev Mineral Geochem. 2013;75(1):47–77. [Google Scholar]
- 12.Woodland AB, Koch M. Variation in oxygen fugacity with depth in the upper mantle beneath the Kaapvaal craton, Southern Africa. Earth Planet Sci Lett. 2003;214(1-2):295–310. [Google Scholar]
- 13.McCammon C, Kopylova MG. A redox profile of the Slave mantle and oxygen fugacity control in the cratonic mantle. Contrib Mineral Petrol. 2004;148(1):55–68. [Google Scholar]
- 14.Stagno V, Ojwang DO, McCammon CA, Frost DJ. The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature. 2013;493(7430):84–88. doi: 10.1038/nature11679. [DOI] [PubMed] [Google Scholar]
- 15.Yaxley GM, Berry AJ, Kamenetsky VS, Woodland AB, Golovin AV. An oxygen fugacity profile through the Siberian Craton: Fe K-edge XANES determinations of Fe3+/Sigma Fe in garnets in peridotite xenoliths from the Udachnaya East kimberlite. Lithos. 2012;140-141:142–151. [Google Scholar]
- 16.Rohrbach A, et al. Metal saturation in the upper mantle. Nature. 2007;449(7161):456–458. doi: 10.1038/nature06183. [DOI] [PubMed] [Google Scholar]
- 17.Rohrbach A, Schmidt MW. Redox freezing and melting in the Earth’s deep mantle resulting from carbon-iron redox coupling. Nature. 2011;472(7342):209–212. doi: 10.1038/nature09899. [DOI] [PubMed] [Google Scholar]
- 18.Merlini M, et al. Structures of dolomite at ultrahigh pressure and their influence on the deep carbon cycle. Proc Natl Acad Sci USA. 2012;109(34):13509–13514. doi: 10.1073/pnas.1201336109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobolev NV, Efimova ES, Pospelova LN. Native iron in Yakutian diamonds and its paragenesis. Soviet Geol Geophys. 1981;22(12):18–21. [Google Scholar]
- 20.Stachel T, Harris JW, Brey GP. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib Mineral Petrol. 1998;132(1):34–47. [Google Scholar]
- 21.Bulanova GP. The formation of diamond. J Geochem Explor. 1995;53(1–3):1–23. [Google Scholar]
- 22.Kaminsky FV, Wirth R. Iron carbide inclusions in lower-mantle diamond from Juina, Brazil. Can Mineral. 2011;49(2):555–572. [Google Scholar]
- 23.Jacob DE, Kronz A, Viljoen KS. Cohenite, native iron and troilite inclusions in garnets from polycrystalline diamonds aggregates. Contrib Mineral Petrol. 2004;146(5):566–576. [Google Scholar]
- 24.Klein-BenDavid O, Izraeli ES, Hauri E, Navon O. Mantle fluid evolution: A tale of one diamond. Lithos. 2004;77(1-4):243–253. [Google Scholar]
- 25.Schrauder M, Navon O. Solid carbon dioxide in natural diamond. Nature. 1993;365(6441):42–44. [Google Scholar]
- 26.Sobolev NV, Logvinova AM, Efimova ES. Syngenetic phlogopite inclusions in kimberlite-hosted diamonds: Implications for role of volatiles in diamond formation. Russ Geol Geophys. 2009;50(12):1234–1248. [Google Scholar]
- 27.Logvinova AM, Wirth R, Fedorova EN, Sobolev NV. Nanometre-sized mineral and fluid inclusions in cloudy Siberian diamonds: new insights on diamond formation. Eur J Mineral. 2008;20(3):317–331. [Google Scholar]
- 28.Zedgenizov DA, Ragozin AL, Shatsky VS, Araujo D, Griffin WL. Fibrous diamonds from the placers of the northeastern Siberian Platform: Carbonate and silicate crystallization media. Russ Geol Geophys. 2011;52(11):1298–1309. [Google Scholar]
- 29.Frost DJ, et al. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature. 2004;428(6981):409–412. doi: 10.1038/nature02413. [DOI] [PubMed] [Google Scholar]
- 30.Frost DJ, McCammon CA. The redox state of Earth’s mantle. Annu Rev Earth Planet Sci. 2008;36:389–420. [Google Scholar]
- 31.Taylor L, Anand M. Diamonds: Time capsules from the Siberian Mantle. Chem Erde. 2004;64(1):1–74. [Google Scholar]
- 32.Arima M, Kozai Y, Akaishi M. Diamond nucleation and growth by reduction of carbonate melts under high-pressure and high-temperature conditions. Geology. 2002;30(8):691–694. [Google Scholar]
- 33.Siebert J, Guyot F, Malavergne V. Diamond formation in metal-carbonate interactions. Earth Planet Sci Lett. 2005;229(3-4):205–216. [Google Scholar]
- 34.Pal'yanov YN, Sokol AG, Borzdov YM, Khokhryakov AF, Sobolev NV. Diamond formation through carbonate-silicate interaction. Am Mineral. 2002;87(7):1009–1013. [Google Scholar]
- 35.Palyanov YN, et al. Reducing role of sulfides and diamond formation in the Earth's mantle. Earth Planet Sci Lett. 2007;260(1-2):242–256. [Google Scholar]
- 36.Strong HM, Chrenko RM. Further studies on diamond growth rates and physical properties of laboratory-made diamond. J Phys Chem. 1971;75(12):1838–1843. [Google Scholar]
- 37.Grassi D, Schmidt MW. Melting of carbonated pelites at 8-13 GPa: Generating K-rich carbonatites for mantle metasomatism. Contrib Mineral Petrol. 2011;162(1):69–191. [Google Scholar]
- 38.Litasov KD, Shatskiy A, Ohtani E, Yaxley GM. Solidus of alkaline carbonatite in the deep mantle. Geology. 2013;41(1):79–82. [Google Scholar]
- 39.Shatskiy A, et al. The system K2CO3-MgCO3 at 6 GPa and 900–1450°C. Am Mineral. 2013;98(8-9):1593–1603. [Google Scholar]
- 40.Foley SF. A reappraisal of redox melting in the Earth's mantle as a function of tectonic setting and time. J Petrol. 2011;52(7-8):1363–1391. [Google Scholar]
- 41.Hirsmann M. Water, melting, and the deep Earth H2O cycle. Annu Rev Earth Planet Sci. 2006;34:629–653. [Google Scholar]
- 42.Wyllie PJ, Ryabchikov ID. Volatile components, magmas, and critical fluids in upwelling mantle. J Petrol. 2000;41(7):1195–1206. [Google Scholar]
- 43.Palyanov YN, Shatsky VS, Sobolev NV, Sokol AG. The role of mantle ultrapotassic fluids in diamond formation. Proc Natl Acad Sci USA. 2007;104(22):9122–9127. doi: 10.1073/pnas.0608134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palyanov YN, Sokol AG. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos. 2009;112(S2):690–700. [Google Scholar]
- 45.Palyanov YuN, Borzdov YuM, Kupriyanov IN, Khokhryakov AF. Effect of H2O on diamond crystal growth in metal-carbon systems. Cryst Growth Des. 2012;12(11):5571–5578. [Google Scholar]
- 46.Dasgupta R, Buono A, Whelan G, Walker D. High-pressure melting relations in Fe–C–S systems: Implications for formation, evolution, and structure of metallic cores in planetary bodies. Geochim Cosmochim Acta. 2009;73(21):6678–6691. [Google Scholar]
- 47.Deng L, Fei Y, Liu X, Gong Z, Shahar A. Effect of carbon, sulfur and silicon on iron melting at high pressure: Implications for composition and evolution of the planetary terrestrial cores. Geochim Cosmochim Acta. 2013;114:220–233. [Google Scholar]
- 48.Palyanov YN, Borzdov YM, Khokhryakov AF, Kupriyanov IN, Sobolev NV. Sulfide melts-graphite interaction at HPHT conditions: Implications for diamond genesis. Earth Planet Sci Lett. 2006;250(1-2):269–280. [Google Scholar]
- 49.Litvin YA. Physicochemical formation conditions of natural diamond deduced from experimental study of the eclogite–carbonatite–sulfide–diamond system. Geol Ore Deposits. 2012;54(6):443–457. [Google Scholar]
- 50.Gunn SC, Luth RW. Carbonate reduction by Fe–S–O melts at high pressure and high temperature. Am Mineral. 2006;91(7):1110–1116. [Google Scholar]
- 51.Stachel T, Harris JW, Brey GP, Joswig W. Kankan diamonds (Guinea) II: Lower mantle inclusion parageneses. Contrib Mineral Petrol. 2000;140(1):16–27. [Google Scholar]
- 52.Brenker FE, et al. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet Sci Lett. 2007;260(1-2):1–9. [Google Scholar]
- 53.Bulanova GP, et al. Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juina, Brazil: Sbducted protoliths, carbonated melts and primary kimberlite magmatism. Contrib Mineral Petrol. 2010;160(4):489–510. [Google Scholar]
- 54.Walter MJ, et al. Primary carbonatite melt from deeply subducted oceanic crust. Nature. 2008;454(7204):622–625. doi: 10.1038/nature07132. [DOI] [PubMed] [Google Scholar]
- 55.Pal'yanov YN, Sokol AG, Borzdov YM, Khokhryakov AF, Sobolev NV. Diamond formation from mantle carbonate fluids. Nature. 1999;400(6743):417–418. [Google Scholar]
- 56.Hayman PC, Kopylova MG, Kaminsky FV. Lower mantle diamonds from Rio Soriso (Juina area, Mato Grosso, Brazil) Contrib Mineral Petrol. 2005;149(4):430–445. [Google Scholar]
- 57.Cartigny P, Harris JW, Javoy M. Diamond genesis, mantle fractionations and mantle nitrogen content: a study of delta C-13-N concentrations in diamonds. Earth Planet Sci Lett. 2001;185(1-2):85–98. [Google Scholar]
- 58.Dobretsov NL, Shatskiy AF. Deep carbon cycle and geodynamics: The role of the core and carbonatite melts in the lower mantle. Russ Geol Geophys. 2012;53(11):1117–1132. [Google Scholar]
- 59.Galimov EM. Istope fractionation related to kimberlite magmatism and diamond formation. Geochim Cosmochim Acta. 1991;55(6):1697–1708. [Google Scholar]
- 60.Boyd SR, Pineau F, Javoy M. Modelling the growth of natural diamonds. Chem Geol. 1994;116(1–2):29–42. [Google Scholar]
- 61.Shiryaev AA, Izraeli ES, Hauri EH, Zakharchenko OD, Navon O. Chemical, optical and isotopic investigation of fibrous diamonds from Brazil. Russ Geol Geophys. 2005;46(12):1185–1201. [Google Scholar]
- 62.Skuzovatov SY, Zedgenizov DA, Ragozin AL, Shatsky VS. Growth medium composition of coated diamonds from the Sytykanskaya kimberlite pipe (Yakutia) Russ Geol Geophys. 2012;53(11):1197–1208. [Google Scholar]
- 63.Wiggers de Vries DF, et al. Micron-scale coupled carbon isotope and nitrogen abundance variations in diamonds: Evidence for episodic diamond formation beneath the Siberian Craton. Geochim Cosmochim Acta. 2013;100:176–199. [Google Scholar]
- 64.Howell D, et al. A spectroscopic and carbon-isotope study of mixed-habit diamonds: Impurity characteristics and growth environment. Am Mineral. 2013;98(1):66–77. [Google Scholar]
- 65.Palyanov YN, Borzdov YM, Khokhryakov AF, Kupriyanov IN, Sokol AG. Effect of nitrogen impurity on diamond crystal growth processes. Cryst Growth Des. 2010;10(7):3169–3175. [Google Scholar]
- 66.Pal’yanov YN, Sokol AG, Borzdov YM, Khokhryakov AF. Fluid-bearing alkaline-carbonate melts as the medium for the formation of diamonds in the Earth’s mantle: An experimental study. Lithos. 2002;60(3-4):145–159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.