Significance
The microtubule (MT) cytoskeleton is a dynamic polymer network that plays a crucial role in cell function and disease. MT assembly and dynamics are precisely controlled; a key regulator is the MT destabilizer known as stathmin. Stathmin’s mechanism of action remains controversial: one well-supported model is that it reduces polymer indirectly by sequestering MT subunits; the alternative is that it acts directly on MTs by an as yet unknown mechanism. We provide a resolution to this debate by presenting experimental evidence that stathmin can act directly on MTs and does so by binding and destabilizing exposed protofilaments. Computer simulations performed in parallel suggest that both the direct and sequestering activities are likely to be significant in a cellular context.
Keywords: Zn-sheets, GMPCPP, T2S complex, computer simulation
Abstract
Regulation of microtubule dynamic instability is crucial for cellular processes, ranging from mitosis to membrane transport. Stathmin (also known as oncoprotein 18/Op18) is a prominent microtubule destabilizer that acts preferentially on microtubule minus ends. Stathmin has been studied intensively because of its association with multiple types of cancer, but its mechanism of action remains controversial. Two models have been proposed. One model is that stathmin promotes microtubule catastrophe indirectly, and does so by sequestering tubulin; the other holds that stathmin alters microtubule dynamics by directly destabilizing growing microtubules. Stathmin’s sequestration activity is well established, but the mechanism of any direct action is mysterious because stathmin binds to microtubules very weakly. To address these issues, we have studied interactions between stathmin and varied tubulin polymers. We show that stathmin binds tightly to Dolastatin-10 tubulin rings, which mimic curved tubulin protofilaments, and that stathmin depolymerizes stabilized protofilament-rich polymers. These observations lead us to propose that stathmin promotes catastrophe by binding to and acting upon protofilaments exposed at the tips of growing microtubules. Moreover, we suggest that stathmin's minus-end preference results from interactions between stathmin's N terminus and the surface of α-tubulin that is exposed only at the minus end. Using computational modeling of microtubule dynamics, we show that these mechanisms could account for stathmin's observed activities in vitro, but that both the direct and sequestering activities are likely to be relevant in a cellular context. Taken together, our results suggest that stathmin can promote catastrophe by direct action on protofilament structure and interactions.
Proper regulation of microtubule (MT) dynamic instability is crucial for cellular processes, ranging from mitosis to membrane transport. One of the most prominent MT regulators in animals is a destabilizer known as stathmin or oncoprotein 18 (Op18) (1–4). Stathmin has been the focus of much attention because increases in its expression are associated with cancer progression (1, 2). However, the mechanism of stathmin action remains controversial. Two models have been proposed. One model is that stathmin reduces MT polymer by sequestering tubulin, indirectly promoting “catastrophe,” which is the transition from growth to depolymerization (3–6). Another model holds that stathmin induces catastrophe directly, presumably by acting on MT tips (6, 7).
Stathmin's sequestration activity is now well documented (3, 4), but the issue of whether stathmin acts directly on MTs has proven difficult to resolve. In particular, one would predict that for stathmin to directly induce catastrophe, it must interact with microtubule polymer, but thus far published reports have shown that binding of stathmin to MT polymer is weak or nonexistent (6, 7). To understand stathmin’s role in cell function and disease, and to develop its therapeutic potential, it is important to establish whether stathmin can induce catastrophe directly or acts by simply sequestering tubulin (3–8).
One potential resolution for this conundrum is to propose that stathmin does not bind significantly to the MT lattice, but interacts instead with some other tubulin conformation that is present at growing MT tips (3, 9). Through interactions with this tip-specific conformation, it could then destabilize the MT tip and cause catastrophe. Here we test this hypothesis using a combination of biochemistry and computational modeling. Based on these data, we propose that stathmin can indeed promote catastrophe directly and does so by direct action on laterally unbound tubulin protofilaments (PFs) exposed at MT tips.
Results and Discussion
To test the hypothesis that stathmin binds to and acts upon a tip-specific polymer conformation, we needed to first identify forms of tubulin polymer that might be expected to mimic this conformation and then test the ability of stathmin to bind to these polymers. Although the specific conformations that occur at the MT tip are unknown, two conformations that are predicted to exist at the tip are the GTP lattice and laterally unbound protofilaments (9, 10). Moreover, growing MTs have protofilaments that are straight or slightly curved, whereas depolymerizing MTs have tightly curved “ram’s horns” (9).
Given this information, we chose to study interactions between stathmin and five MT preparations (Fig. 1 and Figs. S1 and S2). Two of these preparations are commonly used. (i) Taxol MTs (Tx-MT), which are typical Taxol MTs made by stepwise addition of Taxol (11); these are composed of a GDP lattice conformation and have few free protofilaments (12). (ii) GMPCPP MTs (CPP-MT), which have a GTP lattice and some free protofilaments (13, 14); these are made by replacing the standard GTP nucleotide with the slowly hydrolysable analog GMPCPP. In addition, we chose to study three more unusual conformations: (iii) GMPCPP protofilaments (CPP-PF), which are produced by treating GMPCPP MTs with calcium; the calcium decomposes many of the MTs into protofilaments, but also leaves sheets and MT-like structures (13, 14); (iv) Dolastatin-10 rings (DL-rings), which are formed by adding the drug Dolastatin-10 to tubulin; these rings are structurally analogous to the curled protofilaments that form during depolymerization (15–18); and (v) Zinc-induced tubulin sheets (Zn-sheets), which are made by adding ZnCl2, and are characterized by laterally associated protofilaments arranged in an antiparallel fashion, resulting in Zn-sheets having two protofilaments exposed at opposite edges (19, 20). Finally, it has been noted that the ability of stathmin to sequester tubulin depends strongly on pH (4, 6). Therefore, we decided to perform most of our tests at both pH 6.8 (where stathmin strongly sequesters tubulin dimers) and pH 7.5 (where sequestering activity is strongly diminished, but stathmin still causes MT catastrophe) (4, 6).
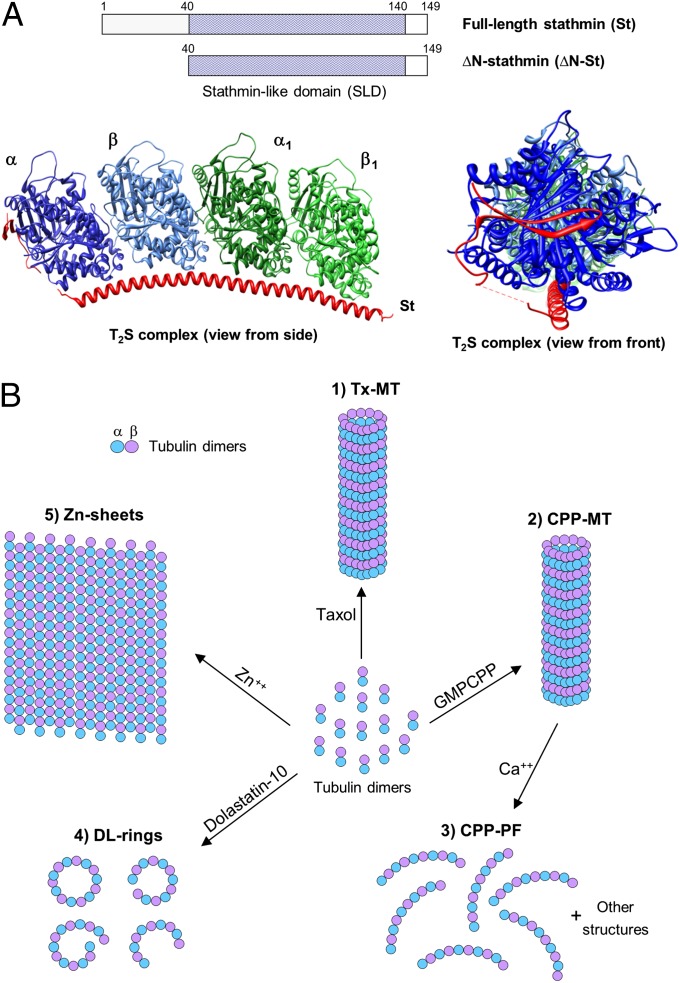
Fig. 1.
Overview of stathmin structures and tubulin polymers used in this work. (A, Upper) Schematic showing the human stathmin constructs used in this work. (Lower) The structure (3RYI) of the complex (T2S complex) formed between the two tubulin heterodimers (blue or green) and stathmin (red) (23, 24). (Left) A view from the side of the protofilament-like structure; (Right) the view from the front (down the axis) of the protofilament. Note that part of stathmin (the N-terminal tail) is bound the exposed surface of α-tubulin; this is the surface that is exposed at the minus end and would bind to the α-tubulin monomer of an incoming tubulin dimer. (B) Chemically induced tubulin polymers with different conformations. 1: Tx-MT, Taxol stabilized tubulin polymers, which are made by stepwise addition of Taxol and consist primarily of MTs (12); 2: CPP-MT, GMPCPP induced tubulin polymers, which are formed by replacing GTP with GMPCPP and consist primarily of MTs (13, 14); 3: CPP-PF, protofilament-rich structures that are made by treating GMPCPP-MTs with calcium (13, 14); 4: DL-rings, Dolastatin-10–induced tubulin rings, which consist of curved protofilament-based rings and other structures similar to the “ram's horns” that form when tubulin depolymerizes (15–18); 5: Zn-Sheets, Zinc-induced tubulin sheets, which are flat sheets formed by antiparallel associations between protofilaments and thus have two exposed protofilaments exposed at both lateral edges (19, 20).
Interactions Between Stathmin and Varied Tubulin Polymers.
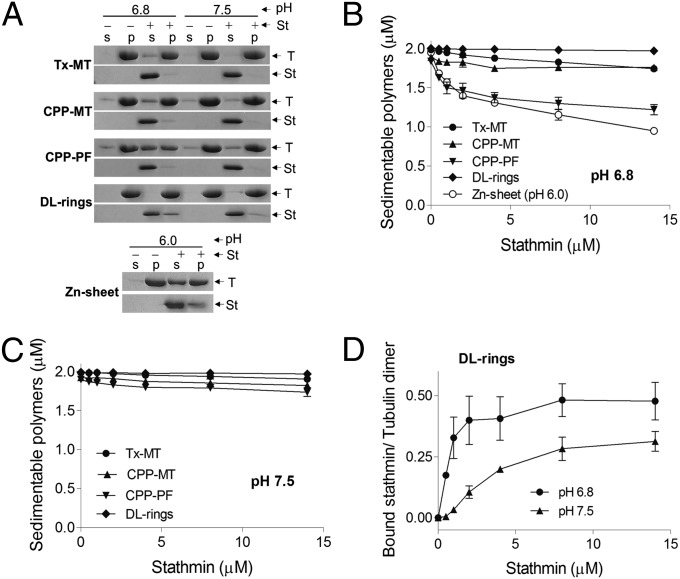
As shown in Fig. 2A and Fig. S3, stathmin bound weakly if at all to Tx-MT at either pH, consistent with previous results (6, 7). More significantly, stathmin also failed to cosediment with CPP-MTs (Fig. 2A and Fig. S3), arguing against the idea that stathmin acts at the tip by binding to GTP polymer. This information about relative binding ability was all we initially planned to extract from these cosedimentation experiments. However, we noticed that addition of stathmin increased the amount of tubulin in the supernatant for both types of MTs, at least at pH 6.8. The effect was minor for the Tx-MT, but it was more significant for the CPP-MTs (Fig. 2 A–C). We were intrigued to observe that with the CPP-MTs, the amount of tubulin in the supernatant increased with the concentration of stathmin, but only up to a point: further increase in stathmin did not cause any further destabilization of the polymer, suggesting that the stathmin was acting on some conformation that was present in limiting quantity (Fig. 2B).
Fig. 2.
Effect of stathmin on different tubulin polymers. (A) Cosedimentation assays and SDS/PAGE analysis showing the effect of 8.0 μM stathmin (St) on different tubulin (T) polymers as indicated (2.0 μM). Note that stathmin induces depolymerization of both CPP-PF and Zn-sheets, but has no effect on DL-rings or Tx-MT (see Fig. 1B and Fig. S2 for details of these different filament types). Depolymerization was stronger at pH 6.8 (pH 6.0 for Zn-sheets) than at pH 7.5. Data for Zn-sheets is shown only at pH 6.0 because Zn-sheets are not stable at higher pH (19, 20). Samples were incubated for 30 min and then sedimented at 165,000 × g for 20 min. Equal fractions of supernatant (s) and pellet (p) were separated by SDS/PAGE, followed by staining with Coomassie blue. Band intensities and data analysis were performed as described in SI Materials and Methods. (B and C) Concentration-dependent effect of stathmin on various tubulin polymers. Increasing concentrations of stathmin (0–14 μM) were incubated with tubulin polymers (2.0 μM). Samples were sedimented and the amount of depolymerized tubulin was measured as described above. Data represent the average of three independent experiments. Values are presented ± SD. (D) Analysis of the tubulin:stathmin stoichiometry for stathmin sedimenting with DL-rings. To determine the binding stoichiometry of stathmin to tubulin dimer in DL-rings, the amount of stathmin bound per sedimentable tubulin dimer at various stathmin concentrations was calculated. Data are the average of three independent experiments. Values are presented ± SD. These data show that stathmin binds to DL-rings with a 2:1 dimer:stathmin stoichiometry, similar to that seen with interactions between stathmin and tubulin dimers (3, 4).
These observations led us to consider the possibility that stathmin might selectively destabilize protofilaments. To test this idea, we examined the effect of stathmin on CPP-PF (as noted above, these are protofilament-rich polymers formed by adding calcium to GMPCPP MTs). Examination of Fig. 2 A and B shows that stathmin had a more significant effect on the CPP-PFs than on the CPP-MTs. In addition, we found that stathmin has a much stronger effect on Tx-MTs when they are made by dilution of preformed MTs into Taxol-containing buffer (Fig. S4A); MTs made by this approach contain a higher proportion of PFs (13). Taken together, these observations suggested that stathmin binds to and depolymerizes stabilized protofilament-like structures, but has little affinity for or effect on normal Taxol-stabilized MTs.
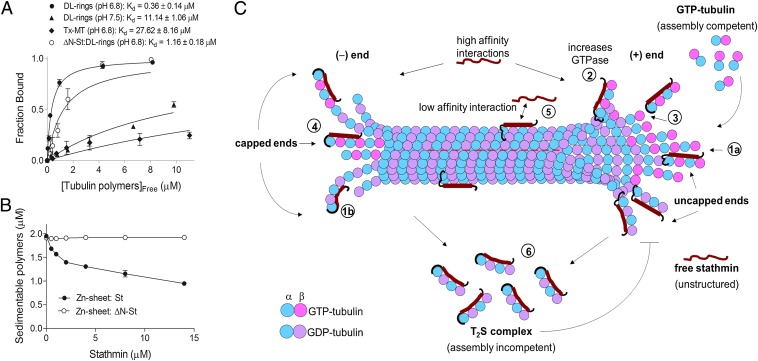
To further test the idea that stathmin binds to and acts upon protofilaments, we examined the effect of stathmin on Dolastatin-10 tubulin rings. Because the rings are composed entirely of protofilaments, one would predict that stathmin should depolymerize them; however, as shown in Fig. 2 A–C, it did not, contrary to our expectation. On the other hand, although stathmin failed to cosediment with any of the other polymers, a significant amount of stathmin did cosediment with the DL-rings (Fig. 2A). More quantitative analysis confirmed that stathmin binds to DL-rings with high affinity (Kd = 0.36 ± 0.14 µM) (Fig. 3A), and showed that the binding saturates with a 2:1 dimer:stathmin ratio similar to that found in the T2S complex (Figs. 1A and 2D) (3, 4). It is notable that the binding affinity (Kd) of stathmin to DL-rings is similar to the affinity range reported for stathmin binding to tubulin dimers (Kd ∼0.1–1.0 µM) (21, 22).
Fig. 3.
Binding affinities of stathmin for tubulin polymers. (A) Binding of stathmin to Tx-MT and DL-rings. The binding of stathmin (2.0 μM) was measured as a function of tubulin polymer concentration (0–12 μM) by cosedimentation assay. The fraction of stathmin bound (in the pellet) was plotted against the concentration of unbound polymerized tubulin [calculated from the total polymerized tubulin assuming a 2:1 ratio (tubulin dimer:stathmin)] (SI Materials and Methods), and the data were fit to the bimolecular binding curve to obtain the apparent Kd. Data are an average of three independent experiments and error bars are ± SD. (B) Effects of full-length and ∆N-stathmin on depolymerization of Zn-sheets. Zn-sheet (2.0 μM) polymer was incubated with various concentrations of stathmin or ∆N-stathmin (0–14 μM) as indicated in Zn-Mes buffer containing 10 μM Taxol. Samples were sedimented and the amount of depolymerized tubulin was measured as described in Fig. 2. Data represent the average of three independent experiments ± SD. These data indicate that the N terminus of stathmin plays a significant role in its ability to depolymerize MTs. (C) Conceptual models for the catastrophe-promoting mechanism by which stathmin depolymerizes microtubules. The data in Figs. 2 and 3 suggest that stathmin can directly promote MT catastrophe by acting on laterally unbound protofilaments at MT tips and does so by some combination of the following three mechanisms (numbers in brackets refer to the figure above): [1], Binding of stathmin to protofilaments could inhibit lateral interactions between protofilaments by steric inhibition [1a] or by inducing curvature [1b] (4, 23, 24), thus destabilizing the tip and increasing the likelihood of catastrophe; [2], Stathmin could increase the GTPase of the tubulin subunits to which it is bound, promoting catastrophe by decreasing the size the GTP cap; [3], The N-terminal peptide of stathmin could promote protofilament severing by binding to the intradimer surface (Fig. 1) (28), promoting catastrophe by removing portions of the GTP cap. All three mechanisms would be expected to operate with equal effectiveness at both the plus and minus ends. A fourth mechanism would be unique to the minus end: [4], The N-terminal peptide of stathmin could bind to and cap the α-tubulin dimer exposed at the minus end, allowing stathmin to bind to the minus end with stronger affinity and preventing the incorporation of new tubulin dimers, thus providing a mechanism for the surprising asymmetry in stathmin’s ability to induce catastrophe. [5], In addition, it remains possible that binding of stathmin to the MT lattice could contribute to catastrophe, as previously suggested (6, 7). [6], Finally, an additional activity that is likely relevant in the cellular context is the demonstrated ability of stathmin to sequester tubulin dimers into the assembly incompetent T2S complex. This activity reduces the amount of tubulin available for MT assembly, the overall polymer mass (3–7, 23–25), and therefore the likelihood that MTs exhibit persistent growth (29, 30).
These observations confirm that stathmin can bind to protofilaments, but they raise two questions: (i) Why doesn’t stathmin depolymerize DL-rings? (ii) What do interactions between stathmin and DL-rings reveal about the mechanism by which stathmin induces MT catastrophe? To answer these questions, we considered existing structural information on the stathmin–tubulin interaction. When stathmin is mixed with unpolymerized tubulin, it assembles into the T2S complex, which is composed of two tubulin dimers arranged in a protofilament-like configuration, with one extended stathmin molecule (Fig. 1A) (3, 4). A striking similarity between the T2S complex and DL-rings is that in both assemblies, the tubulin protofilaments are laterally unbound; they are not attached to other protofilaments as they would be in a MT. This observation suggests that stathmin might bind to the laterally unbound protofilaments that are predicted to exist at the tips of growing MTs (9, 10) and induce catastrophe by inhibiting interactions between them. Such an inhibition could occur because stathmin’s binding site is close to the interface between protofilaments (23–25). Alternatively, because both DL-rings and the T2S complex are curved (15–18, 26), stathmin could inhibit lateral interactions by inducing in protofilaments a degree of curvature that is incompatible with their incorporation into the lattice.
To test the idea that stathmin acts on exposed protofilaments, we treated Zn-sheets with stathmin. Zinc-sheets are flat structures composed of protofilaments arranged in an antiparallel fashion, leaving unbound protofilaments exposed at each edge. The fact that exposed protofilaments are part of the fundamental structure of the polymer suggested that stathmin might have a particularly strong effect on Zn-sheets, and indeed, Fig. 2 A and B shows that stathmin has a greater effect on Zn-sheets than on the other polymers. Moreover, the effect of stathmin on Zn-sheets continued to increase with additional stathmin: unlike the situation with the other polymers, the effect did not saturate even at high stathmin concentration (Fig. 2B). This is the outcome that would be predicted if stathmin binds to and acts upon exposed protofilaments, which are a fundamental characteristic of Zn-sheets but exist only in limiting quantities in the other polymer types (e.g., at MT tips). As would be predicted from the idea that stathmin acts on protofilaments exposed at MT tips, the fraction of the MT population that is sensitive to stathmin can be increased by shearing the MTs (Fig. S4B).
Taken together, these observations suggest that stathmin can directly promote catastrophe by binding to and acting upon the exposed protofilaments that are predicted to exist at the tips of growing MTs (9); consideration of the existing stathmin–tubulin complex structures (23–26) suggests that it acts by inhibiting lateral interactions between the PFs. It is also possible that stathmin increases the GTPase of the tubulin subunits in the protofilaments to which it is bound (27). Although these ideas are attractive, they fail to explain how stathmin depolymerizes preexisting stabilized structures like the Zn-sheets, or why stathmin is unable to alter DL-rings. To address these issues, we need to consider another aspect of the T2S structure, which is that the N-terminal region of stathmin binds the dimer-dimer interface of α-tubulin (Fig. 1A) (3, 28). Based on this observation, we propose that the N-terminal region of stathmin interferes with the longitudinal bond between dimers in the protofilament, leading to severing of single protofilaments and destabilization of structures like Zn-sheets.
To test this idea, we examined the ability of a stathmin construct that is missing the N terminus (∆N-stathmin, aa40-149) to depolymerize Zn-sheets. Fig. 3B shows that ∆N-stathmin has significantly less ability to depolymerize Zn-sheet polymer than does the full-length protein (Fig. 3B), demonstrating that the stathmin N terminus plays a key role in the mechanism by which stathmin acts on these polymers. The observation that ∆N-stathmin has significantly reduced catastrophe activity (6) suggests that this mechanism also contributes to catastrophe promotion in dynamic systems.
To address the question of why stathmin does not depolymerize DL-rings, we tested the ability of ∆N-stathmin to bind to DL-rings. We found a significant reduction in binding ability when the N-terminal peptide is removed (Fig. 3A). This observation suggests that the N-terminal peptide is able to engage in normal binding interactions in the context of the DL-rings. One potential explanation is that stathmin binds to the subset of rings that are open and thus have an available N-terminal peptide-binding site, but this idea conflicts with the observation that stathmin binds to the rings with a 2:1 dimer:stathmin ratio (Fig. 2D). Therefore, we suggest that something about the structure of Dolastatin-stabilized polymers, perhaps their curvature, allows them to accommodate binding of the stathmin N terminus without destabilizing the polymers, thus providing an explanation for why stathmin binds to the rings but does not depolymerize them.
A Conceptual Model for the Mechanism of Direct Catastrophe Promotion by Stathmin.
The combination of results from our experiments and the existing literature lead to a model where stathmin binds to protofilaments exposed at the tips of growing MTs and directly promotes catastrophe by one or more (perhaps all) of the following activities (Fig. 3C): (i) stathmin interferes with lateral bonding between protofilaments sterically, as might be predicted from the proximity of the stathmin binding site to the protofilament-protofilament interface (shown as 1a in Fig. 3C) (23–25) and/or by inducing curvature in protofilaments, increasing the interference with lateral bonding (shown as 1b in Fig. 3C); (ii) stathmin increases the GTPase rate of the dimers to which it is bound, as expected from the observation that stathmin increases the GTPase rate (27); (iii) the stathmin N-terminal tail promotes severing of the protofilament to which it is bound, as predicted from the binding of the N-terminal tail to the surface between dimers (Fig. 1A); and (iv) the stathmin N-terminal tail caps protofilaments with exposed α-tubulins, preventing incorporation of new dimers (Fig. 3C). The first three mechanisms could act at either end of the MT, but the fourth one would be minus-end specific, potentially providing an explanation for the surprising observation that stathmin has stronger effects on the minus end than on the plus end in vitro (3, 7).
Testing the Model Through Computer Simulations.
The experiments above provide compelling evidence that stathmin can bind to and depolymerize protofilament-like structures, but could such a model account for stathmin’s observed ability to promote catastrophe and to do so asymmetrically (i.e., more strongly at the minus end)? If so, which of the mechanisms in Fig. 3C are most likely to contribute to stathmin’s observed effects? To address these questions, we turned to computational modeling. We have previously established a dimer-scale model of MT dynamics that explicitly considers lateral and longitudinal bonds between subunits and exhibits the full range of dynamic instability behaviors (10, 29). To incorporate stathmin into this model, we initially assumed that stathmin binds only to regions of protofilaments that are laterally unbonded on both sides, and that the Kd for the interaction between stathmin and these free PFs is 1.0 µM [a conservative estimate given that the affinity of stathmin for DL-rings is ∼0.3 µM (Fig. 3A)]. Using 10 µM unpolymerized tubulin, and 1.0 µM free stathmin [both assumed to be constant, these concentrations are similar to those used in previous experiments (7)], we tested the effect on MT dynamics of giving the stathmin the various activities depicted in Fig. 3C.
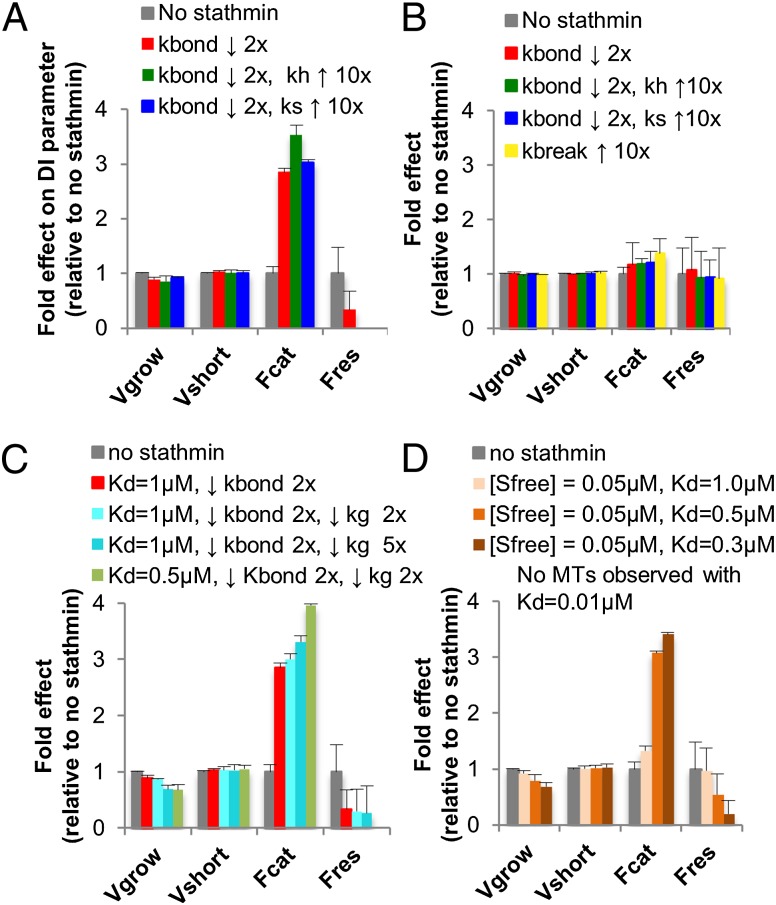
In preliminary simulations, we found that when the stathmin increased the hydrolysis rate or dimer detachment rate (severing rate) as much as 10×, there was little effect on the dynamics of the simulated MTs at the concentrations tested. However, when the stathmin decreased the lateral bonding rate 10×, the effect was so strong that MT polymerization was completely inhibited. Therefore, we investigated more moderate inhibition of lateral binding. Fig. 4A shows that when the stathmin reduced the lateral bonding rate (kbond) by a factor of 2, the catastrophe rate of the simulated MTs increased almost threefold relative to controls without stathmin (Fig. 4A; see Table S1 for the measured dynamic instability parameters). Adding to this 2× lateral bonding effect a 10× effect on the hydrolysis rate (kh) increased the catastrophe rate modestly, whereas instead adding a 10× effect on the dimer detachment rate (ks) had little additional effect on dynamics (Fig. 4A). The 2.5× to 3× increase in catastrophe when stathmin is present, as seen in the simulations, is similar to what has been reported for similar concentrations of stathmin and tubulin in vitro (7).
Fig. 4.
Effect of stathmin on dynamic instability (DI) behavior in simulations of MT dynamics. Using our previously established computational model of MT dynamics (10), we tested the hypothesis that binding of stathmin to free protofilaments could account for stathmin’s observed effects on MT dynamics by examining how adding stathmin molecules with varied activities alter the behavior of the simulated MTs. (A) Effects observed when stathmin (1 μM free, activities as indicated) binds with moderate affinity (Kd = 1 μM) to laterally unbonded regions of PF. (B) Effects observed when stathmin (1 μM free, activities as indicated) binds with weak affinity (Kd = 25 μM) anywhere on the lattice. Examination of the data in A shows that binding of stathmin to PFs can dramatically increase catastrophe frequency, and that DI is more sensitive to effects of stathmin on lateral bond formation than on the hydrolysis rate or the dimer detachment rate. Comparison of A and B shows that weak binding of stathmin to the lattice is less effective than stronger binding of stathmin to PFs. (C) Possible mechanism for stathmin's activity asymmetry. Simulations were conducted as in A, except that in some cases stathmin was given a capping activity (simulated as decreased kgrow) or an increased affinity. These data show that the higher affinity and capping activity that are predicted to result from binding of the stathmin N terminus to α-tubulin increase stathmin’s catastrophe promoting ability, and thus could account for the experimental observation (7) that stathmin has stronger effects at the minus end than at the plus end. (D) Effect of 0.05 µM free stathmin on DI (this is the concentration expected when binding of stathmin (1 μM) to free tubulin dimers (10 μM) is taken into account; see SI Materials and Methods for calculations). These simulations assume that stathmin binds to laterally unbound PFs and that stathmin’s main activity is to prohibit lateral bond formation. These data show that stathmin molecules with affinity values similar to those measured in Fig. 3A can have strong effects on DI even in the presence of free tubulin. Data in all panels represent the average ± SD for three independent simulations of a single MT, each corresponding to more than 1 h of simulated time. The raw dynamic instability data corresponding to these bars graphs are provided in Table S1. Fcat, catastrophe frequency; Fres, rescue frequency; kbond, rate constant for lateral bonding between tubular dimers; kg, rate constant for the formation of the longitudinal bond between tubulin dimers; kh, GTP hydrolysis rate constant; ks, rate constant for breakage of the longitudinal bond between tubulin dimers; Sfree, stathmin free; Vgrow, macroscopically observed MT growth rate; Vshort, macroscopically observed MT depolymerization rate.
These results are significant for two reasons. First, they show that a mechanism where stathmin binds to and acts upon laterally unbound protofilaments at the tips of MTs is in principal capable of giving rise to stathmin’s ability to increase the catastrophe rate. Second, the observation that the simulations are very sensitive to effects on lateral bonding and less sensitive to effects on hydrolysis rate and severing rate suggests that stathmin’s primary activity is to inhibit lateral bonding. However, it is important to point out that these relative sensitivities might shift as the parameter set used for the MT dynamics simulation is changed (e.g., to adjust for different type of tubulin or to refine the parameter set).
We next tested the hypothesis that stathmin’s preferential effect on MT minus ends is a result of capping activity caused by interaction of the stathmin N terminus with the exposed surface of α-tubulin. We were surprised to see that adding a 2× capping activity (i.e., reducing kgrow twofold in addition to the 2× reduction of kbond) had relatively little additional effect on catastrophe, and that a 5× effect was only a little stronger (Fig. 4C). This observation cast doubt on the idea that the capping mechanism could account for the ability of stathmin to induce catastrophe asymmetrically. Further reflection led us to realize that we had not considered an additional source of asymmetry; the increased affinity of stathmin for the minus end that should result from unimpeded interactions between the stathmin N-terminal domain and the minus-end exposed surface of α-tubulin. As noted above, removal of this N-terminal tail reduces the affinity of stathmin for DL-rings by more than twofold (Fig. 3A). Therefore, we took this additional interaction into account by setting the activities as before (2× decrease in kbond and kgrow), but also increasing the affinity for unbound protofilaments 2×, from 1.0 µM to 0.5 µM. We found that this change caused a significant increase in catastrophe frequency (Fig. 4C). Moreover, when the effect on kgrow was increased to 5×, leaving the other parameters the same (2× decrease in kbond and kgrow, Kd = 0.5 µM), the stathmin activity prevented MT growth entirely (Fig. 4C). Taken together, these data lead us to propose that stathmin’s preferential effect at minus ends results from a combination of its higher affinity for MT minus ends and its ability to cap MT minus ends, both of which are predicted from interactions between the N-terminal domain and the exposed surface of α-tubulin (Fig. 1A) (3, 7).
These simulations left open the question of whether binding of stathmin along the MT lattice could contribute to stathmin’s effect on MT dynamics. To address this issue, we repeated the simulations of Fig. 4A, but this time we allowed the stathmin to bind anywhere with a similar weak affinity (Kd = 25 µM), chosen to match the measured affinity of stathmin for Taxol-stabilized MTs (Fig. 3A). The simulations showed that binding of stathmin to the lattice at this Kd had little effect on MT dynamics (Fig. 4B, and Table S1), in contrast to when a similar concentration of stathmin with similar activities bound with higher affinity to protofilaments (compare Fig. 4 B to A). These observations suggest that weak binding of stathmin to the MT lattice makes relatively little contribution to stathmin’s effect on MT dynamics compared with the effects caused by binding to exposed protofilaments.
Although these simulations both support and extend the conceptual model outlined in Fig. 3, there is a limitation to this work: the simulations discussed above fail to take into account interactions between stathmin and unpolymerized tubulin. If one assumes that the Kd of stathmin for tubulin dimers is 0.5 µM, and that this is the same as the Kd of stathmin for PFs, then only ∼0.05 µM stathmin will be free to interact with MTs in a steady-state system consisting of 10 µM free tubulin and 1.0 µM total stathmin (see SI Materials and Methods for calculations). In preliminary work, when we stipulated that the concentration of free stathmin was 0.05 µM in simulations otherwise identical to those of Fig. 4A, we saw relatively little effect on catastrophe. However, when we stipulated that the bound stathmin completely inhibited lateral bond formation, the effect was dramatic: catastrophe increased by a factor of three (Fig. 4D). These simulation results further support the idea that action of stathmin on exposed PFs can promote MT catastrophe to a degree consistent with experimental results, and suggest that the effect of stathmin on lateral bond formation is likely to be relatively strong.
Simulations in a Cell-Like Environment.
These results suggest that stathmin can directly promote MT catastrophe by binding to and acting on tubulin protofilaments exposed at growing tips. However, this work leaves open the issue of how this direct activity of stathmin might compare in significance to its established tubulin-sequestering activity. In vitro, once steady-state has been reached (i.e., polymer mass has stopped increasing), a simple sequestering activity would be expected to alter only the mass of tubulin polymer and not the catastrophe frequency, because the steady-state concentration of free (not sequestered or polymerized) tubulin subunits should be similar with and without stathmin (this logic comes from classic understanding of polymer-critical concentrations). However, in an interphase cell with persistently growing MTs, a relatively small reduction in the concentration of active tubulin subunits through sequestration might be predicted to alter the MT network significantly by dropping the concentration of available unpolymerized tubulin below that needed for persistent growth (see SI Results and Discussion).
To investigate the effect of stathmin activities in these different environments, we performed a set of simulations with the same parameters as in Fig. 4D, except this time we performed them in both cell-like (spatially constrained) and in vitro-like (not constrained) environments: We let the MTs compete with each other for a limited pool of tubulin subunits, and we started with a higher concentration of tubulin to approximate more in vivo-like conditions (Fig. S5 and Table S2). In a “cell” with 15 µM total tubulin and no stathmin, MTs grew persistently, similar to interphase MTs in vivo (30), as evidenced both by their steady-state length distribution (Fig. S5A) and positive drift coefficient (1.2 ± 0.4 µM/min) (Table S2; see SI Results and Discussion). Addition of either a 1 µM stathmin sequestering activity or a direct activity like that of Fig. 4D shifted the MT system to a state that grew less persistently (drift coefficient = <0.1 μM/min) and had a relatively flat length distribution (Fig. S5 B and C and Table S2). However, combination of the sequestering and direct activities shifted the system to a completely nonpersistent state as assessed by a drift coefficent of zero, and a length distribution that approximates an exponential decay (Fig. S5D and Table S2). Although physiological systems differ from this simulation and from each other in quantitative details, these simulations suggest that both the sequestration and direct activities of stathmin could contribute to stathmin’s functions in vivo.
Taken together, these experimental and simulation-based observations lead us to propose that stathmin can directly promote MT catastrophe, and that it does so by binding to and acting on tubulin protofilaments exposed at MT tips. We suggest that both this direct mechanism and stathmin's well-established tubulin-sequestering ability work together to create stathmin’s observed activities in vitro and in vivo.
Materials and Methods
Pipes and Taxol (paclitaxel) were obtained from Sigma. All other chemicals were of analytical grade. Methods for tubulin polymer preparation, protein binding measurements and the computational work are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Erin Jonasson for critical reading of the manuscript. This work was supported initially by National Institutes of Health Grant R01 GM065420 (to H.V.G.), and by National Science Foundation Grants NSF-MCB-0951264 and NSF MCB-1244593 (to H.V.G. and M.S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309958110/-/DCSupplemental.
References
- 1.Murphy ME, Cassimeris L. A novel cancer therapy approach targeting microtubule function. Cancer Biol Ther. 2006;5(12):1721–1723. doi: 10.4161/cbt.5.12.3736. [DOI] [PubMed] [Google Scholar]
- 2.Rana S, Maples PB, Senzer N, Nemunaitis J. Stathmin 1: A novel therapeutic target for anticancer activity. Expert Rev Anticancer Ther. 2008;8(9):1461–1470. doi: 10.1586/14737140.8.9.1461. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz MO. Structure and thermodynamics of the tubulin-stathmin interaction. J Struct Biol. 2007;158(2):137–147. doi: 10.1016/j.jsb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14(1):18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- 5.Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84(4):623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 6.Howell B, Larsson N, Gullberg M, Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol Biol Cell. 1999;10(1):105–118. doi: 10.1091/mbc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manna T, Thrower D, Miller HP, Curmi P, Wilson L. Stathmin strongly increases the minus end catastrophe frequency and induces rapid treadmilling of bovine brain microtubules at steady state in vitro. J Biol Chem. 2006;281(4):2071–2078. doi: 10.1074/jbc.M510661200. [DOI] [PubMed] [Google Scholar]
- 8.Amayed P, Pantaloni D, Carlier MF. The effect of stathmin phosphorylation on microtubule assembly depends on tubulin critical concentration. J Biol Chem. 2002;277(25):22718–22724. doi: 10.1074/jbc.M111605200. [DOI] [PubMed] [Google Scholar]
- 9.Nogales E, Wang HW. Structural intermediates in microtubule assembly and disassembly: How and why? Curr Opin Cell Biol. 2006;18(2):179–184. doi: 10.1016/j.ceb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Margolin G, et al. The mechanisms of microtubule catastrophe and rescue: Implications from analysis of a dimer-scale computational model. Mol Biol Cell. 2012;23(4):642–656. doi: 10.1091/mbc.E11-08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta KK, et al. Minimal plus-end tracking unit of the cytoplasmic linker protein CLIP-170. J Biol Chem. 2009;284(11):6735–6742. doi: 10.1074/jbc.M807675200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnal I, Wade RH. How does taxol stabilize microtubules? Curr Biol. 1995;5(8):900–908. doi: 10.1016/s0960-9822(95)00180-1. [DOI] [PubMed] [Google Scholar]
- 13.Elie-Caille C, et al. Straight GDP-tubulin protofilaments form in the presence of taxol. Curr Biol. 2007;17(20):1765–1770. doi: 10.1016/j.cub.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 14.Müller-Reichert T, Chrétien D, Severin F, Hyman AA. Structural changes at microtubule ends accompanying GTP hydrolysis: Information from a slowly hydrolyzable analogue of GTP, guanylyl (alpha,beta)methylenediphosphonate. Proc Natl Acad Sci USA. 1998;95(7):3661–3666. doi: 10.1073/pnas.95.7.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moores CA, Milligan RA. Visualisation of a kinesin-13 motor on microtubule end mimics. J Mol Biol. 2008;377(3):647–654. doi: 10.1016/j.jmb.2008.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulder AM, et al. A new model for binding of kinesin 13 to curved microtubule protofilaments. J Cell Biol. 2009;185(1):51–57. doi: 10.1083/jcb.200812052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boukari H, Sackett DL, Schuck P, Nossal RJ. Single-walled tubulin ring polymers. Biopolymers. 2007;86(5-6):424–436. doi: 10.1002/bip.20752. [DOI] [PubMed] [Google Scholar]
- 18.Cormier A, Marchand M, Ravelli RB, Knossow M, Gigant B. Structural insight into the inhibition of tubulin by vinca domain peptide ligands. EMBO Rep. 2008;9(11):1101–1106. doi: 10.1038/embor.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamimura S, Mandelkow E. Tubulin protofilaments and kinesin-dependent motility. J Cell Biol. 1992;118(4):865–875. doi: 10.1083/jcb.118.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawachi A, et al. Different protofilament-dependence of the microtubule binding between MAP2 and MAP4. Biochem Biophys Res Commun. 2003;305(1):72–78. doi: 10.1016/s0006-291x(03)00707-1. [DOI] [PubMed] [Google Scholar]
- 21.Krouglova T, Amayed P, Engelborghs Y, Carlier MF. Fluorescence correlation spectroscopy analysis of the dynamics of tubulin interaction with RB3, a stathmin family protein. FEBS Lett. 2003;546(2–3):365–368. doi: 10.1016/s0014-5793(03)00636-7. [DOI] [PubMed] [Google Scholar]
- 22.Carlier MF. Measurements of stathmin-tubulin interaction in solution. Methods Mol Med. 2007;137:103–110. doi: 10.1007/978-1-59745-442-1_7. [DOI] [PubMed] [Google Scholar]
- 23.Gigant B, et al. The 4 A X-ray structure of a tubulin:stathmin-like domain complex. Cell. 2000;102(6):809–816. doi: 10.1016/s0092-8674(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 24.Ravelli RB, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428(6979):198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 25.Nawrotek A, Knossow M, Gigant B. The determinants that govern microtubule assembly from the atomic structure of GTP-tubulin. J Mol Biol. 2011;412(1):35–42. doi: 10.1016/j.jmb.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Barbier P, et al. Stathmin and interfacial microtubule inhibitors recognize a naturally curved conformation of tubulin dimers. J Biol Chem. 2010;285(41):31672–31681. doi: 10.1074/jbc.M110.141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Cormier A, Gigant B, Knossow M. Insight into the GTPase activity of tubulin from complexes with stathmin-like domains. Biochemistry. 2007;46(37):10595–10602. doi: 10.1021/bi701147f. [DOI] [PubMed] [Google Scholar]
- 28.Clément MJ, et al. N-terminal stathmin-like peptides bind tubulin and impede microtubule assembly. Biochemistry. 2005;44(44):14616–14625. doi: 10.1021/bi0512492. [DOI] [PubMed] [Google Scholar]
- 29.Gregoretti IV, Margolin G, Alber MS, Goodson HV. Insights into cytoskeletal behavior from computational modeling of dynamic microtubules in a cell-like environment. J Cell Sci. 2006;119(Pt 22):4781–4788. doi: 10.1242/jcs.03240. [DOI] [PubMed] [Google Scholar]
- 30.Komarova YA, Vorobjev IA, Borisy GG. Life cycle of MTs: Persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary. J Cell Sci. 2002;115(Pt 17):3527–3539. doi: 10.1242/jcs.115.17.3527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.