Significance
Bats are a frequent source of pathogen spillover to humans and livestock, and a reservoir for emerging infectious diseases. Transmission mechanisms within bat populations remain enigmatic, precluding effective management of zoonotic infections. Vampire bats transmit rabies virus throughout Latin America, causing lethal human rabies and thousands of livestock deaths every year. By selecting among competing transmission models applied to spatially replicated, longitudinal field data, we find that most rabies virus exposures are nonlethal and instead immunize bats, thus facilitating viral persistence. Further, frequent interactions among bats from different colonies are necessary to maintain the chain of transmission. We also evaluate the efficacy of bat culling and demonstrate that it has minimal effects on seroprevalence when spatially coordinated control is absent.
Keywords: Desmodus rotundus, zoonotic disease, host–pathogen dynamics, spatial processes, wildlife culling
Abstract
Bats are important reservoirs for emerging infectious diseases, yet the mechanisms that allow highly virulent pathogens to persist within bat populations remain obscure. In Latin America, vampire-bat–transmitted rabies virus represents a key example of how such uncertainty can impede efforts to prevent cross-species transmission. Despite decades of agricultural and human health losses, control efforts have had limited success. To establish persistence mechanisms of vampire-bat–transmitted rabies virus in Latin America, we use data from a spatially replicated, longitudinal field study of vampire bats in Peru to parameterize a series of mechanistic transmission models. We find that single-colony persistence cannot occur. Instead, dispersal of bats between colonies, combined with a high frequency of immunizing nonlethal infections, is necessary to maintain rabies virus at levels consistent with field observations. Simulations show that the strong spatial component to transmission dynamics could explain the failure of bat culls to eliminate rabies and suggests that geographic coordination of control efforts might reduce transmission to humans and domestic animals. These findings offer spatial dynamics as a mechanism for rabies persistence in bats that might be important for the understanding and control of other bat-borne pathogens.
Bats (Chiroptera) host some of the most significant newly emerging viruses affecting humans and domestic animals, and have been suggested as the evolutionary origin of several endemic human infections (1, 2). Predicting the spatiotemporal distribution of pathogen transmission from bats to other species requires understanding both the ecological factors that encourage cross-species exposures and the transmission dynamics within bat populations (3). Understanding viral persistence in bat populations is challenging because seemingly ideal ecological traits for explosive pathogen transmission such as high mobility and colonial aggregation contrast with the limited supply of new susceptible individuals generated by characteristically long-lived and slow-reproducing host species. Consequently, epizootiological models of bat viruses have required complex immunological or behavioral mechanisms to achieve long-term persistence, such as waning maternal immunity or an extended incubation period through hibernation (4–6). These studies also demonstrated the power of a combined field, experimental, and modeling approach for identifying persistence mechanisms in bats, but such holistic investigations are still absent for most systems (3).
In Latin America, vampire bat (Desmodus rotundus) transmitted rabies virus (VBRV) is among the most significant wildlife zoonoses for agricultural development and human health (7). Growth in the livestock industry likely exacerbates VBRV outbreaks by providing an almost unlimited food source for these blood-feeding bats, fueling population growth and range expansions (8–10). The combination of large vampire bat populations and frequent contacts with livestock as bats bite to drink blood contributes to losses of ca. $30 million (US dollars) per year in livestock mortality alone (7). Simultaneously, lethal human rabies outbreaks are increasingly recognized in remote areas of the Amazon rainforest; these may be linked to a combination of human encroachment into forested areas, natural prey depletion, and improved detection (11). Attempts to control vampire bat populations and VBRV transmission have been in place since the 1960s, and include indiscriminate killing of bats and a topical anticoagulant poison, “vampiricide,” that kills conspecifics that groom treated bats (12). A similar vehicle has been proposed—but not attempted in natural populations—for oral vaccination of vampire bats (13). To date, no control method has eliminated viral circulation as evidenced by recurrent cases in livestock and humans, even in areas where culling is performed regularly.
Developing effective control strategies for VBRV relies on understanding the transmission dynamics within the reservoir host (14), an issue that has been largely neglected despite recognition of VBRV and its health risks since the early 1900s (15). Spatiotemporal patterns of VBRV mortality in livestock at the edge of the vampire bats’ range in northern Argentina suggested traveling waves of infection in vampire bats that were speculated to recur upon recovery of an unknown threshold density of susceptible bats (16, 17). However, in many regions of Peru, Brazil, and Mexico, VBRV continuously affects livestock, suggesting enzootic persistence rather than invasion. Several possible but untested mechanisms of persistence have been suggested, including sufficiently large bat population sizes (i.e., above the critical community size, ref. 18), a healthy carrier state, and a variety of immunological scenarios (19–21). Distinguishing these competing scenarios is fundamental to understanding persistence and improving control.
We evaluated the determinants of viral persistence in vampire bat colonies by developing a maximum likelihood framework to parameterize and evaluate stochastic epizootiological models. This was achieved using data from infection studies in captive vampire bats and a unique longitudinal field study in wild vampire bats, where rabies exposures were monitored in individually marked bats from 17 colonies across four departments of Peru between 2007 and 2010 (Fig. 1). Because culling is the most common practice currently used to control VBRV in vampire bat populations, we simulated potential culling practices to examine their impact on both the seroprevalence and its expected exposure rate to livestock.
Fig. 1.
(Right) Map of South America with Peru indicated by gray shading. Antibody prevalence field data on VBRV were sampled across 17 sites in four departments of Peru [Apurimac (red), Cajamarca (orange), Lima (blue), and Madre de Dios (green)] and are provided for 2007, 2009, and 2010 (19). Sites were sampled approximately yearly. For each site in a given year, the proportion of seropositive bats is denoted by off-white with the proportion seronegative denoted by the color associated with the department where the colony is located. The radius of each observation is proportional to sample size. See SI Appendix for the complete dataset.
Results
Model Formulation and Statistical Inference.
We explore four models of rabies dynamics within single bat colonies, representing alternative hypotheses based on field and experimental studies for the biology of rabies infection. Each model is described through modifications of the classic susceptible–exposed–infected–recovered paradigm (22, 23), and assumes that following exposure to VBRV, bats enter an exposed state (E) and subsequently either enter a noninfectious state with temporary immunity (T) with probability 1 − α, or enter a lethal infectious state with probability α (see SI Appendix for further discussion on immunizing exposures and infectious states). Bats that develop a lethal infection initially enter an infectious, but clinically silent state, IN, where bats have normal social and feeding behaviors before progressing to a rabid infectious state, IR, where they are aggressive or withdrawn (24). Model I made no further assumptions. Additional models captured recovery from an infectious state (model II), immune-boosting upon reexposure (model III), and lifelong immunity (model IV). Schematic representations for each model are provided in Fig. 2A and full details provided in the SI Appendix.
Fig. 2.

(A) Schematic diagram of each model where the force of infection  . Demographic processes are omitted for clarity. Filled states have seroconverted, and white states are seronegative. Model IV (lifelong immunity) is a base model that includes all black lines and states. Model I (temporary immunity and lethal infection) additionally includes the blue text, model II (nonlethal infection) includes red and blue text where 1 − ρ is the probability of recovery from infection, and model III (immune boosting) includes yellow and blue where c is the strength of immune boosting that depends on the force of infection. (B) Here 95% confidence intervals (CIs) for lethal infection probability versus the effect of immigration on the force of infection. As in A, model I is colored blue, model II is colored red, model III is colored yellow, and model IV is represented by black. Models I–III arrive at the same MLE for α and ϕ (blue star), and the MLE for model IV is indicated by a black star. (C) Change in likelihood from the MLE for each model across all R0 values. Points above the horizontal gray line fall within the 95% CI (found using the likelihood ratio test), and the vertical line indicates
. Demographic processes are omitted for clarity. Filled states have seroconverted, and white states are seronegative. Model IV (lifelong immunity) is a base model that includes all black lines and states. Model I (temporary immunity and lethal infection) additionally includes the blue text, model II (nonlethal infection) includes red and blue text where 1 − ρ is the probability of recovery from infection, and model III (immune boosting) includes yellow and blue where c is the strength of immune boosting that depends on the force of infection. (B) Here 95% confidence intervals (CIs) for lethal infection probability versus the effect of immigration on the force of infection. As in A, model I is colored blue, model II is colored red, model III is colored yellow, and model IV is represented by black. Models I–III arrive at the same MLE for α and ϕ (blue star), and the MLE for model IV is indicated by a black star. (C) Change in likelihood from the MLE for each model across all R0 values. Points above the horizontal gray line fall within the 95% CI (found using the likelihood ratio test), and the vertical line indicates  . The MLE of R0 and associated 95% CIs are also shown. (D) Log likelihood (log ℒ) and ΔAIC score (with zero associated with the best-fitting model) corresponding to the MLE for each model. The ΔAIC score accounts for differences in the number of estimated parameters.
. The MLE of R0 and associated 95% CIs are also shown. (D) Log likelihood (log ℒ) and ΔAIC score (with zero associated with the best-fitting model) corresponding to the MLE for each model. The ΔAIC score accounts for differences in the number of estimated parameters.
In each model, transmission to susceptible individuals S occurs by bites from infectious bats (IN or IR). Transmission is assumed to be frequency dependent because seroprevalence in the field was not significantly associated with the size of these bat colonies (19). Immigration of infectious bats also contributes to the force of infection λ at a constant rate ϕ so that
where N is the total population size and βN and βR are the transmission rates from bats in the IN and IR states, respectively. Here, ϕ is a term that arises externally and represents infectious bats entering a colony and exposing susceptible bats before either leaving or dying. It can also capture interactions with infectious noncolony members during foraging.
Although many model parameters were inferred from challenge studies and knowledge of vampire bat life history (specifically, parameters describing the time bats spend in each state and all mortality parameters; SI Appendix, Table S2), the probability of developing lethal infection (α) versus temporary immunity following exposure and the impact of movement or interactions between colonies ϕ are unknown, but likely crucial determinants of transmission dynamics. Therefore, we used likelihood-based statistical inference methods (25) to confront the seroprevalence data from Peru with our transmission models to obtain maximum likelihood estimates (MLEs) and associated confidence bounds for α and ϕ when optimized over the transmission parameters, βN and βR (Materials and Methods).
We compared parameter estimates for each model (I–IV) by overlaying the 95% confidence intervals for α versus ϕ (Fig. 2B). Although model I (temporary immunity and lethal infection) is the best fitting model, the associated ΔAkaike Information Criterion (ΔAIC) indicates that models II and III (recovery from an infectious state and immune boosting) also create plausible representations of the data. It is important to note that the confidence intervals and the associated MLE for models I–III consistently point to a low probability of developing lethal infection—implying that most exposures are subclinical and immunizing—and frequent immigration of infectious bats. The role of immigration in VBRV dynamics is highlighted by the intrinsic basic reproductive ratio R0. In the absence of imports, the R0 values corresponding to the MLE are all less than one (0.61, 0.34, and 0.51 for models I, II, and III, respectively), and confidence intervals associated with each model confirm that enzootic persistence relies on spatial structure and immigration of infectious individuals (Fig. 2C).
In contrast to models I–III, the model including lifelong immunity (model IV) suggests viral persistence across most feasible values of α with lower levels of immigration (Fig. 2B). However, the ΔAIC score of 7.38 indicates that this model provides a considerably less plausible explanation of the data (26). Further, although the presence of lifelong immunity would imply a different relationship between ϕ and α than that observed in models I–III, the conclusion that immigration is required for persistence within a colony is maintained; the estimated intrinsic R0 remains substantially less than 1 (0.61) at the MLE.
Given the consistency of parameter estimates across models I–III, we further explored the dynamics of the most parsimonious model (model I): temporary immunity and lethal infection. To confirm the efficacy of our maximum likelihood estimation, we averaged over 1,000 simulations using the parameter values associated with the MLE across sample sites, which yields a mean global seroprevalence of ∼10.8%—nearly identical to the observed data (∼10.5%). Further, Fig. 3 displays a contour plot of the complete likelihood surface of the lethal infection probability α and the contribution of immigration to the force of infection ϕ. The MLE for the probability of lethal infection is surprisingly low (0.1) with immigration contributing notably to the force of infection. The simulated dynamics from this region of parameter space suggest that long-term rabies persistence is enabled by a high frequency of immunizing exposures, with the chain of transmission maintained by immigration events that lead to sporadic but lethal VBRV outbreaks (Fig. 3D). These dynamics are consistent with three observations of VBRV in the field. First, they capture the sporadic nature of local rabies outbreaks in livestock, as described by anecdotal reports from ranchers in Peru. Second, by again averaging over 1,000 simulations, the prevalence of active infection in vampire bats is found to be low (<1%), as has been reported in a variety of bat species in the Americas (27). Third, consistent with our field observations (Fig. 1), such dynamics allow a continuous presence of seropositive individuals with relatively stable colony sizes.
Fig. 3.

Likelihood of VBRV dynamics in response to changes in α and ϕ shows that the most likely parameter combination is a low lethal infection probability (α) combined with a high effect of immigration on the force of infection (ϕ). (Center) Contour plot of the log likelihood for α versus ϕ for model 1. The reported likelihood values are maximized over βN and βR. The star indicates the MLE and the thick gray line represents the 95% CI. Above the contour plot is the profile likelihood for ϕ and to the right of the contour plot is the profile likelihood for α. The MLE corresponds to 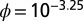 and
and  with 95% CIs (10−3.51, 10−2.83) and (0, 0.29), respectively, noting 0 is not included in the CI for α. Parameters for sample simulations: (A)
with 95% CIs (10−3.51, 10−2.83) and (0, 0.29), respectively, noting 0 is not included in the CI for α. Parameters for sample simulations: (A)  ,
,  ,
, 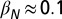 ,
,  ; (B)
; (B)  ,
,  ,
,  ,
, 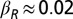 ; (C)
; (C)  ,
,  ,
,  ,
,  ; and (D)
; and (D)  ,
,  ,
,  ,
,  (the MLE). In A–D, shading indicates the proportion of seropositive individuals with temporary immunity (T, light red) and the proportion of seropositive individuals that are infectious (
(the MLE). In A–D, shading indicates the proportion of seropositive individuals with temporary immunity (T, light red) and the proportion of seropositive individuals that are infectious ( , light blue). In contrast to D, simulations in A and C fail to consistently produce seropositive bats, while the dynamics in B drive the colony to extinction. At the MLE, ∼ 2 bats for every 10 susceptible bats present in a colony are expected to be exposed to VBRV by immigrant bats each year. Based on simulations this accounts for approximately half (53%) of all exposures, with the remaining exposures arising from bats within the colony. Department-specific parameters were consistent with the dynamics at the national scale (SI Appendix).
, light blue). In contrast to D, simulations in A and C fail to consistently produce seropositive bats, while the dynamics in B drive the colony to extinction. At the MLE, ∼ 2 bats for every 10 susceptible bats present in a colony are expected to be exposed to VBRV by immigrant bats each year. Based on simulations this accounts for approximately half (53%) of all exposures, with the remaining exposures arising from bats within the colony. Department-specific parameters were consistent with the dynamics at the national scale (SI Appendix).
Thus, our estimation work indicates that the enzootic viral persistence suggested by serology in bats and recurrent spillover to livestock requires both movement of infectious bats among colonies and a high frequency of immunizing nonlethal exposures. It is important to note that these observations contrast sharply from the dynamics expected under qualitatively different and less likely regions of parameter space (Fig. 3 A–C). When both the infection probability and contribution of immigration to the force of infection (ϕ and α) are low (Fig. 3C), seropositive bats are observed only sporadically and at very low levels throughout the time series. In contrast, if α is increased with low immigration, rabies is present only for the duration of the infectious period—on average less than two weeks—and in contrast to the field data, seropositive individuals are rarely present (Fig. 3A). Under this scenario, infectious cases occasionally cause large epizootics that deplete the susceptible pool but such large-scale bat die-offs from rabies have not been reported. If both α and ϕ are high, seroprevalence falls within the range observed in the field, but the population approaches extinction owing to the high rate of lethal infections (Fig. 3B).
Effect of Culling on VBRV Dynamics.
Although the probability of lethal infection (α) is likely to be a relatively fixed trait of the vampire bat–rabies virus interaction, bat dispersal (ϕ) may respond on ecological timescales, providing a behavioral mechanism that might alter VBRV dynamics. In particular, vampire bat culls, a widespread activity for VBRV control, might increase bat immigration into depleted areas. Notably, the efficacy of culling for VBRV control has been questioned due to observations in Peru that culling either had no effect or positive effects on seroprevalence in vampire bats (19). One possible explanation is that culling via the vampiricide paste preferentially targets adult bats due to population age structure and behavioral factors, but that adults may be more likely than juveniles to have acquired immunity from past exposures. An alternate explanation is that potentially unintended outcomes of culling might reflect increases in ϕ following disturbance. To distinguish these possibilities, we used our model to simulate the outcomes of bat culls with a range of biases in which individuals were culled and across a range of immigration levels. Although we are unable to parameterize a spatially explicit model given that the sampled colonies in our field design were distant enough that they do not interact (19); we used model I to explore scenarios ranging from one extreme in which culling is indiscriminate among nonrabid bats to the opposite extreme, where only previously exposed and immune bats are removed. We assumed that rabid bats are not affected by vampiricide because they are either withdrawn and lethargic or aggressive, and consequently are less likely to be exposed to vampiricide by allogrooming. For a given proportion of bats culled r and degree to which culling is indiscriminate ξ, seroprevalence (P) becomes
 |
immediately following culling. Here,  corresponds to indiscriminate culling and
corresponds to indiscriminate culling and  reflects no culling of susceptible bats. Mean seroprevalence is trivially reduced during the first year following culling relative to the no-cull case across values of ξ. It is important to note that seroprevalence increases dramatically with immigration (Fig. 4A). This is crucial, because this implies that under our model conditions, disproportionate culling of immune bats cannot independently explain the inability of culling to reduce seroprevalence, and instead, increased bat dispersal in response to culling might better explain observed seroprevalence in the field.
reflects no culling of susceptible bats. Mean seroprevalence is trivially reduced during the first year following culling relative to the no-cull case across values of ξ. It is important to note that seroprevalence increases dramatically with immigration (Fig. 4A). This is crucial, because this implies that under our model conditions, disproportionate culling of immune bats cannot independently explain the inability of culling to reduce seroprevalence, and instead, increased bat dispersal in response to culling might better explain observed seroprevalence in the field.
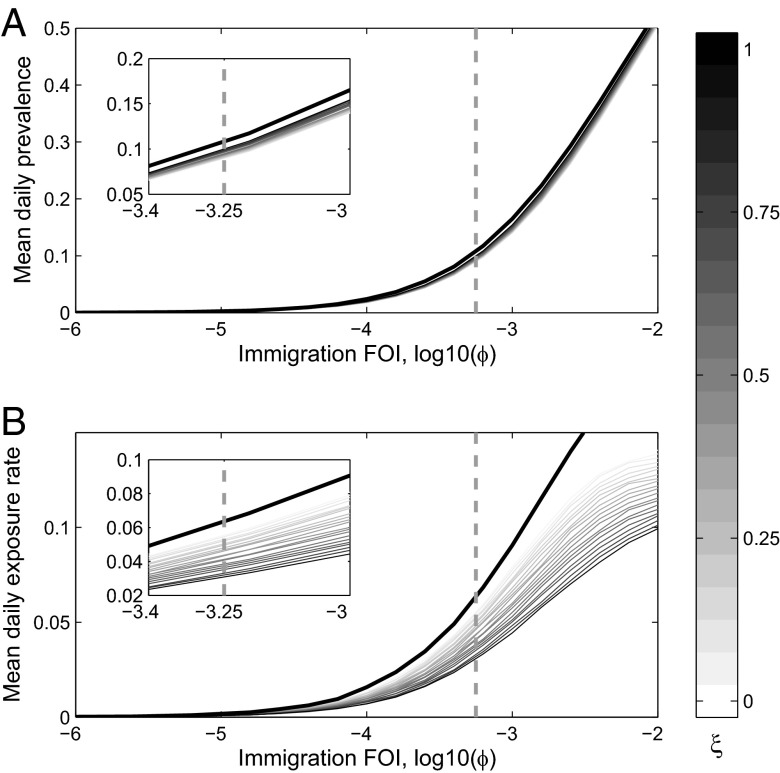
Fig. 4.
The effect of culling for different levels of immigration was evaluated by averaging over 1,000 stochastic simulations for each of the 17 sampled bat colonies. All other parameters were fixed at the MLE. Here, 50% culling is assumed (sensitivity analysis provided in the SI Appendix). We tested a series of culling strategies that, at one extreme, target all uninfected bats indiscriminately (darkest curve,  ) and at the other, do not affect susceptible bats (lightest curve,
) and at the other, do not affect susceptible bats (lightest curve,  ). On each plot, the thick black line indicates the no-cull scenario, and the vertical gray dashed line is the MLE of ϕ; Insets magnify the results near the MLE. (A) Seroprevalence initially declines the most when susceptible bats are not culled because Eq. 1 is highest when
). On each plot, the thick black line indicates the no-cull scenario, and the vertical gray dashed line is the MLE of ϕ; Insets magnify the results near the MLE. (A) Seroprevalence initially declines the most when susceptible bats are not culled because Eq. 1 is highest when  , but in subsequent years the greatest decline corresponds to indiscriminate culling (SI Appendix). (B) Exposure rate of livestock to bats in the IN state, assuming one blood meal per night. Here, a significant decline in exposure rate is observed when culling is indiscriminate.
, but in subsequent years the greatest decline corresponds to indiscriminate culling (SI Appendix). (B) Exposure rate of livestock to bats in the IN state, assuming one blood meal per night. Here, a significant decline in exposure rate is observed when culling is indiscriminate.
Under the assumptions of our model, we also evaluated the risk of spillover to livestock and other species over a range of immigration levels both in the absence and presence of culling. Before the presentation of clinical signs, infected vampire bats (IN) continue to feed, with each bat biting approximately one animal per night. Therefore, the mean number of bats in the IN state provides a proxy for evaluating the daily spillover risk. Although culling results in negligible differences in seroprevalence and infection prevalence, it proportionately decreases the actual number of infectious bats—or spillover risk—when culling is indiscriminate (Fig. 4B). However, as culling becomes more biased toward previously exposed individuals, the predicted efficacy of control diminishes. This is alarming because it implies that any increases in immigration between colonies—such as those caused by behavioral responses to colony disturbance or culling—could increase seroprevalence and the risk of spillover regardless of culling efforts (Fig. 4). This highlights the urgency of understanding how a variety of anthropogenic factors, from land use change to climate change, influence the dispersal behavior of vampire bats. Further, the sporadic timing of infectious cases suggested by our model indicates that although we can evaluate the general systemwide potential for spillover, the temporal dynamics of cross-species transmission at any given site are likely unpredictable without highly resolved, spatially explicit models.
Discussion
By statistically integrating longitudinal field surveillance data with mechanistic mathematical models, we were able to both distinguish among competing scenarios of VBRV persistence and estimate previously unidentified parameters that have crucial effects on transmission dynamics. Collectively, our findings indicate that rabies persistence among vampire bat colonies in Peru depends on immigration of infectious individuals from neighboring colonies and frequent immunizing but nonlethal exposures. This central conclusion was robust to a range of pathological and immunological assumptions. It is important to note that such outcomes require spatial asynchrony in viral dynamics among networks of bat colonies to maintain long-term viral persistence. In addition, our analysis suggests that in contrast to rabies in carnivores and mammals other than bats (28), the probability of developing a lethal infection upon exposure to rabies is quite low for vampire bats (∼10%), enabling viral persistence in slowly reproducing bat colonies. Similarly high seroprevalence to rabies virus in other bat species suggests that frequent survival after exposure may be a general feature of bat rabies (29). The apparent rarity of lethal infection following natural exposures calls into question the infection dynamics and pathology observed in experimental challenge studies, which often generate 50–90% mortality using high inoculation doses (20, 24, 30), and suggests that rabies virus may not be as markedly exceptional among bat-borne viral zoonoses in having high virulence in its natural host as previously believed (1, 3). The impact of more realistic lower challenge doses on within-host dynamics, such as incubation and infectious periods, remains an important unknown.
Bat rabies persistence has not generally been considered a consequence of spatial processes. This opinion is reflected in field studies that have consistently suggested enzootic persistence at low prevalence (27), and more recently in modeling studies that have argued that interactions between herd immunity and seasonal activity allow local persistence without immigration (4). Moreover, reviews of bat rabies persistence omit spatial dynamics, instead focusing on seasonal processes such as birth pulses, migration, and hibernation (28, 31). Our results provide evidence that metapopulation persistence can explain both relatively high seroprevalence and low infection prevalence in wild bats.
It is important to note that the critical role of immigration between bat colonies predicted by our analysis indicates that current culling practices, often reactive to outbreaks in livestock or haphazardly implemented, are unlikely to eliminate VBRV. Although programs targeting specific colonies may limit local spillover from bats to humans or domestic animals, regional viral persistence will likely remain unaffected due to high connectivity between bat colonies. Moreover, if culling increases movement due to freeing up space or disturbance-mediated dispersal, culling could have the opposite of the intended effect on rabies transmission. This phenomenon has recently been noted in controlled badger culls in the UK, where disruption of badger social dynamics and subsequent dispersal led to increased bovine tuberculosis transmission in neighboring sites (32). Our work suggests, therefore, that efforts aimed at reducing spillover through viral elimination must likely be spatially coordinated with a view to defining and synchronizing transmission dynamics within enzootic regions (23).
Materials and Methods
Collection of Field Data.
Field data were collected between 2007 and 2010 from 17 colonies in four departments of Peru (Apurimac, Cajamarca, Lima, and Madre de Dios), which spanned coastal deserts, inter-Andean valleys and the Amazon rainforest (Fig. 1). In brief, vampire bats were captured over 3–6 consecutive nights using mist nets and harp traps, and marked with individually numbered wing bands. Blood samples were collected by peripheral venipuncture, centrifuged to isolate serum and tested for rabies virus neutralizing antibodies using the micro-Rapid Fluorescent Focus Inhibition Test. Sites were sampled 1–2 times per year, and a total of 1,436 unique bats were captured over the study duration. Colony sizes were estimated using standard capture–mark–recapture approaches. Complete descriptions of colonies and methods are found in ref. 19. Notably, this dataset is the most spatially and temporally complete record of rabies exposure patterns ever collected for any bat species. The University of Georgia’s Institutional Animal Care and Use Committee approved protocols for the capture and handling of bats (Animal Use Protocol no. A2009-10003) and collection and exportation permits were obtained from the Peruvian government (103-2008-INRENA-IFFS-DCB; RD-222–2009-AG-DGFFS-DGEFFS; RD-0299–2010-AG-DGFFS-DGEFFS; 003851-AG-DGFFS; 004692-AG-DGFFS; and 005216-AG-DGFFS).
Parameter Estimation from the Field Data.
For each model, we estimated the parameters in  , and imposed the restrictions that
, and imposed the restrictions that  as suggested by current estimates (20, 24, 30) and
as suggested by current estimates (20, 24, 30) and  . After implementing fully stochastic versions of our models using the τ-leap method (an approximation to Gillespie’s algorithm) we used particle filtering, implemented from the partially observed Markov processes (POMP) 0.36–1 package of the statistical computing language R (25, 33), to perform a grid search over all parameters in θ to compute the log likelihood at each point. A grid search was then performed over the relevant ranges for α and ϕ, and for each parameter combination we performed an additional search to identify the optimal transmission parameters, βN and βR. For all models, we find that for a fixed α and ϕ the relative magnitudes of βN and βR are nearly indistinguishable but that the associated intrinsic R0 is identifiable, allowing us to create a likelihood profile for R0 (Fig. 2C and SI Appendix).
. After implementing fully stochastic versions of our models using the τ-leap method (an approximation to Gillespie’s algorithm) we used particle filtering, implemented from the partially observed Markov processes (POMP) 0.36–1 package of the statistical computing language R (25, 33), to perform a grid search over all parameters in θ to compute the log likelihood at each point. A grid search was then performed over the relevant ranges for α and ϕ, and for each parameter combination we performed an additional search to identify the optimal transmission parameters, βN and βR. For all models, we find that for a fixed α and ϕ the relative magnitudes of βN and βR are nearly indistinguishable but that the associated intrinsic R0 is identifiable, allowing us to create a likelihood profile for R0 (Fig. 2C and SI Appendix).
Particle filtering requires specification of the “process” model that describes the true transmission dynamics within a single bat colony and an “observation” model that relates model variables to field data. The process model is given by our transmission models, and we assumed that the field data follow a binomial distribution, so that for a given study site s and sample date t, the number of seropositive individuals k is given by  , where n is the number of samples collected, and p is the seroprevalence predicted by the process model for a given parameter set θ.
, where n is the number of samples collected, and p is the seroprevalence predicted by the process model for a given parameter set θ.
Supplementary Material
Acknowledgments
We thank the P.R. laboratory and two anonymous reviewers for their feedback on earlier drafts of this manuscript. We also thank the Office of Epidemiology of the Ministry of Health of Peru, the National Service of Agricultural Health of the Ministry of Agriculture of Peru, and the Centers for Disease Control (CDC) Rabies Program for logistical support of field and lab work. This research was supported by National Science Foundation (NSF) Grant DEB-1020966 and a University of Georgia (UGA)–CDC Infectious Disease Seed Grant FID-908 to S.A. and P.R. In addition, D.G.S. was supported by an NSF Graduate Research Fellowship, a UGA Dissertation Completion Award, and a Young Explorers Grant from the National Geographic Committee for Research and Exploration. P.R. was supported by the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. O.N.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308817110/-/DCSupplemental.
References
- 1.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19(3):531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drexler JF, et al. Bats host major mammalian paramyxoviruses. Nat Commun. 2012;3(796):796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayman DTS, et al. Ecology of zoonotic infectious diseases in bats: Current knowledge and future directions. Zoonoses and Public Health. 2012;60(1):2–21. doi: 10.1111/zph.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George DB, et al. Host and viral ecology determine bat rabies seasonality and maintenance. Proc Natl Acad Sci USA. 2011;108(25):10208–10213. doi: 10.1073/pnas.1010875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plowright RK, et al. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.) Proc Biol Sci. 2011;278(1725):3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulliam JRC, et al. Henipavirus Ecology Research Group (HERG) Agricultural intensification, priming for persistence and the emergence of Nipah virus: A lethal bat-borne zoonosis. J R Soc Interface. 2012;9(66):89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization (2005) WHO expert consultation on rabies: First report 5-8 Oct 2004. Geneva, Switzerland: World Health Organisation. Technical Report 931, 1–88. [PubMed]
- 8.Delpietro H, Marchevsky N, Simonetti E. Relative population densities and predation of the common vampire bat (Desmodus rotundus) in natural and cattle-raising areas in north-east Argentina. Prev Vet Med. 1992;14:13–20. [Google Scholar]
- 9.Voigt C, Kelm D. Host preference of the common vampire bat (Desmodus rotundus; chiroptera) assessed by stable isotopes. J Mammal. 2006;87:1–6. [Google Scholar]
- 10.Lee DN, Papeş M, Van den Bussche RA. Present and potential future distribution of common vampire bats in the Americas and the associated risk to cattle. PLoS ONE. 2012;7(8):e42466. doi: 10.1371/journal.pone.0042466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider MC, et al. Rabies transmitted by vampire bats to humans: An emerging zoonotic disease in Latin America? Rev Panam Salud Publica. 2009;25(3):260–269. doi: 10.1590/s1020-49892009000300010. [DOI] [PubMed] [Google Scholar]
- 12.Arellano-Sota C. Vampire bat-transmitted rabies in cattle. Rev Infect Dis. 1988;10(Suppl 4):S707–S709. doi: 10.1093/clinids/10.supplement_4.s707. [DOI] [PubMed] [Google Scholar]
- 13.Sétien AA, et al. Experimental rabies infection and oral vaccination in vampire bats (Desmodus rotundus) Vaccine. 1998;16(11-12):1122–1126. doi: 10.1016/s0264-410x(98)80108-4. [DOI] [PubMed] [Google Scholar]
- 14.Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: A conceptual and practical challenge. Emerg Infect Dis. 2002;8(12):1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawan J. Rabies in the vampire bat in Trinidad with special reference to the clinical course and the latency of infection. Ann Trop Med Parasitol. 1936;30:401–422. [PubMed] [Google Scholar]
- 16.Delpietro H, Russo R. Ecological and epidemiological aspects of attacks by vampire bats in relation to paralytic rabies in Argentina, and an analysis of proposals for control. Rev Sci Tech. 1996;15:971–984. [PubMed] [Google Scholar]
- 17.Lord RD, et al. Observations on the epizootiology of vampire bat rabies. Bull Pan Am Health Organ. 1975;9(3):189–195. [PubMed] [Google Scholar]
- 18.Bartlett M. Measles periodicity and community size. J R Stat. Soc Ser. 1957;120:48–70. [Google Scholar]
- 19.Streicker DG, et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: Implications for transmission and control. Proc Biol Sci. 2012;279(1742):3384–3392. doi: 10.1098/rspb.2012.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguilar-Setien A, et al. Salivary excretion of rabies virus by healthy vampire bats. Epidemiol Infect. 2005;133(3):517–522. doi: 10.1017/s0950268805003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massad E, Coutinho FA, Burattini MN, Sallum PC, Lopez LF. A mixed ectoparasite—microparasite model for bat-transmitted rabies. Theor Popul Biol. 2001;60(4):265–279. doi: 10.1006/tpbi.2000.1494. [DOI] [PubMed] [Google Scholar]
- 22.Anderson R, May R. Infectious Diseases of Humans. New York: Oxford Univ Press; 1991. [Google Scholar]
- 23.Keeling M, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton, NJ: Princeton Univ Press; 2008. [Google Scholar]
- 24.Moreno JA, Baer GM. Experimental rabies in the vampire bat. Am J Trop Med Hyg. 1980;29(2):254–259. doi: 10.4269/ajtmh.1980.29.254. [DOI] [PubMed] [Google Scholar]
- 25.Ionides EL, Bretó C, King AA. Inference for nonlinear dynamical systems. Proc Natl Acad Sci USA. 2006;103(49):18438–18443. doi: 10.1073/pnas.0603181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnham KP, Anderson D. Model Selection and Multi-Model Inference. New York: Springer; 2002. [Google Scholar]
- 27.Constantine DG. Bat rabies in the southwestern United States. Public Health Rep. 1967;82(10):867–888. [PMC free article] [PubMed] [Google Scholar]
- 28.Rupprecht CE, Turmelle A, Kuzmin IV. A perspective on lyssavirus emergence and perpetuation. Curr Opin Virol. 2011;1(6):662–670. doi: 10.1016/j.coviro.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Steece R, Altenbach JS. Prevalence of rabies specific antibodies in the Mexican free-tailed bat (Tadarida brasiliensis mexicana) at Lava Cave, New Mexico. J Wildl Dis. 1989;25(4):490–496. doi: 10.7589/0090-3558-25.4.490. [DOI] [PubMed] [Google Scholar]
- 30.Almeida MF, et al. Experimental rabies infection in haematophagous bats Desmodus rotundus. Epidemiol Infect. 2005;133(3):523–527. doi: 10.1017/s0950268804003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Constantine D (2009) Bat Rabies and Other Lyssavirus Infections. U.S. Geological Survey Circular (Reston, VA), Vol 1329.
- 32.Donnelly CA, et al. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature. 2006;439(7078):843–846. doi: 10.1038/nature04454. [DOI] [PubMed] [Google Scholar]
- 33. King A, Ionides E, Bretó C, Ellner S, Kendall B (2009) POMP: Statistical inference for partially observed Markov processes (R package). http://pomp.r-forge.r-rproject.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.