Abstract
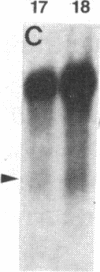
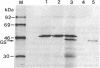
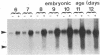
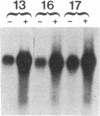
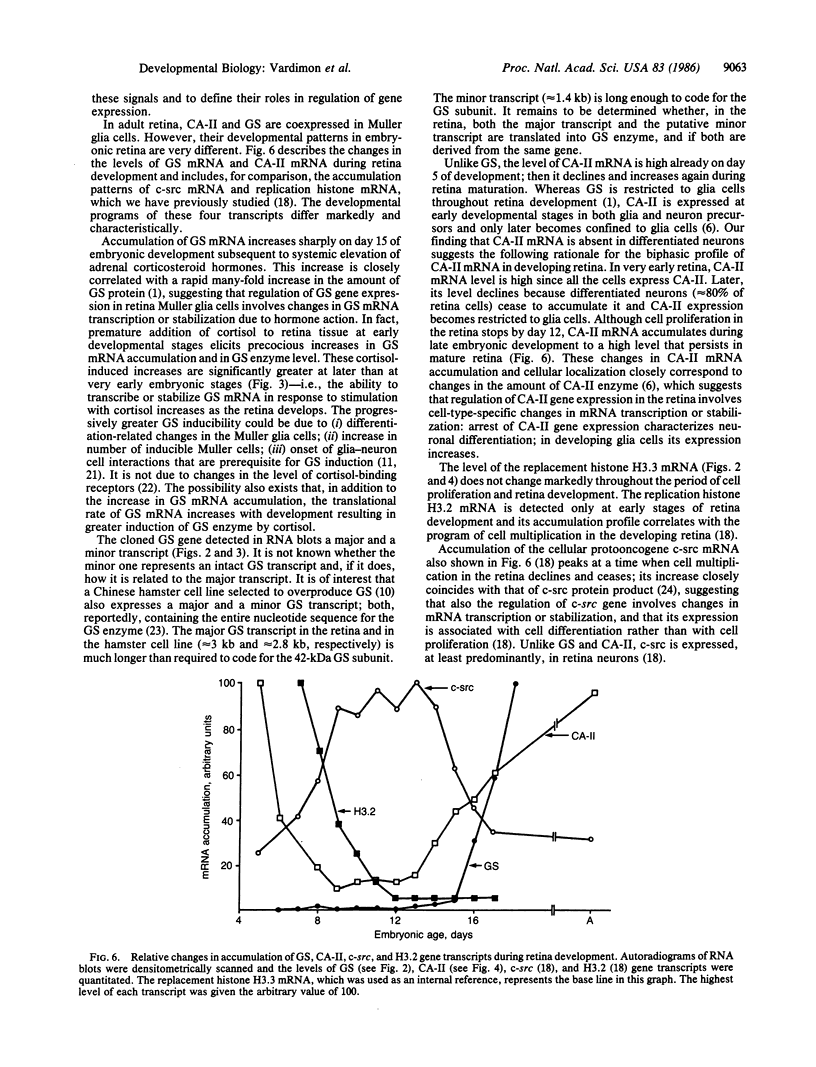
Glutamine synthetase (GS) is expressed in the neural retina only in Muller glia cells and is inducible with cortisol. A chicken genomic clone that contains at least part of the coding region for the GS enzyme was used to investigate developmental changes in the level of GS mRNA in embryonic chicken retina. A major GS transcript (approximately equal to 3 kilobases) detected by the probe begins to accumulate sharply on day 15 of embryonic development. When cortisol is prematurely supplied to early embryonic retina, it induces precocious accumulation of GS mRNA and of the GS enzyme. At later ages, these effects of cortisol are significantly greater, which suggests that competence to transcribe or stabilize GS mRNA in response to stimulation with cortisol increases with development. Carbonic anhydrase II (CA-II) is expressed in early retina in all the cells, but it becomes later restricted to Muller glia. Using cloned CA-II cDNA, we detected a high level of CA-II mRNA in early retina, followed by a decline due to arrest of CA-II mRNA accumulation in differentiated neurons. As glia cells mature, CA-II mRNA and the enzyme increase to a new high level. Therefore, changes in CA-II gene expression during retina development reflect differentiation-dependent cell-type-specific control of CA-II mRNA accumulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. T., Wellman S. E., Sittman D. B. Changes in the levels of three different classes of histone mRNA during murine erythroleukemia cell differentiation. Mol Cell Biol. 1985 Nov;5(11):2879–2886. doi: 10.1128/mcb.5.11.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush D., Dodgson J. B., Choi O. R., Stevens P. W., Engel J. D. Replacement variant histone genes contain intervening sequences. Mol Cell Biol. 1985 Jun;5(6):1307–1317. doi: 10.1128/mcb.5.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Sugarman B. J., Dodgson J. B. A chicken histone H3 gene contains intervening sequences. Nature. 1982 Jun 3;297(5865):434–436. doi: 10.1038/297434a0. [DOI] [PubMed] [Google Scholar]
- Hayward B. E., Hussain A., Wilson R. H., Lyons A., Woodcock V., McIntosh B., Harris T. J. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res. 1986 Jan 24;14(2):999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P. J., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: its suppression by the gliatoxic agent alpha-aminoadipic acid. Brain Res. 1981 Jan;227(1):103–119. doi: 10.1016/0165-3806(81)90097-3. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Carbonic anhydrase C in the neural retina: transition from generalized to glia-specific cell localization during embryonic development. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7190–7194. doi: 10.1073/pnas.78.11.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Hormonal induction of glutamine synthetase in cultures of embryonic retina cells: requirement for neuron-glia contact interactions. Dev Biol. 1983 Apr;96(2):529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: localization in Müller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. A., Linser P. Developmental and experimental changes in retinal glia cells: cell interactions and control of phenotype expression and stability. Curr Top Dev Biol. 1983;18:155–188. doi: 10.1016/s0070-2153(08)60582-7. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Moscona M. H., Saenz N. Enzyme induction in embryonic retina: the role of transcription and translation. Proc Natl Acad Sci U S A. 1968 Sep;61(1):160–167. doi: 10.1073/pnas.61.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona M., Frenkel N., Moscona A. A. Regulatory mechanisms in the induction of glutamine synthetase in the embryonic retina: immunochemical studies. Dev Biol. 1972 May;28(1):229–241. doi: 10.1016/0012-1606(72)90140-6. [DOI] [PubMed] [Google Scholar]
- Moscona M., Moscona A. A. The development of inducibility for glutamine synthetase in embryonic neural retina: inhibition by BrdU. Differentiation. 1979;13(3):165–172. doi: 10.1111/j.1432-0436.1979.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Piddington R., Moscona A. A. Precocious induction of retinal glutamine synthetase by hydrocortisone in the embryo and in culture. Age-dependent differences in tissue response. Biochim Biophys Acta. 1967 Jul 25;141(2):429–432. doi: 10.1016/0304-4165(67)90120-1. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A. D., Moscona A. A. Cortisol receptors and inducibility of glutamine synthetase in embryonic retina. Cell Differ. 1985 Jun;16(4):241–250. doi: 10.1016/0045-6039(85)90574-3. [DOI] [PubMed] [Google Scholar]
- Sanders P. G., Wilson R. H. Amplification and cloning of the Chinese hamster glutamine synthetase gene. EMBO J. 1984 Jan;3(1):65–71. doi: 10.1002/j.1460-2075.1984.tb01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P. K., Fischman D. A., Goldwasser E., Moscona A. A. Isolation and characterization of glutamine synthetase from chicken neural retina. J Biol Chem. 1972 Dec 10;247(23):7743–7749. [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Yoshihara C. M., Federspiel M., Dodgson J. B. Isolation of the chicken carbonic anhydrase II gene. Ann N Y Acad Sci. 1984;429:332–334. doi: 10.1111/j.1749-6632.1984.tb12357.x. [DOI] [PubMed] [Google Scholar]