Significance
MuSK myasthenia gravis (MG) is a debilitating autoimmune disease: one-third of MuSK MG patients experience a life-threatening respiratory crisis, and long-term immunosuppression is the only current treatment option. Here we show that pathogenic human IgG4 MuSK antibodies bind to the first Ig-like domain in MuSK and prevent Lrp4 from binding MuSK, thereby inhibiting Agrin-stimulated MuSK phosphorylation. This inhibitory mechanism is likely responsible for disrupting the structure of the synapse, compromising synaptic transmission and causing disease. Our findings therefore suggest that therapeutic strategies designed to increase MuSK activity may prove effective in treating MuSK MG. Moreover, our studies provide mechanistic understanding of an IgG4-mediated autoimmune disease and may shed light on the mechanisms of other IgG4-mediated autoimmune diseases.
Keywords: neuromuscular junction, Rapsyn, Dok7, activation loop, insulin receptor
Abstract
Myasthenia gravis (MG) is a severely debilitating autoimmune disease that is due to a decrease in the efficiency of synaptic transmission at neuromuscular synapses. MG is caused by antibodies against postsynaptic proteins, including (i) acetylcholine receptors, the neurotransmitter receptor, (ii) muscle-specific kinase (MuSK), a receptor tyrosine kinase essential for the formation and maintenance of neuromuscular synapses, and (iii) low-density lipoprotein receptor-related protein 4 (Lrp4), which responds to neural Agrin by binding and stimulating MuSK. Passive transfer studies in mice have shown that IgG4 antibodies from MuSK MG patients cause disease without requiring complement or other immune components, suggesting that these MuSK antibodies cause disease by directly interfering with MuSK function. Here we show that pathogenic IgG4 antibodies to MuSK bind to a structural epitope in the first Ig-like domain of MuSK, prevent binding between MuSK and Lrp4, and inhibit Agrin-stimulated MuSK phosphorylation. In contrast, these IgG4 antibodies have no direct effect on MuSK dimerization or MuSK internalization. These results provide insight into the unique pathogenesis of MuSK MG and provide clues toward development of specific treatment options.
Myasthenia gravis (MG) is an autoimmune disease caused by autoantibodies to proteins in the postsynaptic membrane at neuromuscular synapses. Most MG patients carry antibodies to acetylcholine receptors (AChRs), the neurotransmitter receptor at vertebrate neuromuscular synapses (1, 2). Autoantibodies to AChRs are largely of the IgG1 and IgG3 subclass (3), which causes muscle weakness by three mechanisms: (i) complement-mediated membrane lysis (4), (ii) cross-linking and depletion of cell-surface AChRs (5), and (iii) to a lesser extent, functional blocking of the ACh-binding site (6). The ability of antibodies to AChRs to recruit complement, dimerize, and modulate AChR expression is an important component of their pathogenic mechanism: animals with experimental autoimmune MG (EAMG) can be rescued from disease with monovalent Fab fragments generated from AChR IgG antibodies, and complement-deficient mice are protected against EAMG (5, 7, 8).
Approximately 20% of patients with MG lack antibodies to AChRs, and ∼40% of these AChR-negative patients carry autoantibodies to muscle-specific kinase (MuSK), a receptor tyrosine kinase that is essential for all aspects of synaptic differentiation and maintenance (9–11). The synaptic defects in MuSK MG overlap with those in AChR MG, including a reduction in the number of functional AChRs at synapses and unreliable synaptic transmission, resulting in muscle fatigue and weakness. In contrast to AChR MG, MuSK MG is caused in large part by IgG4 antibodies (12–14) that fail to engage complement and are considered functionally monovalent (12–15). Consequently, the accumulation of complement and muscle membrane damage, hallmark pathological features of AChR MG, appear insignificant in MuSK MG (12, 16). Despite the paucity or absence of complement and cell damage in MuSK MG, the structural and functional deficits of synapses are extensive in MuSK MG, which highlights the key role that MuSK plays in organizing all aspects of synaptic differentiation (9, 17).
AChR clustering and synapse formation are orchestrated by neuronally released Agrin, which binds to low-density lipoprotein receptor-related protein 4 (Lrp4), a member of the lipoprotein receptor-related protein family, causing Lrp4 to bind and activate MuSK (18–20). Once tyrosine-phosphorylated, MuSK recruits Dok-7, an adaptor protein that becomes phosphorylated and recruits additional signaling molecules essential for synapse formation (21–23).
The extracellular region of MuSK contains three Ig-like domains and a Frizzled-like domain (9). The first Ig-like domain in MuSK is required for MuSK to bind Lrp4. Mutation of a single residue, I96, on a solvent-exposed surface of the first Ig-like domain, prevents MuSK from binding Lrp4 and responding to Agrin (20, 24). A hydrophobic surface on the opposite side of the first Ig-like domain mediates MuSK homodimerization, which is essential for MuSK transphosphorylation (24). Although MuSK is expressed by muscle and not by motor neurons, MuSK is critical for presynaptic as well as postsynaptic differentiation (9). In mice lacking MuSK, motor axons fail to stop and differentiate and instead wander aimlessly throughout the muscle (10). MuSK regulates presynaptic differentiation, at least in part, by clustering Lrp4 in muscle, which functions bidirectionally by serving not only as a receptor for Agrin and as a ligand for MuSK but also as a direct retrograde signal for presynaptic differentiation (25). In addition to its role during synapse formation, MuSK is also required to maintain adult synapses, because inhibition of MuSK expression in adult muscle leads to profound defects in presynaptic and postsynaptic differentiation (26, 27).
Because IgG4 antibodies do not engage complement and are thought to be incapable of cross-linking and modulating expression of cell-surface antigens, we reasoned that pathogenic IgG4 autoantibodies to MuSK may directly interfere with MuSK function.
Here we demonstrate that human IgG4 MuSK antibodies bind to the first Ig-like domain in MuSK and prevent Lrp4 from binding MuSK, thereby inhibiting Agrin-stimulated MuSK phosphorylation. We show that inhibiting the association between Lrp4 and MuSK seems to be the major mechanism by which the MuSK IgG4 antibodies disrupt MuSK signaling and cause MG, because these antibodies neither modulate MuSK surface expression nor have a direct effect on MuSK dimerization.
Results
The Main Immunogenic Region Includes Structural Epitopes Contained in the First Ig-Like Domain of MuSK.
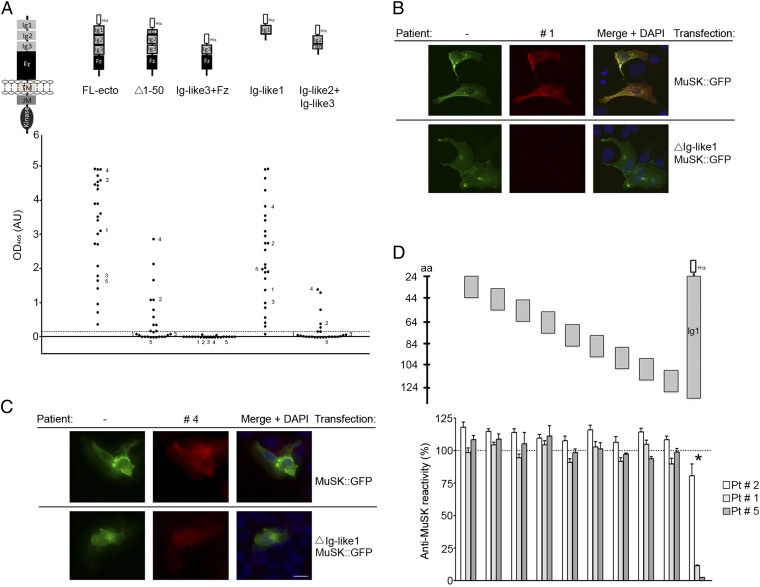
The earliest available serum samples from 25 MuSK MG patients and sera from 18 controls were tested for immunoreactivity against human MuSK recombinant proteins using an ELISA (Fig. 1A). All patients but no controls had high immunoreactivity against the Ig-like domain 1, although the level of binding varied among patients (Fig. 1A and Fig. S1). This variation likely reflects differences in antibody titer and/or affinity for MuSK. For 20 patients the immunoreactivity was limited to the first Ig-like domain (Fig. 1A). Five patients showed additional immunoreactivity against the Ig-like domain 2 (Fig. 1A). We did not detect reactivity to the Ig-like domain 3 or the Frizzled-like domain, although we cannot exclude the possibility that this is due to the experimental conditions (Fig. 1A).
Fig. 1.
MuSK MG IgG4 antibodies bind predominantly to the first Ig-like domain in MuSK. (A) An ELISA shows that antibodies from all 25 patients bind to the extracellular region of MuSK. The predominant binding sites reside in the first Ig-like domain, because antibodies bind to this domain nearly as well as the entire extracellular region. Moreover, deletion of the N-terminal half of the first Ig-like domain substantially reduces antibody binding. Antibodies from five patients have additional reactivity to the second Ig-like domain. Data shown reflect the average binding per patient determined in three independent assays (Fig. S1). (B and C) Antibodies that bind selectively to the first Ig-like domain stain cells expressing full-length MuSK-GFP but not ∆Ig-like1-MuSK-GFP, whereas antibodies with additional reactivity bind to cells expressing either construct. (D) An ELISA shows that antibody-binding to the extracellular region of MuSK is strongly inhibited by the first Ig-like domain but poorly by 20-mer overlapping peptides from this domain.
In addition, we expressed full-length MuSK-GFP or a mutant form of MuSK-GFP, lacking the first Ig-like domain (ΔIg-like1 MuSK::GFP) in nonmuscle cells and stained transfected cells with patient sera. Antibodies that bound selectively to the first Ig-like domain stained cells expressing wild-type MuSK but not the mutant form lacking the first Ig-like domain (Fig. 1B). In contrast, antibodies that showed reactivity to the second Ig-like domain stained cells expressing wild-type MuSK as well as cells expressing MuSK lacking the first Ig-like domain (Fig. 1C). In conclusion, all patients in this Dutch cohort harbor antibodies against the first Ig-like domain in MuSK at disease onset. Therefore, this region is likely to represent the main immunogenic region (MIR) of MuSK. A small group of patients have additional autoantibodies against the second Ig-like domain.
In AChR MG and other autoimmune diseases, autoantibodies often require a discontinuous stretch of amino acids that comprise a structural epitope (28–32). To determine whether the autoantibodies to MuSK recognize a linear epitope in the first Ig-like domain of MuSK, we used a competition ELISA with overlapping 20-mer peptides from the first Ig-like domain (Fig. 1D and Table S1). Preincubation of patient antibodies with the complete Ig-like domain 1 inhibited binding of the IgG4 fractions to full-length recombinant MuSK. Inhibition was nearly complete for patients 1 and 5 who harbored antibodies that bind exclusively to the first Ig-like domain, whereas competition was incomplete for patient 2 with reactivity to the second Ig-like domain, confirming our findings from the direct ELISA. Preincubation of the patient antibodies with 20-mer peptides, covering the first Ig-like domain, was without effect (Fig. 1D and Table S2). These findings indicate that the antibodies bind to a structural epitope, formed either by noncontiguous sequences within the first Ig-like domain or folding of a linear amino acid sequence, which is poorly represented in short peptides. Thus, similar to antibodies in AChR MG, antibodies to MuSK recognize linear sequences poorly, if at all.
MuSK Patient IgG4 Antibodies Interfere with Agrin-Dependent Association Between MuSK and Lrp4.
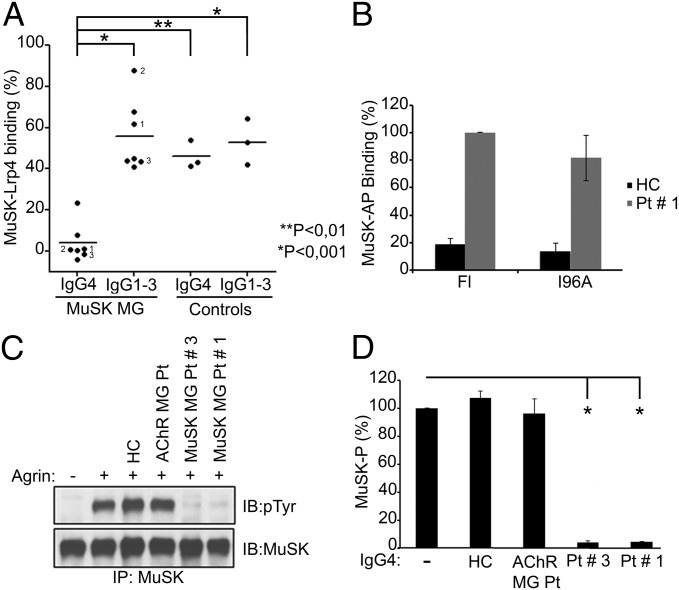
One face of the first Ig-like domain in MuSK is solvent-exposed and binds Lrp4. Because pathogenic IgG4 antibodies to MuSK bind the first Ig-like domain, we asked whether the autoantibodies interfered with the association between Lrp4 and MuSK. We measured binding between Lrp4 and MuSK using a solid-phase binding assay in which the extracellular region of MuSK, fused to Fc (ecto-MuSK-Fc), was adsorbed to protein A plates. The extracellular region of Lrp4 (ecto-Lrp4), fused to human alkaline phosphatase (AP), binds specifically but weakly to ecto-MuSK in the absence of neural Agrin; neural Agrin binds Lrp4 and stimulates strong and specific binding of AP-ecto-Lrp4 to ecto-MuSK (20). We tested IgG4 as well as IgG1-3 antibodies from seven MuSK MG patients and found that the IgG4 autoantibodies from all MuSK MG patients strongly inhibited binding between Lrp4 and MuSK, reducing binding by as much as 80–100%, in a dose-dependent manner (Fig. 2A and Fig. S2), whereas IgG1-3 patient antibodies had little effect, similar to IgG4 antibodies from healthy controls (Fig. 2A). The patient antibodies that were the most effective inhibitors of MuSK-Lrp4 association were the most potent inducers of myasthenia in vivo in a passive transfer model (12). Because the association between MuSK and Lrp4 is crucial for maintaining neuromuscular synapses, these findings raise the possibility that the autoantibodies cause myasthenia by interfering with binding between MuSK and Lrp4.
Fig. 2.
MuSK MG IgG4 antibodies block binding between Lrp4 and MuSK and inhibit Agrin-stimulated MuSK phosphorylation in muscle. (A) IgG4 antibodies from MuSK MG patients but not IgG1-3 from the same patients significantly inhibit Agrin-dependent binding between MuSK and Lrp4. IgG1-3 antibodies from MuSK MG patients and IgG4 and IgG1-3 antibodies from AChR MG and healthy controls moderately and equally reduce association between MuSK and Lrp4. The values for these control groups do not differ from one another but differ significantly from those for MuSK MG patient IgG4 (P < 0.05, n = 3). (B) Mutation of MuSK I96 fails to reduce binding of AP-ecto-MuSK to IgG4 antibodies from patient 1 (P > 0.05, n = 3). (C) IgG4 antibodies from MuSK MG patients but not from an AChR MG patient or a healthy control inhibit Agrin-stimulated MuSK phosphorylation in C2 myotubes. (D) IgG4 antibodies from MuSK MG patients reduce MuSK phosphorylation (*P < 0.01, n = 4).
Given these findings, we wondered whether binding of patient IgG4 antibodies to MuSK required MuSK I96, which is required for MuSK to bind Lrp4. We used an ELISA, in which patient antibodies were attached to a protein A plate, which was probed with AP-MuSK fusion proteins, encoding either the entire extracellular region from wild-type MuSK or MuSK I96A. Fig. 2B shows that mutation of MuSK I96 had no significant effect on antibody binding. These findings demonstrate that the patient antibodies and Lrp4 bind distinctly to the first Ig-like domain in MuSK.
Because binding between Lrp4 and MuSK is essential for Agrin to stimulate MuSK phosphorylation, we asked whether the pathogenic IgG4 autoantibodies to MuSK prevented Agrin-induced MuSK phosphorylation. We added IgG4 antibodies from patients with MuSK MG to cultured C2 myotubes, together with neural Agrin, and measured MuSK phosphorylation. Patient IgG4 antibodies to MuSK blocked MuSK phosphorylation (Fig. 2 C and D). Together, these data indicate that the MuSK antibodies cause disease by preventing Lrp4 from binding MuSK and blocking MuSK phosphorylation.
MuSK Patient IgG4 Antibodies Do Not Inhibit MuSK Dimerization.
Because the first Ig-like domain of MuSK also contains a hydrophobic surface that functions as a dimerization interface, which is important for Agrin to induce MuSK phosphorylation (24), we considered the possibility that pathogenic IgG4 antibodies to MuSK might also directly interfere with MuSK homodimerization. We therefore sought to determine whether IgG4 antibodies to MuSK inhibit MuSK phosphorylation in fibroblasts expressing MuSK but not Lrp4, a context whereby MuSK phosphorylation is dependent upon MuSK dimerization and not facilitated by Lrp4.
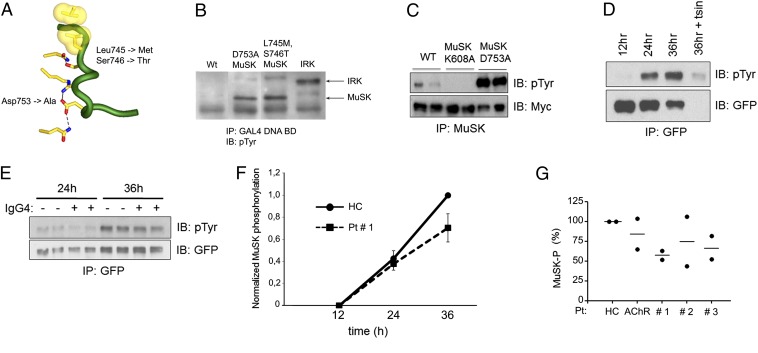
Previously we found that MuSK is poorly tyrosine phosphorylated in transfected mammalian nonmuscle cells and in yeast. Because MuSK has an unusually high Km for ATP (33), similar to the ATP concentration in muscle but higher than the ATP concentration in most cell types, we considered the possibility that this high Km for ATP restrained MuSK phosphorylation in nonmuscle cell types. Because the insulin receptor has a lower Km for ATP, typical for receptor tyrosine kinases, we mutated two amino acids in the activation loop of MuSK to the corresponding residues in the insulin receptor, reasoning that these substitutions might destabilize the activation loop, lower the Km for ATP, and increase MuSK phosphorylation in nonmuscle cells.
We generated an activation loop double mutant, MuSK L746M, S747T, and found that this activation loop mutant, unlike wild-type MuSK, was efficiently tyrosine phosphorylated in nonmuscle cells (Fig. 3 A–C). We transfected 3T3 cells with the activated form of MuSK and measured MuSK phosphorylation 12, 24, and 36 h after transfection and found that MuSK phosphorylation was undetectable at 12 h but increased in a linear manner over the next 24 h (Fig. 3D). Nearly all tyrosine phosphorylated MuSK was on the cell surface, because mild trypsin treatment degraded MuSK, leading to the disappearance of the tyrosine phosphorylated MuSK band at ∼110 kd (Fig. 3D). We therefore treated transfected 3T3 cells with IgG4 antibodies to MuSK, beginning at 12 h after transfection, and measured MuSK phosphorylation at 24 and 36 h. Fig. 3 shows that IgG4 from MuSK MG patients failed to inhibit MuSK phosphorylation (Fig. 3 E–G). Because these antibodies completely block binding between Lrp4 and MuSK but have no detectable effect on MuSK dimerization, inhibition of MuSK dimerization likely plays little if any role in pathogenesis.
Fig. 3.
IgG4 antibodies from MuSK MG patients fail to reduce MuSK phosphorylation in 3T3 cells transfected with MuSK but not Lrp4. (A) The diagram of the MuSK activation loop shows the substitutions in rodent MuSK that increase MuSK kinase activity. (B) MuSK tyrosine phosphorylation in yeast is enhanced to levels that are comparable to the insulin receptor (IRK) by mutation of D753 or L745/S746 in rodent MuSK (L746/S747 in human MuSK). Yeast were transformed with plasmids encoding fusion proteins between the DNA binding domain (BD) of GAL4 and MuSK or IRK. (C) MuSK tyrosine phosphorylation in 293 cells is enhanced 50-fold by mutation of D753. 293 cells were transfected with wild-type Myc-MuSK (21), Myc-MuSK K608A, a kinase-inactive form of MuSK, or Myc-MuSK D753A. (D) Tyrosine phosphorylation of MuSK L746M/S747T-GFP is detectable 24 h after transfection and increases over the next 12 h. Nearly all tyrosine phosphorylated MuSK is on the cell surface, because it is removed by digestion of cell-surface proteins with trypsin. (E–G) IgG4 antibodies from MuSK MG patients, a healthy control (HC), or an AChR MG patient were added to cells 12 h after transfection. (F) Differences in MuSK phosphorylation in cells treated with patient 1 or control antibodies are not significant (P > 0.05) at 24 or 36 h (n = 4). (G) The level of MuSK phosphorylation in cells treated with patient or control antibodies (●, value from separate experiments; -, mean value).
MuSK Patient IgG4 Antibodies Do Not Deplete MuSK Cell-Surface Expression.
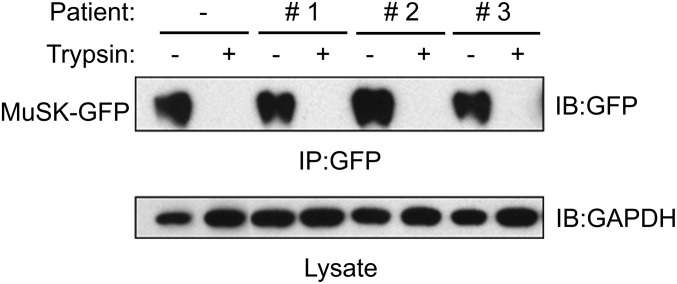
Nearly all MuSK expressed in 3T3 cells was on the cell surface and removed by mild trypsin treatment (Fig. 3D). Treatment of these cells with IgG4 patient antibodies did not alter the amount of MuSK expressed by 3T3 cells (Fig. 3E), which was likewise found almost entirely on the cell surface and removed by mild trypsin treatment (Fig. 4). In contrast, intracellular proteins, such as GAPDH, were not degraded by trypsin (Fig. 4). Thus, the pathogenic IgG4 antibodies to MuSK do not modulate and reduce MuSK cell-surface expression in this context.
Fig. 4.
IgG4 antibodies from MuSK MG patients do not reduce MuSK cell-surface expression. 3T3 cells, which were transiently transfected with MuSK-GFP, were treated with MuSK MG IgG4 antibodies for 24 h (Fig. 3). Cells were harvested or treated with trypsin before harvesting. MuSK-GFP was immunoprecipitated from lysates and detected in Western blots using antibodies to GFP, and the level of GAPDH in lysates was determined by Western blotting.
Discussion
MuSK MG is an IgG4-mediated autoimmune disease. Transfer of purified IgG4 from MuSK MG patients into immune-deficient mice causes myasthenic weakness that mimics the pathophysiology of patients with MuSK MG (13). Thus, these autoantibodies exert their pathogenic effects independent of the immune system by binding to MuSK and interfering with normal neuromuscular physiology. Our studies show that pathogenic IgG4 antibodies to MuSK prevent Agrin from stimulating MuSK by blocking association between Lrp4 and MuSK. This inhibitory mechanism likely plays a key role in disrupting the structure of the synapse, compromising synaptic transmission and causing disease. Because the pathogenic antibodies neither decrease MuSK surface expression nor directly interfere with MuSK dimerization, therapeutic strategies designed to increase MuSK activity may prove effective in treating MuSK MG.
All Dutch MuSK MG patients tested had strong immunoreactivity against the first Ig-like domain of MuSK. Five patients harbored additional reactivity against the Ig-like domain 2 in the ELISA. Others have reported that patients harbor autoantibodies outside of the Ig-like domains, including the Frizzled-like domain (13, 34, 35). These differences might be caused by racial differences and/or different disease states of the patients.
Other studies have explored an active immunization model of MuSK MG instead of a passive transfer model with patient autoantibodies. These studies have shown that bivalent MuSK antibodies activate MuSK phosphorylation and inhibit Agrin-dependent AChR clustering (16, 36), whereas monovalent Fab fragments, generated from these antibodies, inhibit MuSK phosphorylation and AChR clustering (37). Because rabbits lack the equivalent of human IgG4 antibodies, and mouse IgG binds complement (38, 39), the active immunization models lead to the production of classic, bivalent antibodies that cross-link antigens, deplete cell-surface expression, and engage complement. In addition, the MuSK epitopes recognized by these polyclonal antibody responses are unknown. As such, the nature of disease in the active immunization model is distinct from MuSK MG caused by human IgG4 antibodies.
Passive transfer of total IgG from MuSK MG patients into mice can stimulate rather than inhibit MuSK phosphorylation, and in contrast to our findings, induce MuSK internalization (40). Given the structure and function of IgG4 antibodies, as well as our findings showing that IgG4 antibodies inhibit MuSK phosphorylation, it seems likely that stimulation of MuSK phosphorylation was due to IgG1-3 rather than IgG4 in these passive transfer experiments. Combining the IgG1-3 and IgG4 fractions might mask individual effects of the different anti-MuSK IgG subclasses. Because IgG4 rather than IgG1-3 antibodies are pathogenic in Dutch MuSK MG patients (12), these findings, taken together, raise the possibility that different ethnic groups generate different immune responses to MuSK and that some MG patients carry IgG1-3 antibodies to MuSK that cause disease by other mechanisms. As such, identifying the optimal therapy for MuSK MG may require an understanding of the mechanism by which MuSK antibodies cause disease in individual MuSK MG patients.
MuSK is essential for all known aspects of presynaptic and postsynaptic differentiation (9, 10, 17). As such, antibodies that inhibit MuSK function would be expected to disrupt the architecture of the neuromuscular synapse as well as perturb neurotransmitter release and reception (9, 18, 19, 34, 41–44). Because the synaptic accumulation of acetylcholinesterase (AChE), like all other postsynaptic proteins, depends on MuSK (10, 42), IgG4 antibodies to MuSK are likely to lower AChE expression at the synapse, which may explain the hypersensitivity of MuSK MG patients to AChE inhibitors.
In AChR MG, the decrease in AChR expression and function leads to a compensatory increase in neurotransmitter release, termed “quantal content.” An increase in quantal content, however, is not evident in MuSK MG (12, 16, 45). These findings suggest that MuSK plays an important role in this homeostatic response. Because muscle inactivity increases MuSK expression (46), antibodies to AChRs may stimulate MuSK expression and MuSK-dependent retrograde signaling, thereby increasing quantal content. Because MuSK signaling is required to cluster Lrp4, which serves as a retrograde signal for presynaptic differentiation (25), antibodies that inhibit MuSK are likely to compromise presynaptic differentiation and prevent a compensatory increase in transmitter release. If so, autoantibodies to Lrp4 may likewise perturb presynaptic and postsynaptic differentiation (12, 40, 47–49).
Traditionally, IgG4 antibodies have been considered to have an antiinflammatory role, because they can compete with IgG1-3, thereby attenuating antigen down-regulation, complement-mediated cell damage, and inflammation. There is growing evidence, however, that IgG4 antibodies can be pathogenic: IgG4 antibodies against desmoglein cause a skin-blistering disease, termed pemphigus (50), and IgG4 antibodies to the phospholipase A2 receptor are thought to contribute to membranous nephropathy (51). Moreover, IgG4 autoantibodies to Leucine-rich glioma inactivated 1 (Lgi1), a regulator of the voltage-gated potassium channel, are thought to be responsible for limbic encephalitis (52). The mechanisms by which these IgG4 antibodies disrupt function and cause disease, however, are not understood. Our studies provide a mechanistic understanding of an autoimmune disease caused by IgG4 antibodies and may shed light on the mechanisms of other IgG4-mediated autoimmune diseases.
Materials and Methods
Patients.
Twenty-five Dutch MuSK MG patients and 18 controls were included for these studies (Table 1). All patients gave written consent according to the Declaration of Helsinki, and the study was approved by the Leiden University Medical Center ethics committee. The patients were diagnosed on the basis of the presence of fatigable muscle weakness with electrophysiological evidence of decrementing compound muscle action potentials in response to low-rate repetitive nerve stimulation or increased jitter on single-fiber electromyography. Furthermore, they tested positive for MuSK antibodies in a standard commercial RIA from RSR. Eighteen controls included 5 patients with AChR MG, 1 with Lambert-Eaton myasthenic syndrome, and 12 healthy controls. Plasmapheresis material from 7 patients became available during regular treatment for MuSK MG. This material was stored at −80 °C until it was further processed for IgG purification. Plasmapheresis material was affinity purified for IgG4 and IgG1-3 as described previously (SI Materials and Methods) (12).
Table 1.
Demographical and clinical characteristics of 25 MuSK MG patients
| Women, n (%) | 15 (60) |
| Age at onset , y, median (range) | 38.5 (2–80) |
| Follow-up, y, median (range) | 5.8 (0.5–33) |
| Predominant weakness, n (%) | |
| Bulbar | 9 (36) |
| Oculobulbar | 12 (48) |
| Generalized | 4 (16) |
| MGFA* at maximum | |
| II, n (b) | 6 (6) |
| III, n (b) | 4 (2) |
| IV, n (b) | 8 (8) |
| V, n (%) | 7 (28) |
| Immunosuppressive treatment at serum sampling, n (%) | 16 (64) |
Myasthenia Gravis Foundation of America score is a quantitative assessment of muscle weakness.
Binding Assays.
Recombinant proteins were generated to cover the complete extracellular region of MuSK or domains of MuSK (SI Materials and Methods). We identified the epitopes recognized by the MuSK MG patient antibodies using an ELISA and by a competition ELISA, using 20-aa overlapping peptide fragments (Leiden University Medical Center peptide facility) that cover the first Ig-like domain (Table S1). We measured binding between patient antibodies, bound to a protein A plate, and AP-ecto MuSK or AP-ecto MuSK I96A, in triplicate, using an ELISA. Binding between MuSK and Lrp4 was measured using a solid-phase binding assay, as described previously (20), except that we used human rather than rat MuSK (SI Materials and Methods). To determine the effect of IgG on MuSK-Lrp4 binding, 10 nM Agrin and 10 nM Lrp4-AP were coincubated with 8.3 μM purified IgG, which is within the reported normal range for IgG4 (0.05–9 μM); moreover, IgG4 levels can be elevated more than 20-fold in IgG4 autoimmune diseases (53). The average value from three independent experiments for each patient was calculated. One-way ANOVA analysis with Bonferroni’s correction was used to compare differences in MuSK-Lrp4 binding in the presence of IgG4 or IgG1-3 and between MuSK MG patients and controls. The values were considered statistically different if P < 0.05.
Tyrosine Phosphorylation Assays.
MuSK L746M/S747T was generated by site-directed mutagenesis and transfected into 3T3 cells with Lipofectamine 2000 (Invitrogen) (SI Materials and Methods). 3T3 cells were treated with 40 μg/mL IgG4 from MuSK MG patients, or controls from 12 to 36 h after transfection; cell-surface proteins from triplicate samples were digested by trypsin (0.05%) for 5 min at 37 °C. Myotubes were stimulated with 0.4 nM neural Agrin or Agrin together with 40 μg/mL IgG4 from MuSK MG patients, or controls for 30 min at 37 °C. MuSK tyrosine phosphorylation in duplicate samples was measured as described previously (54). PJ69-4A yeast were transformed with plasmids encoding the GAL4 DNA binding domain fused to wild-type rat MuSK, MuSK D753A, MuSK L745M, S746T, or the insulin receptor. Fusions proteins were immunoprecipitated with antibodies to GAL4, and Western blots were probed with antibodies to phosphotyrosine (SI Materials and Methods).
Immunostaining.
U2O cells were transfected with human MuSK-GFP or ΔIg-like1-MuSK-GFP, and fixed cells were stained with patient antibodies, followed by Alexa 594-conjugated mouse anti-human IgG (Invitrogen). Stained cells were viewed with a Leica DM 5500B microscope, and images were analyzed with LAS AF software (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Prof. André Deelder and Carolien Koeleman for technical support in the IgG purifications; Ingrid Hegeman, Dr. Jan Kuks, and Dr. Aad Verrips for a longstanding collaboration in collecting MuSK MG material; and Stevan Hubbard for his keen insight and suggesting substitutions that generated a more active form of MuSK. This research was supported by funding of the Prinses Beatrix Spierfonds, Association Française contre les Myopathies, a short-term fellowship from the European Molecular Biology Organization (to M.G.H.), National Institutes of Health Grant NS36193 (to S.J.B.), and a Veni fellowship (to M.L.) from the Netherlands Organization for Scientific Research and a fellowship of the Brain Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313944110/-/DCSupplemental.
References
- 1.Lennon VA, Lindstrom JM, Seybold ME. Experimental autoimmune myasthenia: A model of myasthenia gravis in rats and guinea pigs. J Exp Med. 1975;141(6):1365–1375. doi: 10.1084/jem.141.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindstrom JM, Engel AG, Seybold ME, Lennon VA, Lambert EH. Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine receptor antibodies. J Exp Med. 1976;144(3):739–753. doi: 10.1084/jem.144.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rødgaard A, Nielsen FC, Djurup R, Somnier F, Gammeltoft S. Acetylcholine receptor antibody in myasthenia gravis: Predominance of IgG subclasses 1 and 3. Clin Exp Immunol. 1987;67(1):82–88. [PMC free article] [PubMed] [Google Scholar]
- 4.Engel AG, Arahata K. The membrane attack complex of complement at the endplate in myasthenia gravis. Ann N Y Acad Sci. 1987;505:326–332. doi: 10.1111/j.1749-6632.1987.tb51301.x. [DOI] [PubMed] [Google Scholar]
- 5.Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978;298(20):1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- 6.Drachman DB, Adams RN, Josifek LF, Self SG. Functional activities of autoantibodies to acetylcholine receptors and the clinical severity of myasthenia gravis. N Engl J Med. 1982;307(13):769–775. doi: 10.1056/NEJM198209233071301. [DOI] [PubMed] [Google Scholar]
- 7.Christadoss P. C5 gene influences the development of murine myasthenia gravis. J Immunol. 1988;140(8):2589–2592. [PubMed] [Google Scholar]
- 8.Papanastasiou D, Poulas K, Kokla A, Tzartos SJ. Prevention of passively transferred experimental autoimmune myasthenia gravis by Fab fragments of monoclonal antibodies directed against the main immunogenic region of the acetylcholine receptor. J Neuroimmunol. 2000;104(2):124–132. doi: 10.1016/s0165-5728(99)00259-3. [DOI] [PubMed] [Google Scholar]
- 9.Burden SJ, Yumoto N, Zhang W. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb Perspect Biol. 2013;5(5):a009167. doi: 10.1101/cshperspect.a009167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeChiara TM, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85(4):501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 11.Hoch W, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- 12.Klooster R, et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 2012;135(Pt 4):1081–1101. doi: 10.1093/brain/aws025. [DOI] [PubMed] [Google Scholar]
- 13.McConville J, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55(4):580–584. doi: 10.1002/ana.20061. [DOI] [PubMed] [Google Scholar]
- 14.Niks EH, et al. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol. 2008;195(1-2):151–156. doi: 10.1016/j.jneuroim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 15.van der Neut Kolfschoten M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, et al. Antibodies against muscle-specific kinase impair both presynaptic and postsynaptic functions in a murine model of myasthenia gravis. Am J Pathol. 2012;180(2):798–810. doi: 10.1016/j.ajpath.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2(11):791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 18.Kim N, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, et al. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Coldefy AS, Hubbard SR, Burden SJ. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK) J Biol Chem. 2011;286(47):40624–40630. doi: 10.1074/jbc.M111.279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallock PT, et al. Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev. 2010;24(21):2451–2461. doi: 10.1101/gad.1977710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamuro J, et al. Mutations causing DOK7 congenital myasthenia ablate functional motifs in Dok-7. J Biol Chem. 2008;283(9):5518–5524. doi: 10.1074/jbc.M708607200. [DOI] [PubMed] [Google Scholar]
- 23.Okada K, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312(5781):1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- 24.Stiegler AL, Burden SJ, Hubbard SR. Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK. J Mol Biol. 2006;364(3):424–433. doi: 10.1016/j.jmb.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yumoto N, Kim N, Burden SJ. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature. 2012;489(7416):438–442. doi: 10.1038/nature11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesser BA, Henschel O, Witzemann V. Synapse disassembly and formation of new synapses in postnatal muscle upon conditional inactivation of MuSK. Mol Cell Neurosci. 2006;31(3):470–480. doi: 10.1016/j.mcn.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Kong XC, Barzaghi P, Ruegg MA. Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep. 2004;5(2):183–188. doi: 10.1038/sj.embor.7400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Im SH, Barchan D, Fuchs S, Souroujon MC. Mechanism of nasal tolerance induced by a recombinant fragment of acetylcholine receptor for treatment of experimental myasthenia gravis. J Neuroimmunol. 2000;111(1-2):161–168. doi: 10.1016/s0165-5728(00)00395-7. [DOI] [PubMed] [Google Scholar]
- 29.Lennon VA, Lambert EH, Leiby KR, Okarma TB, Talib S. Recombinant human acetylcholine receptor alpha-subunit induces chronic experimental autoimmune myasthenia gravis. J Immunol. 1991;146(7):2245–2248. [PubMed] [Google Scholar]
- 30.Lindstrom J, Einarson B. Antigenic modulation and receptor loss in experimental autoimmune myasthenia gravis. Muscle Nerve. 1979;2(3):173–179. doi: 10.1002/mus.880020304. [DOI] [PubMed] [Google Scholar]
- 31.Luo J, et al. Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J Neurosci. 2009;29(44):13898–13908. doi: 10.1523/JNEUROSCI.2833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahler M, Fritzler MJ. Epitope specificity and significance in systemic autoimmune diseases. Ann N Y Acad Sci. 2010;1183:267–287. doi: 10.1111/j.1749-6632.2009.05127.x. [DOI] [PubMed] [Google Scholar]
- 33.Till JH, et al. Crystal structure of the MuSK tyrosine kinase: Insights into receptor autoregulation. Structure. 2002;10(9):1187–1196. doi: 10.1016/s0969-2126(02)00814-6. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami Y, et al. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology. 2011;77(20):1819–1826. doi: 10.1212/WNL.0b013e318237f660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamori M, Nakamura T, Motomura M. Antibodies against Wnt receptor of muscle-specific tyrosine kinase in myasthenia gravis. J Neuroimmunol. 2013;254(1-2):183–186. doi: 10.1016/j.jneuroim.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Shigemoto K, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest. 2006;116(4):1016–1024. doi: 10.1172/JCI21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori S, et al. Divalent and monovalent autoantibodies cause dysfunction of MuSK by distinct mechanisms in a rabbit model of myasthenia gravis. J Neuroimmunol. 2012;244(1-2):1–7. doi: 10.1016/j.jneuroim.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Dangl JL, et al. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7(7):1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oi VT, et al. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984;307(5947):136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- 40.Cole RN, et al. Patient autoantibodies deplete postsynaptic muscle-specific kinase leading to disassembly of the ACh receptor scaffold and myasthenia gravis in mice. J Physiol. 2010;588(Pt 17):3217–3229. doi: 10.1113/jphysiol.2010.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amenta AR, et al. Biglycan is an extracellular MuSK binding protein important for synapse stability. J Neurosci. 2012;32(7):2324–2334. doi: 10.1523/JNEUROSCI.4610-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cartaud A, et al. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J Cell Biol. 2004;165(4):505–515. doi: 10.1083/jcb.200307164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo ZG, et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35(3):489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 44.Ngo ST, Cole RN, Sunn N, Phillips WD, Noakes PG. Neuregulin-1 potentiates agrin-induced acetylcholine receptor clustering through muscle-specific kinase phosphorylation. J Cell Sci. 2012;125(Pt 6):1531–1543. doi: 10.1242/jcs.095109. [DOI] [PubMed] [Google Scholar]
- 45.Viegas S, et al. Passive and active immunization models of MuSK-Ab positive myasthenia: Electrophysiological evidence for pre and postsynaptic defects. Exp Neurol. 2012;234(2):506–512. doi: 10.1016/j.expneurol.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Valenzuela DM, et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15(3):573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69(2):418–422. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- 48.Pevzner A, et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol. 2012;259(3):427–435. doi: 10.1007/s00415-011-6194-7. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 2012;69(4):445–451. doi: 10.1001/archneurol.2011.2393. [DOI] [PubMed] [Google Scholar]
- 50.Di Zenzo G, et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J Clin Invest. 2012;122(10):3781–3790. doi: 10.1172/JCI64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck LH, Jr, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irani SR, et al. Morvan syndrome: Clinical and serological observations in 29 cases. Ann Neurol. 2012;72(2):241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- 53.Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62(6):1755–1762. doi: 10.1002/art.27435. [DOI] [PubMed] [Google Scholar]
- 54.Herbst R, Burden SJ. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 2000;19(1):67–77. doi: 10.1093/emboj/19.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.