Significance
The Arctic Ocean is a bellwether for ocean acidification, yet few direct Arctic studies have been carried out and limited observations exist, especially in winter. We present unique under-ice physicochemical data showing the persistence of a mid water column area of high CO2 and low pH through late winter, Zooplankton data demonstrating that the dominant copepod species are distributed across these different physicochemical conditions, and empirical data demonstrating that these copepods show sensitivity to pCO2 that parallels the range of natural pCO2 they experience through their daily vertical migration behavior. Our data, collected as part of the Catlin Arctic Survey, provide unique insight into the link between environmental variability, behavior, and an organism’s physiological tolerance to CO2 in key Arctic biota.
Keywords: climate change, diel vertical migration, ecophysiology, pH response
Abstract
The Arctic Ocean already experiences areas of low pH and high CO2, and it is expected to be most rapidly affected by future ocean acidification (OA). Copepods comprise the dominant Arctic zooplankton; hence, their responses to OA have important implications for Arctic ecosystems, yet there is little data on their current under-ice winter ecology on which to base future monitoring or make predictions about climate-induced change. Here, we report results from Arctic under-ice investigations of copepod natural distributions associated with late-winter carbonate chemistry environmental data and their response to manipulated pCO2 conditions (OA exposures). Our data reveal that species and life stage sensitivities to manipulated OA conditions were correlated with their vertical migration behavior and with their natural exposures to different pCO2 ranges. Vertically migrating adult Calanus spp. crossed a pCO2 range of >140 μatm daily and showed only minor responses to manipulated high CO2. Oithona similis, which remained in the surface waters and experienced a pCO2 range of <75 μatm, showed significantly reduced adult and nauplii survival in high CO2 experiments. These results support the relatively untested hypothesis that the natural range of pCO2 experienced by an organism determines its sensitivity to future OA and highlight that the globally important copepod species, Oithona spp., may be more sensitive to future high pCO2 conditions compared with the more widely studied larger copepods.
Ocean acidification (OA) has been highlighted as one of the most pervasive human impacts on the ocean (1). However, observational datasets that link oceanic carbonate chemistry with biotic responses on which to ground predictions of OA impacts remain limited, especially in the most susceptible and rapidly changing ocean, the Arctic (2). Recent observations indicate that several locations in the Arctic already experience seasonal undersaturation with respect to aragonite, concomitantly with elevated pCO2 and lowered pH conditions (3), and such incidences are predicted to increase as OA progresses (4). However, knowledge of current seasonal and interannual variability in carbonate system parameters for the Arctic Ocean, particularly under winter sea ice, remains limited. Furthermore, information about the ecology of organisms that live in Arctic waters is primarily restricted to summer studies, with only a few investigations being conducted during the ice-covered winter period (5). Hence, predicting how organisms and ecosystems respond to OA is currently restricted to studies from subarctic and/or ice-free Arctic systems because of the technical difficulties and costs involved in sampling remote ice-associated Arctic locations. Given that the Arctic is recognized as a “bellwether” for global OA processes (2) and that polar species are potentially more sensitive to these changes due to their reduced metabolic scope (6), this lack of data on Arctic under-ice zooplankton responses to changes in current and future carbonate chemistry represents a serious knowledge gap and limits predictive modeling capabilities of future scenarios.
Copepods generally make up the dominant zooplankton of Arctic waters, exerting significant influences on primary production and pelagic fisheries (e.g., ref. 7). Due to their large body size, high lipid content, and dominant biomass, calanoid copepods, in particular, are an important high-quality food source for many pelagic Arctic fish (8, 9); hence, their responses to OA have important implications for Arctic ecosystems. Copepods have a mainly chitinous exoskeleton, so they are not as vulnerable to calcium carbonate undersaturation as other calcifying Arctic organisms, such as pteropods (10). However, evidence for impacts of elevated CO2 have been demonstrated for a number of temperate copepod species and life history stages (11–13) [although only one study (11) showed responses occurring at levels projected for the year 2100], whereas others, including temperate calanoids, appear more resilient (14, 15). OA impacts are most likely to occur as a result of increased energetic costs of maintaining homeostasis of physiological processes [e.g., acid–base balance (16)] under elevated CO2 conditions, with resultant shifts in growth, fecundity, and survival, yet these responses remain relatively understudied for this ecologically important group. During the Arctic winter, there is low food availability; therefore, overcoming negative OA impacts through more energy intake (17) may not be possible for copepods that are present in late winter or for nonfeeding, early life stages.
Previous work has suggested that organism sensitivity to stress can be inferred from a combination of knowledge of the organism’s ecology in relation to the variability of its environment (e.g., refs. 18, 19), with the hypothesis being that organisms will have more effective mechanisms to cope with stress if they frequently experience a more variable environment. One example of this hypothesis for pelagic zooplankton comes from knowledge of mesozooplankton (20) distributions in relation to oxygen minimum zones (OMZs), and thus their sensitivity to low oxygen (20). Childress (21) showed the midwater migratory copepod Gaussia princeps had lower metabolic rates during the day when it was found at depth in the OMZ. More recently, Maas et al. (22) showed that pteropods that migrate vertically through a tropical OMZ reduced their metabolic rate under low oxygen and low temperature conditions in the laboratory. Thus, under oxygen stress, organisms previously exposed to low oxygen conditions have mechanisms, including metabolic depression or consuming more protein, that allow them to survive. With respect to OA, high CO2 and low pH conditions are often also found in the OMZs, and Maas et al. (23) showed that nonmigratory pteropods were affected during OA experiments, whereas migratory pteropods showed no response to high CO2, although this result may be confounded by the temperature at which the experiments were conducted.
Here, we examine the natural distributions of the dominant Arctic copepods found under winter sea ice in relation to the current seawater carbonate chemistry conditions and compare these with their short-term responses to future high CO2 conditions. We measured under-ice seawater carbonate chemistry and zooplankton dynamics at the temporary Catlin Arctic Survey Ice Base (CIB) during late winter to early spring in 2011. The zooplankton were dominated by adult calanoid copepods, comprising mainly the Arctic endemics Calanus glacialis and Calanus hyperboreus but also the smaller, globally occurring Oithona similis, together with the nauplii of various copepod species. A series of OA experiments were then conducted using these copepod species and life history stages to compare their response to future high CO2 conditions with natural under-ice pCO2 exposures.
Materials and Methods
The CIB was located at 78°43.11′ N, 104°47.44′ W in Deer Bay, off Ellef Ringnes Island in the high Canadian Arctic (Fig. S1). All samples were collected from a 1 × 1-m hole cut through 1.6 m of sea ice.
Zooplankton dynamics were monitored over two 24-h periods: March 20–21, 2011 and April 23–24, 2011. A 53-μm net was used to trawl vertically from 50 m to the surface (through the ice hole) every hour during the 24-h period. All samples were sorted and preserved using buffered 4% (vol/vol) formaldehyde and returned to the laboratory for quantification and identification to the lowest taxonomic level possible. Additionally, 200-m vertical trawls were carried out at 1300 hours on all routine sample days.
Discrete water samples were collected through the ice hole using a 5-L Niskin (model 1010; General Oceanics) from 1, 3, 10, and 50 m under the ice every 4 d for 40 d beginning on March 19, 2011, and additionally from 100 and 200 m on March 24, March 31, and April 25, 2011. Conductivity, temperature, and depth (CTD) measurements were made on each of these sample days using a SBE19plus SeaCAT CTD (Sea-Bird Electronics Inc.) instrument and were calibrated using bottle salinity samples analyzed on a Guildline Autosal 8400B at the Institute of Ocean Sciences.
Seawater samples for total dissolved inorganic carbon (DIC) and total alkalinity (TA) were collected in triplicate into 250-mL borosilicate glass bottles with ground-glass stoppers and poisoned with 100 μL of saturated HgCl2, according to Dickson et al. (24). Samples were stored at 4 °C to the extent possible (sample storage temperature was difficult to control at the ice camp) in the dark and analyzed at the Institute of Ocean Sciences, using Versatile INstrument for the Determination of Titration Alkalinity (VINDTA) and Single Operator Multiparameter Metabolic Analyzer (SOMMA) instrumentation for DIC and an open-cell potentiometric titration with nonlinear least squares curve fitting for TA (24) Certified reference materials were provided by Andrew Dickson (Scripps Institute of Oceanography, San Diego, CA) and used for calibration. The seawater carbonate system program CO2sys (25) was used to calculate the pCO2 and pHT (total scale) from DIC, TA, in situ temperature, and salinity. Carbonic acid dissociation constants of Mehrbach et al. (26) refit by the method of Dickson and Millero (27) and the HSO4− constant of Dickson (28) were used.
A series of OA experiments were conducted using the two dominant calanoid copepods from the trawls (adult C. glacialis and C. hyperboreus in combination), adult O. similis, and the two size classes of nauplii. The nauplii were not identifiable to species level; hence, they were differentiated into size classes, although based on size, morphology (particularly head shape), and abundance of adults, nauplii were tentatively identified as being either Calanus spp. (>250 μm) or Oithona spp. (<60 μm). OA experiments were conducted using unfiltered seawater collected from a depth of 25 m to replicate the conditions within the middle of the upper mixed layer (UML; defined here as approximately 0–50 m). Seawater was unfiltered so that it contained naturally available phytoplankton and microzooplankton food conditions. Carbonate chemistry was manipulated in header tanks (25 L) using HCl and NaHCO3 additions, based on measurements of pH and alkalinity, and using the Seacarb program (Seawater Carbonate chemistry with R) to calculate the acid–base concentrations required (29). Three pCO2 scenarios were used: ambient (∼370 μatm), midfuture (∼700 μatm), and high future (∼1,000 μatm).
Copepods were collected from trawls using 200 μm (for adults) and 53 μm (for nauplii) plankton nets. Thirty adult Calanus spp. (comprising 22 C. glacialis and 8 C. hyperboreus), 30 adult O. similis, 30 large nauplii, and 50 small nauplii individuals were counted into culture bottles (60 mL for nauplii and 500 mL for adults; Polyethylene terephthalate glycol-modified (PETG), transparent square, narrow neck; Thermo Scientific Nalgene) filled with treatment seawater from the header tanks (three to six replicates per treatment). Exposures ran for 7 d at ambient temperature (−1.6 °C) by hanging the culture bottles through a hole in the sea ice, with water changes performed every other day. Survivorship was determined for all experiments by comparing the number of individuals alive at the end of the exposure (7 d) with the number of individuals at the beginning of the exposure.
The pH, temperature, and salinity were measured at the beginning and the end of the experiment and at each water change in water samples taken from each of the incubation bottles. Temperature and salinity were measured using a handheld WTW LF197 multimeter (Xylem Inc., Germany) with a Tetra con 325 electrode (VWR International Ltd.), and pH was measured using a handheld a Metrohm 826 pH meter and pH electrode (6.0228.000) (Metrohm UK Ltd.), and using Amp and Tris buffers, to give pH on total scale, following the method of Dickson et al. (24). Samples for DIC and TA were collected at the beginning and end of each exposure and returned with the environmental water samples to be analyzed as described above at the Institute of Ocean Sciences. Environmental conditions within the experiment are reported in Table 1.
Table 1.
Seawater (under-ice) conditions from sampled depths for the period March 28–April 25, 2011
| Seawater parameter | 0–10 m | 50 m | 100 m | 200 m |
| Temperature, °C | −1.672 ± 0.008 | −1.460 ± 0.006 | −1.270 ± 0.014 | −0.253 ± 0.066 |
| Salinity | 30.57 ± 0.14 | 31.79 ± 0.06 | 32.99 ± 0.08 | 34.60 ± 0.33 |
| DIC, μmol⋅kg−1 | 2,044.7 ± 13.1 | 2,109.3 ± 7.7 | 2,180.1 ± 11.1 | 2,193.9 ± 1.4 |
| TA, μmol⋅kg−1 | 2,156.4 ± 11.5 | 2,200.1 ± 6.6 | 2,242.9 ± 0.4 | 2,282.3 ± 2.5 |
| pH, total | 8.116 ± 0.026 | 8.024 ± 0.022 | 7.906 ± 0.039 | 7.952 ± 0.007 |
| pCO2, μatm | 308.7 ± 20.1 | 391.6 ± 20.9 | 528.4 ± 50.6 | 472.9 ± 8.5 |
| Ω Calcite | 2.11 ± 0.12 | 1.81 ± 0.09 | 1.48 ± 0.12 | 1.74 ± 0.04 |
| Ω Aragonite | 1.31 ± 0.07 | 1.14 ± 0.06 | 0.93 ± 0.08 | 1.10 ± 0.02 |
| HCO3−, μmol⋅kg−1 | 1,937.4 ± 14.8 | 2,007.5 ± 9.0 | 2,082.7 ± 13.0 | 2,088.4 ± 2.6 |
| CO32− , μmol⋅kg−1 | 86.1 ± 4.7 | 75.3 ± 3.6 | 62.2 ± 5.4 | 75.6 ± 1.9 |
Values are the average ± SD (0–10 m is the average of samples taken immediately under the ice at 3 m and at 10 m). Measured values were temperature, salinity, DIC, and TA. The pH, pCO2, calcite and aragonite saturation states, and bicarbonate and carbonate ion concentrations were all calculated, using CO2sys, from temperature, salinity, DIC, and TA.
Differences between treatments for survival (%) of all examined species and nauplii in the OA experiments were determined using ANOVA tests, followed by post hoc Tukey’s tests. All parameters were initially tested for normality (Kolmogorov–Smirnov test) and homogeneity of variance (Bartlett’s test). Survival data were arcsine-transformed to conform to normality and variance homogeneity.
Results
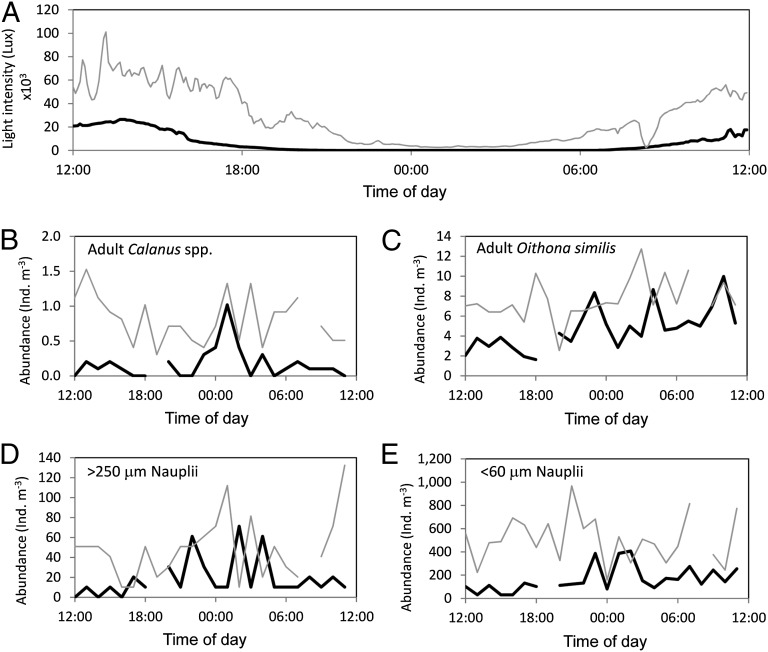
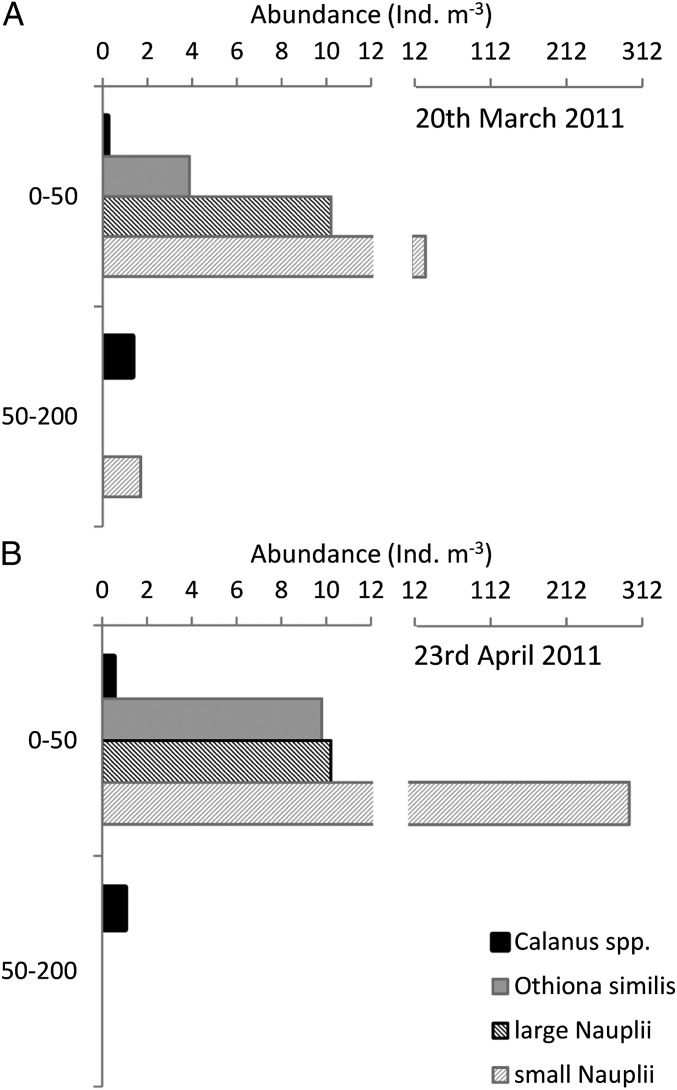
At the beginning of the field campaign (first 24-h zooplankton investigation), the day length was 12 h and astronomical twilight lasted all night. However, in late April (during the second 24-h investigation), the day length had increased to 20 h and civil twilight persisted throughout the night (Fig. 1 A–D and Table S1). These changes in light duration and intensity were reflected in a corresponding shift in copepod abundances beneath the sea ice (Fig. 1 A–D). Adult Calanus copepods, although present in relatively low numbers, had a distinct peak in abundance throughout the hours of darkness during the March sampling (Fig. 1A), suggesting they were vertically migrating through the halocline from deeper waters (>50 m) at this time (compare with Fig. 2). In April, adult Calanus abundance increased in the surface layers (Fig. 1A) and individuals were present over the whole 24-h period, suggesting vertical migration had become less pronounced, or at least less structured (i.e., at specific times of the day) as the length of darkness decreased (compare with Fig. 2). Nauplii numbers had also increased, with nearly a 10-fold increase in small nauplii within the surface layers in later April compared with March (Fig. 2).
Fig. 1.
Twenty-four–hour time series in the surface water (0–50 m) at the CIB over the periods March 20–21, 2011 (thick black line) and April 23–24, 2011 (thin gray line) for surface light intensity (lux) (A), adult Calanus spp. abundance [Individual (Ind.) m−3] (B), adult O. similis abundance (Ind. m−3) (C), large (>250 μm) nauplii abundance (Ind. m−3) (D), and small (<60 μm) nauplii abundance (Ind. m−3) (E).
Fig. 2.
Abundance of organisms [adult Calanus spp., adult O. similis, large (>250 μm) nauplii, and small (<60 μm) nauplii] found in the different depth trawls (0–50 m and 50–200 m) at 1300 hours on March 20, 2011 (A) and 1300 hours on April 24, 2011 (B). Values are number of individuals per cubic meter trawled using a 53-μm mesh size net.
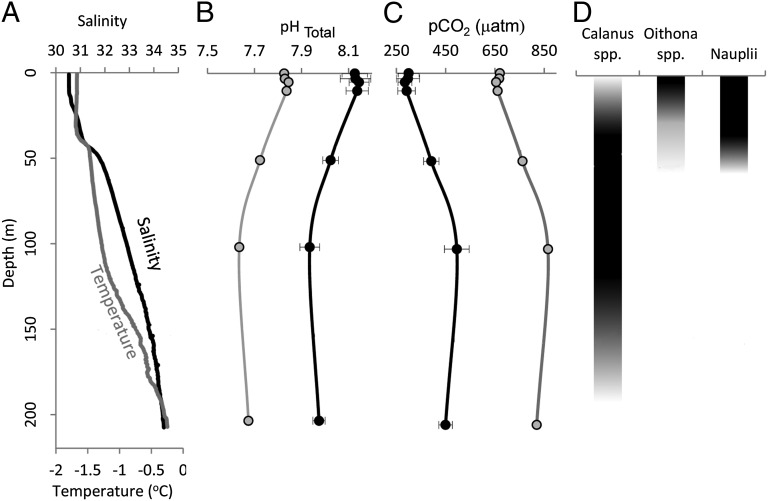
Physicochemical conditions demonstrated that the water column under the ice had distinct layers. From immediately under the ice (0 m) to 40–50 m was the polar mixed layer (PML, or surface layer), a cold, fresh, low-density layer of water. From 40 to 50 m to about 120 m was the upper halocline layer (UHL), a layer increasing in temperature and salinity, and below this was the lower halocline layer (LHL) (Fig. 3 and Table 1). Data here are compared with PML, UHL, and LHL end members in a study by Jones and Anderson (30). With increasing depth, the pCO2 increased to a maximum (maximum pCO2 = 564.2 μatm) and pH decreased to a minimum (minimum pH = 7.87) at around 100 m within the UHL. This water circulates from the Pacific, where a high CO2 signal comes from remineralization of organic matter. The PML surface waters had low pCO2 and high pH conditions (minimum pCO2 = 240 μatm, maximum pH = 8.21).
Fig. 3.
Temperature (°C) and salinity profiles through the water column at the CIB (A); water column profile of pH from the 2011 data (mean ± SD; black circles) calculated from DIC and alkalinity and the projected pH for year 2100 (gray circles), assuming a 0.4-unit decrease across the 200-m water column (B); water column profile of pCO2 from the 2011 data (mean ± SD; black circles) calculated from DIC and alkalinity and the projected pCO2 for year 2100 (gray circles), assuming a doubling of atmospheric CO2 (C); and illustration of the depth of vertical migration of copepod species observed at the CIB and investigated in the OA experiments (D).
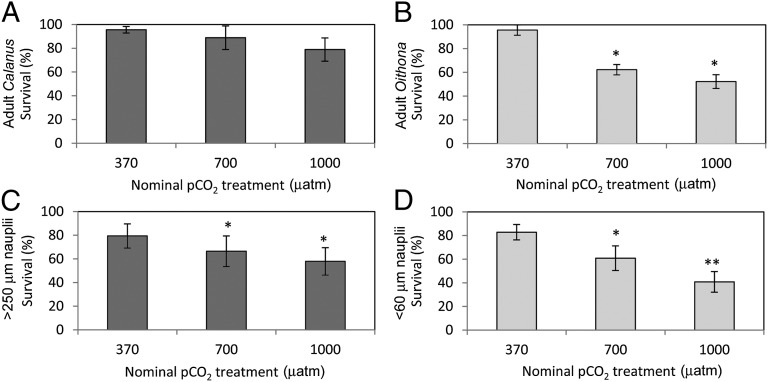
Clear differences in sensitivity to short-term pCO2 exposures were observed between the large Calanus (size range: 3–8 mm) compared with the smaller O. similis (size range: 0.5–1 mm) and the nauplii larvae. Elevated pCO2 levels had no significant impact on survival of the adult Calanus (Fig. 4A; Fdf = 2 = 3.33, P = 0.064). In contrast, the adult O. similis was found to have a significant reduction in survival in both the 700-μatm and 1,000-μatm treatments (Fig. 4B; Fdf = 2 = 30.74, P = 0.001). Both size classes of nauplii were also found to be sensitive to the high pCO2: Survival in the larger nauplii was significantly reduced in the 700-μatm and 1,000-μatm treatments compared with the ambient treatment at 370 μatm (Fig. 4C; Fdf = 2 = 4.07, P = 0.028). The smaller nauplii showed a similar response but with significant decreases in survival between all treatments (Fig. 4D; Fdf = 2 = 22.19, P < 0.001).
Fig. 4.
Survival response [survival (%)] for the dominant under-ice copepod adults and nauplii to short-term (7 d) OA exposure experiments: adult Calanus spp. (A), adult O. similis (B), large (>250 μm) nauplii (C), and small (<60 μm) nauplii (D). Values are the mean ± 95% confidence interval. An asterisk represents a significant difference from the ambient (370 μatm) treatment, and a double asterisk represents a significant difference from the midwater (700 μatm) treatment.
Discussion
The 24-h zooplankton data presented here, which align with previous studies, show that adult calanoid copepods perform diel migrations under sea ice during the Arctic winter (8) but cease this synchronized migration under the Arctic midnight sun (5); instead, they undergo continuous individual movement up and down between the surface and >200 m, which results in little observed change in the median depth of the overall biomass (31, 32). Calanus spp. have previously been found throughout the water column depending on stage. In Arctic domains of the Norwegian Sea, progressively deeper migration appeared to occur with increasing calanoid stage, such that copepods at young calanoid stages (CI–CIII) were generally confined to the upper 30 m during both day and night, whereas CV stage copepods and adult females displayed the most significant migrations (33). Our data support this further, with the calanoid nauplii stages appearing to reside mainly in the upper 50 m throughout all our sampling points. In contrast, no evidence was found in our data of adult O. similis or their nauplii making similar migrations during the sampling period, because they were observed consistently throughout both 24-h sampling periods in the upper 50 m, and data from additional trawls to 200 m confirmed the lack of O. similis and nauplii below the surface waters (Fig. 2). Again, these observations align with previous summer Arctic studies showing O. similis is most prevalent in the UML, with abundance positively correlated to UML temperature (34). This behavior is also observed in Oithona spp. found in the Antarctic (e.g., ref. 35).
The associated under-ice water column carbonate chemistry data (Table 2) reveal that in the surface layers (0–50 m), pCO2 is relatively low (<330 μatm), with correspondingly high pH (pH 8.10; Fig. 3 A–C). As depth increases, the accumulation and remineralization of organic matter (36), stratification (37), and brine release (38) cause pCO2 to increase significantly to >550 μatm at about 100 m, with correspondingly low pH (pH 7.87), even during this late-winter period. This mid water column area of high pCO2 has previously only been reported during the summer period (3). Together with zooplankton data, this demonstrates that vertical migration through the halocline exposes adult Calanus to a daily pH range of >0.15 units and a daily pCO2 range of >140 μatm (Fig. 3 A–C). Remaining above the halocline exposes O. similis and nauplii to a much smaller average daily pH range of about 0.08 units and to a pCO2 range of about 73 μatm (Fig. 3 A–C and Table 2).
Table 2.
Experimental conditions for the three OA treatments (mean ± SD)
| Seawater parameter | Ambient CO2 | Mid-CO2 | High CO2 |
| Temperature, °C | −1.67 | −1.68 | −1.69 |
| Salinity | 30.89 ± 1.41 | 29.97 ± 0.59 | 29.38 ± 0.48 |
| DIC, μmol⋅kg−1 | 2,067.5 ± 2.3 | 2,183.1 ± 9.7 | 2,156.2 ± 12.8 |
| TA, μmol⋅kg−1 | 2,156.4 ± 9.3 | 2,201.9 ± 3.8 | 2,122.5 ± 29.9 |
| pH, total | 8.04 ± 0.03 | 7.80 ± 0.05 | 7.60 ± 0.09 |
| pCO2, μatm | 371 ± 26.7 | 698 ± 76.8 | 1,092 ± 202.8 |
| Ω Calcite | 1.82 ± 0.10 | 1.08 ± 0.12 | 0.67 ± 0.14 |
| Ω Aragonite | 1.13 ± 0.06 | 0.67 ± 0.08 | 0.41 ± 0.09 |
In situ seawater temperature (°C) was measured in the ice hole, and salinity, DIC, and TA were measured in the header tanks. Measured parameters were used to calculate, using CO2sys, the additional parameters: pH (total) in situ temperature (°C), pCO2, and saturation states for calcite (Ω Calcite) and aragonite (Ω Aragonite).
These experiences appear to be reflected in the results from the manipulated OA experiments: The large Calanus spp. were relatively robust to short-term exposures to manipulated OA conditions predicted for the year 2300, with no impact on survival observed. Conversely, O. similis adults and nauplii, as well as Calanus nauplii, which experience much narrower physicochemical gradients, had significantly lower survival and seemingly greater sensitivity to elevated pCO2. Although data for this species from other regions remains limited, studies to date do not suggest Oithona spp. undergo the diel vertical migrations of other larger copepod species but dominate surface waters (e.g., ref. 39). Oithona spp. are considered one of the most important and abundant copepods occurring globally within the epipelagic zone. Their occurrence in the surface waters means they have been exposed to relatively low levels of CO2 (high pH) evolutionarily; therefore, their relative sensitivity to changes in CO2 conditions warrants further investigation into the global impact of OA on these small copepods.
Further, to assess the wider relevance of our findings, we conducted a review of the current literature on OA responses of copepod species from other regions (incorporating only studies using a year 2100 OA relevant level of pH or pCO2) and compared these data with the existing knowledge of their vertical migration behaviors (summarized in Table 3). This clearly highlights that, as we found in the high Arctic, the species and life history stages that are known to exhibit vertical migration behavior are generally reported to be robust to predicted OA conditions. This literature review also reveals a general lack of studies on species that do not undergo diel vertical migrations, such as Oithona, and on small species, that are more difficult to maintain in laboratories. Further, there seems a lack of consideration for the environment from which these organisms, used in experiments, were obtained. The only other study of a nonvertically migrating copepod species that we found in the literature was that by Fitzer et al. (11) on the benthic copepod Tisbe, who found them to be sensitive to year 2100 relevant OA conditions over multiple generations; however, these responses were long-term responses rather than short-term responses to variability on a daily scale. Our finding for O. similis and those of Fitzer et al. (11) suggest that more OA studies should focus on species and/or life history stages with more restricted spatial ranges, and therefore potentially narrower extant natural pCO2 exposure conditions
Table 3.
Summary of previous studies on copepod OA responses and whether the species used are known to exhibit diel vertical migration
| Author | Species | pH range | Sensitivity to year 2100 level |
Adult DVM? | |||||
| EP | H | N | C | Adult | |||||
| This study | Calanus glacialis/hyperborealis | 8.04–7.60 | √ | x | Hays (51), Falk-Petersen et al. (52) | √ | |||
| This study | Oithona similis | 8.04–7.60 | √ | √ | Coyle and Pinchuk (53) | x | |||
| Weydmann et al. (54) | Calanus glacialis | 8.20–6.90 | x | Falk-Petersen et al. (55) | √ | ||||
| Fitzer et al. (56) | Tisbe battagliai | 8.06–7.67 | √ | √ | √ | Hicks and Coull (57) | x | ||
| Kurihara and Ishmatsu (58) | Acartia tsuensis | 8.23–7.31* | x | x | x | x | x | Sakaguchi et al. (59) | ? |
| Kurihara et al. (60) | Acartia steueri | 8.09–6.82* | x | x | No data | ? | |||
| Kurihara et al. (60) | Acartia erythraea | 8.09–6.82* | x | x | x | x | Tang et al. (61) | √ | |
| Vehmaa et al. (62) | Acartia bilifosa | 8.17–7.77 | x | White et al. (63) | √ | ||||
| Li and Gao (64) | Centropages tenuiremis | 8.18–7.83 | † | Boyd et al. (65) | √ | ||||
| Mayor et al. (66) | Calanus helgolandicus | 8.08–7.77 | x | x | Williams (67) | √ | |||
| McConville et al. (68) | Centropages typicus | 8.04–7.78 | x | x | White et al. (63) | √ | |||
| McConville et al. (68) | Temora longicornis | 8.04–7.78 | x | x | Harding et al. (69) | √ | |||
| Pedersen et al. (70) | Calanus finmarchicus | 8.20–6.85* | x | x | x | Falkenhaug et al. (71) | √ | ||
C, copepodite stages; EP, egg production; H, hatching; N, nauplii stages; x, no response to OA or does not exhibit diel vertical migration (DVM); √, response to OA or does exhibit DVM; ?, no data available.
No year 2100 pCO2 scenario was used in the cited experiment; however, no response was recorded for the more extreme experimental pCO2 scenario.
Physiological adjustment to conditions was observed, but no clear negative health impact was associated with this change.
The hypothesis that experience influences sensitivity could indeed explain the high level of variability found in previous investigations into the impacts of pCO2 on copepods, with some species (particularly early life stages) being more sensitive (e.g., refs. 11–13) and others, including temperate calanoids, appearing more resilient (e.g., refs. 14, 40). The influence of natural variation of physicochemical conditions has recently been studied in the copepod Arcartia tonsa along the upwelling off the southern coast of Chile (41), where a clear negative influence of low pH (pH 7.7) sea waters on copepod reproductive outcomes was observed. An additional study using much higher pCO2 levels than predicted for OA (deep ocean CO2 storage scenarios) but comparing high CO2 responses of copepods from different depths and regions (42) also supports the idea that experience, in terms of natural physicochemical conditions, influences the CO2 response, because the study clearly showed a greater short-term tolerance to high pCO2 in deeper water copepods than observed for shallower water species, which, again, aligns with our findings. Indeed, the two Calanus spp. investigated here, C. glacialis and C. hyperboreus, also have different life histories and vertical migrations. Although both are known to pass from at least 200 m up to the surface, thereby being exposed to the middepth high CO2, the larger C. hyperboreus is known to occur at depths >1,000–3,000 m (43), whereas the slightly smaller C. glacialis occurs at depths between 200 and 500 m (44). Therefore, the two species will experience different environmental regimes and, consequently, we would expect them to have differing sensitivities, with C. glacialis being more sensitive.
Underlying the size and behavioral differences between Calanus and Oithona are differences in their physiologies. Any shifts in physicochemical gradients in seawater can have a significant impact on gas exchange in small organisms (45) In pelagic copepods, gas exchange occurs across the integument and hindgut, and only larger adult Calanus species have a heart (46). The presence of a more developed cardiovascular system in Calanus species is likely related to the need for better internal CO2 transport to support their more active lifestyles (47). C. hyperboreus, for example, has a 13-fold smaller surface area-to-volume (SA/V) ratio than O. similis. A greater SA/V ratio increases CO2 diffusion rates; hence, smaller organisms generally have low extracellular pCO2 levels that reflect the external (seawater) pCO2 conditions that are maintained low by the large diffusion gradient, (i.e., if CO2 is produced internally, the lower seawater pCO2 allows rapid removal by diffusion). Larger, active organisms have higher extracellular pCO2 levels due to less efficient diffusion resulting from a smaller SA/V ratio, and hence often use alternative mechanisms to remove CO2. An increase in seawater pCO2 will therefore cause a greater relative change in extracellular pCO2 of smaller organisms than in larger organisms (48). Hence, smaller organisms, such as Oithona spp., are hypothesized to be more sensitive to OA than larger organisms.
Furthermore, crustaceans are generally able to buffer short-term exposure to high CO2 through increasing hemolymph HCO3− levels, but ionoregulating ability differs greatly between species and is energetically expensive (e.g., ref. 6). More active animals with higher natural extracellular pCO2 levels should already possess efficient ionoregulatory machinery for acid–base regulation for dealing with metabolic CO2; hence, such animals are proposed to be better placed to cope with any change in external pCO2 (48). Because adult calanoids actively undergo large vertical migrations, their acid–base regulatory physiology might be predicted to be more enhanced than that of O. similis, which does not undertake long-distance migrations (49). The physiological constraints for a small organism are likely to play a role in its behavior, and therefore in determining its sensitivity to OA.
Traditionally, larvae have been considered much poorer at acid–base regulation than their adult counterparts, possibly accounting for the high sensitivity to OA observed across our nauplii experiments, yet this remains poorly understood for most species. Nonmigrating nauplii stages that inhabit stable physiochemical conditions may simply not “waste” energetic investment in expensive, unnecessary ion regulation mechanisms, making them vulnerable to changes in conditions.
Our study clearly highlights the importance of understanding the in situ environmental conditions and variability, together with organism behavior and life history strategy, to assess the vulnerability and sensitivity of Arctic species to a rapidly changing environment. Short-term experiments cannot simply be scaled-up for century-scale responses of organisms to OA, but they do provide us with an understanding of a species scope for acclimation. Importantly, different populations, with local adaptation, may have different phenotypic plasticity underpinning their ability to perform under near-future pCO2 scenarios. Our findings imply that migratory zooplankton, by virtue of their daily exposure to a wide range of pCO2 conditions, might not require evolutionary adaptation to future pCO2 scenarios. In contrast, nonmigratory zooplankton are more likely to experience local extinction in the absence of evolutionary adaptation (50). Direct Arctic studies are therefore required to assess organism and species sensitivities rather than assuming that responses will be the same as those from tropical or midlatitude studies. Certainly, ubiquitous species in their adult form, living across a range of physicochemical conditions, are likely capable of surviving change, but the apparent bottleneck to long-term species survival is yet again found at the early life stage. Larvae of many marine organisms are released at very specific times to coincide with favorable environmental or food conditions, and we show here that even a short-term shift in the pCO2 conditions results in decreased survival of nauplii. Finally, the overall spatial range of pCO2 conditions will change into the future, thus exposing organisms to even greater values than the surface averages often used in experiments.
Supplementary Material
Acknowledgments
The authors thank the Ice Base and Geo Missions staff for assistance in carrying out fieldwork, and M. Cole, G. Cripps, Dr. L. Miller (Institute of Ocean Sciences, Canada), Dr. R. Wilson (University of Exeter, United Kingdom), and Dr. A. Atkinson (Plymouth Marine Laboratory, United Kingdom), as well as two anonymous referees, for discussion and comments. These data were collected as part of the Catlin Arctic Survey funded by Catlin Ltd. and coordinated by Geo Mission Ltd. C.N.L. was supported by Natural Environment Research Council (NERC) UK Fellowship NE/G014728/1. H.S.F. was supported by the PML Lord Kingsland Fellowship and was in receipt of the Ralph Brown Expedition Grant from the Royal Geographical Society. L.A.E. was supported by NERC’s National Centre for Earth Observation and received fieldwork funds from the World Wide Fund for Nature. G.C. was supported by Fisheries and Oceans Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315162110/-/DCSupplemental.
References
- 1.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319(5865):948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 2.Fabry VJ, McClintock JB, Mathis JT, Grebmeier JM. Ocean acidification at high latitudes: The bellweather. Oceanography (WASH D C) 2009;22(4):160–171. [Google Scholar]
- 3.Bates NR, Orchowska MI, Garley R, Mathis JT. Seasonal calcium carbonate undersaturation in shelf waters of the Western Arctic Ocean; how biological processes exacerbate the impact of ocean acidification. Biogeosciences Discussions. 2012;9:14255–14290. [Google Scholar]
- 4.Steinacher M, Joos F, Froelicher TL, Plattner GK, Doney SC. Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences. 2009;6(4):515–533. [Google Scholar]
- 5.Blachowiak-Samolyk K, et al. Arctic zooplankton do not perform diel vertical migration (DVM) during periods of midnight sun. Mar Ecol Prog Ser. 2006;308:101–116. [Google Scholar]
- 6.Whiteley NM. Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser. 2011;430:257–271. [Google Scholar]
- 7.Campbell RG, et al. Mesozooplankton prey preference and grazing impact in the western Arctic Ocean. Deep Sea Res Part 2 Top Stud Oceanogr. 2009;56(17):1274–1289. [Google Scholar]
- 8.Falk-Petersen S, Mayzaud P, Kattner G, Sargent JR. Lipids and life strategy of Arctic Calanus. Marine Biology Research. 2009;5(1):18–39. [Google Scholar]
- 9.Wassmann P, et al. Food webs and carbon flux in the Barents Sea. Prog Oceanogr. 2006;71(2-4):232–287. [Google Scholar]
- 10.Comeau S, Jeffree R, Teyssié J-L, Gattuso J-P. Response of the Arctic pteropod Limacina helicina to projected future environmental conditions. PLoS ONE. 2010;5(6):e11362. doi: 10.1371/journal.pone.0011362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzer SC, et al. Ocean acidification induces multi-generational decline in copepod naupliar production with possible conflict for reproductive resource allocation. J Exp Mar Biol Ecol. 2012;418:30–36. [Google Scholar]
- 12.Kurihara H, Ishimatsu A. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar Pollut Bull. 2008;56(6):1086–1090. doi: 10.1016/j.marpolbul.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Weydmann A, Soreide JE, Kwasniewski S, Widdicombe S. Influence of CO2-induced acidification on the reproduction of a key Arctic copepod Calanus glacialis. J Exp Mar Biol Ecol. 2012;428:39–42. [Google Scholar]
- 14.Mayor DJ, Everett NR, Cook KB. End of century ocean warming and acidification effects on reproductive success in a temperate marine copepod. J Plankton Res. 2012;34(3):258–262. [Google Scholar]
- 15.Mayor DJ, Matthews C, Cook K, Zuur AF, Hay S. CO2-induced acidification affects hatching success in Calanus finmarchicus. Mar Ecol Prog Ser. 2007;350:91–97. [Google Scholar]
- 16.Widdicombe S, Spicer JI. Predicting the impact of ocean acidification on benthic biodiversity: What can animal physiology tell us? J Exp Mar Biol Ecol. 2008;366(1-2):187–197. [Google Scholar]
- 17.Thomsen J, Casties I, Pansch C, Körtzinger A, Melzner F. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: Laboratory and field experiments. Glob Change Biol. 2013;19(4):1017–1027. doi: 10.1111/gcb.12109. [DOI] [PubMed] [Google Scholar]
- 18.Pane EF, Barry JP. Extracellular acid-base regulation during short-term hypercapnia is effective in a shallow-water crab, but ineffective in a deep-sea crab. Mar Ecol Prog Ser. 2007;334:1–9. [Google Scholar]
- 19.Seibel BA. Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J Exp Biol. 2011;214(Pt 2):326–336. doi: 10.1242/jeb.049171. [DOI] [PubMed] [Google Scholar]
- 20.Childress JJ, Seibel BA. Life at stable low oxygen levels: Adaptations of animals to oceanic oxygen minimum layers. J Exp Biol. 1998;201(Pt 8):1223–1232. doi: 10.1242/jeb.201.8.1223. [DOI] [PubMed] [Google Scholar]
- 21.Childress JJ. Effects of pressure, temperature and oxygen on oxygen-consumption rate of midwater copepod Gaussia princeps. Marine Biology. 1977;39(1):19–24. [Google Scholar]
- 22.Maas AE, Wishner KF, Seibel BA. Metabolic suppression in thecosomatous pteropods as an effect of low temperature and hypoxia in the eastern tropical North Pacific. Marine Biology. 2012;159(9):1955–1967. [Google Scholar]
- 23.Maas AE, Wishner KF, Seibel BA. The metabolic response of pteropods to acidification reflects natural CO2-exposure in oxygen minimum zones. Biogeosciences. 2012;9(2):747–757. [Google Scholar]
- 24.Dickson AG, Sabine CL, Christion JR. Guide to best practices for ocean CO2 measurements. North Pacific Marine Science Organisation (PICES) Special Publication. 2007;3:1–191. [Google Scholar]
- 25. Pierrot D, Lewis E, Wallace DWR (2006) MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105 (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN)
- 26.Mehrbach C, Culberso CH, Hawley JE, Pytkowic RM. Measurement of apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18(6):897–907. [Google Scholar]
- 27.Dickson AG, Millero FJ. A comparison of the equilibrium-constants for the dissociation of carbonic-acid in seawater media. Deep Sea Res A. 1987;34(10):1733–1743. [Google Scholar]
- 28.Dickson AG. Standard potential of the reaction—AgCl(s) + 1/2H2(g) = Ag(s) + HCl(aq) and the standard acidity constant of the ion HSO4− in synthetic sea-water from 273.15-k to 318.15-k. J Chem Thermodyn. 1990;22(2):113–127. [Google Scholar]
- 29. Lavigne H, Proye A, Gattuso JP (2009) Package seacarb [Laboratoire d'Oceanographie de Villefranche (LOV) France]
- 30.Jones EP, Anderson LG. On the origin of the chemical-properties of the arctic-ocean halocline. J Geophys Res Oceans. 1986;91(C9):759–767. [Google Scholar]
- 31.Cottier FR, Tarling GA, Wold A, Falk-Petersen S. Unsynchronized and synchronized vertical migration of zooplankton in a high arctic fjord. Limnol Oceanogr. 2006;51(6):2586–2599. [Google Scholar]
- 32.Wallace MI, Cottier FR, Brierley AS, Tarling GA. Modelling the influence of copepod behaviour on faecal pellet export at high latitudes. Polar Biol. 2013;36(4):579–592. [Google Scholar]
- 33.Dale T, Kaartvedt S. Diel patterns in stage-specific vertical migration of Calanus finmarchicus in habitats with midnight sun. ICES Journal of Marine Science. 2000;57(6):1800–1818. [Google Scholar]
- 34.Coyle KO, Pinchuk AI. Seasonal cross-shelf distribution of major zooplankton taxa on the northern Gulf of Alaska shelf relative to water mass properties, species depth preferences and vertical migration behavior. Deep Sea Res Part II Top Stud Oceanogr. 2005;52(1-2):217–245. [Google Scholar]
- 35.Atkinson A, Ward P, Murphy EJ. Diel periodicity of Subantarctic copepods: Relationships between vertical migration, gut fullness and gut evacuation rate. J Plankton Res. 1996;18(8):1387–1405. [Google Scholar]
- 36.Junge K, Eicken H, Deming JW. Bacterial Activity at -2 to -20 degrees C in Arctic wintertime sea ice. Appl Environ Microbiol. 2004;70(1):550–557. doi: 10.1128/AEM.70.1.550-557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmack E, Wassmann P. Food webs and physical-biological coupling on pan-Arctic shelves: Unifying concepts and comprehensive perspectives. Prog Oceanogr. 2006;71(2-4):446–477. [Google Scholar]
- 38. Miller LA, et al. (2011) Carbon dynamics in sea ice: A winter flux time series. J Geophys Res Oceans 116 C02028, 10.1029/2009JC006058.
- 39.Yamaguchi A, et al. Community and trophic structures of pelagic copepods down to greater depths in the western subarctic Pacific (WEST-COSMIC) Deep Sea Res Part I Oceanogr Res Pap. 2002;49(6):1007–1025. [Google Scholar]
- 40.McConville K, et al. Effects of elevated CO2 on the reproduction of two calanoid copepods. Mar Pollut Bull. 2013;73(2):428–434. doi: 10.1016/j.marpolbul.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Aguilera VM, Vargas CA, Manriquez PH, Navarro JM, Duarte CM. Low pH freshwater discharges drive spatial and temporal variations in life history traits of neritic copepod Acartia tonsa. Estuaries Coasts. 2013;36(5):1084–1092. [Google Scholar]
- 42.Watanabe Y, et al. Lethality of increasing CO2 levels on deep-sea copepods in the western North Pacific. Journal of Oceanography. 2006;62(2):185–196. [Google Scholar]
- 43.Hirche HJ, Niehoff B. Reproduction of the Arctic copepod Calanus hyperboreus in the Greenland Sea-field and laboratory observations. Polar Biol. 1996;16(3):209–219. [Google Scholar]
- 44.Tang KW, et al. Metazooplankton community structure, feeding rate estimates, and hydrography in a meltwater-influenced Greenlandic fjord. Mar Ecol Prog Ser. 2011;434:77–90. [Google Scholar]
- 45.Hofmann AF, Peltzer ET, Brewer PG. Kinetic bottlenecks to chemical exchange rates for deep-sea animals—Part 2: Carbon dioxide. Biogeosciences. 2013;10(4):2409–2425. [Google Scholar]
- 46.Mauchline J, Blaxter JHS, Southward AJ, Tyler PA. Advances in marine biology—The biology of calanoid copepods—Introduction. Adv Mar Biol. 1998;33:14–48. [Google Scholar]
- 47.Seibel BA, Drazen JC. The rate of metabolism in marine animals: Environmental constraints, ecological demands and energetic opportunities. Philos Trans R Soc Lond B Biol Sci. 2007;362(1487):2061–2078. doi: 10.1098/rstb.2007.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melzner F, et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeosciences. 2009;6(10):2313–2331. [Google Scholar]
- 49.Metz C. Seasonal-variation in the distribution and abundance of Oithona and Oncaea species (Copepoda, Crustacea) in the southeastern Weddell sea, Antarctica. Polar Biol. 1995;15(3):187–194. [Google Scholar]
- 50.Dam HG. Evolutionary adaptation of marine zooplankton to global change. Ann Rev Mar Sci. 2013;5:349–370. doi: 10.1146/annurev-marine-121211-172229. [DOI] [PubMed] [Google Scholar]
- 51.Hays GC. Diel vertical migration behavior of Calanus hyperboreus at temperate latitudes. Mar Ecol Prog Ser. 1995;127(1-3):301–304. [Google Scholar]
- 52.Falk-Petersen S, Mayzaud P, Kattner G, Sargent JR. Lipids and life strategy of Arctic Calanus. Marine Biology Research. 2009;5(1):18–39. [Google Scholar]
- 53.Coyle KO, Pinchuk AI. Seasonal cross-shelf distribution of major zooplankton taxa on the northern Gulf of Alaska shelf relative to water mass properties, species depth preferences and vertical migration behavior. Deep Sea Res Part II Top Stud Oceanogr. 2005;52(1-2):217–245. [Google Scholar]
- 54.Weydmann A, Soreide JE, Kwasniewski S, Widdicombe S. Influence of CO2-induced acidification on the reproduction of a key Arctic copepod Calanus glacialis. J Exp Mar Biol Ecol. 2012;428:39–42. [Google Scholar]
- 55.Falk-Petersen S, et al. Vertical migration in high Arctic waters during autumn 2004. Deep Sea Res Part II Top Stud Oceanogr. 2008;55(20-21):2275–2284. [Google Scholar]
- 56.Fitzer SC, et al. Ocean acidification induces multi-generational decline in copepod naupliar production with possible conflict for reproductive resource allocation. J Exp Mar Biol Ecol. 2012;418:30–36. [Google Scholar]
- 57.Hicks GRF, Coull BC. The ecology of marine meiobenthic harpacticoid copepods. Oceanography and Marine Biology: An Annual Review. 1983;21:67–175. [Google Scholar]
- 58.Kurihara H, Ishimatsu A. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar Pollut Bull. 2008;56(6):1086–1090. doi: 10.1016/j.marpolbul.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 59.Sakaguchi SO, Ueda H, Ohtsuka S, Soh HY, Yoon YH. Zoogeography of planktonic brackish-water calanoid copepods in western Japan with comparison with neighboring Korean fauna. Plankton and Benthos Research. 2011;6(1):18–25. [Google Scholar]
- 60.Kurihara H, Shimode S, Shirayama Y. Effects of raised CO2 concentration on the egg production rate and early development of two marine copepods (Acartia steueri and Acartia erythraea) Mar Pollut Bull. 2004;49(9-10):721–727. doi: 10.1016/j.marpolbul.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Tang KW, Chen QC, Wong CK. Diel vertical migration and gut pigment rhythm of Paracalanus parvus, P. crassirostris, Acartia erythraea and Eucalanus subcrassus (Copepoda, Calanoida) in Tolo Harbour, Hong Kong. Hydrobiologia. 1994;292-293:389–396. [Google Scholar]
- 62.Vehmaa A, Brutemark A, Engström-Öst J. Maternal effects may act as an adaptation mechanism for copepods facing pH and temperature changes. PLoS ONE. 2012;7(10):e48538. doi: 10.1371/journal.pone.0048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White HH, Heaton JS, Schmitz KB. Vertical migration of Centropagus typicus (Cpoepoda) in Chesapeake Bay, with some thoughts on migration studies. Estuaries. 1979;2(1):61–63. [Google Scholar]
- 64.Li W, Gao K. A marine secondary producer respires and feeds more in a high CO2 ocean. Mar Pollut Bull. 2012;64(4):699–703. doi: 10.1016/j.marpolbul.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 65.Boyd CM, Smith SL, Cowles TJ. Grazing patterns of copepods in the upwelling system off Peru. Limnol Oceanogr. 1980;25(4):583–596. [Google Scholar]
- 66.Mayor DJ, Everett NR, Cook KB. End of century ocean warming and acidification effects on reproductive success in a temperate marine copepod. J Plankton Res. 2012;34(3):258–262. [Google Scholar]
- 67.Williams R, Conway DVP. Vertical-distribution, and seasonal and diurnal migration of Calanus helgolandicus in the Celtic sea. Marine Biology. 1984;79(1):63–73. [Google Scholar]
- 68.McConville K, et al. Effects of elevated CO2 on the reproduction of two calanoid copepods. Mar Pollut Bull. 2013;73(2):428–434. doi: 10.1016/j.marpolbul.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Harding GC, Vass WP, Hargrave BT, Pearre S. Diel vertical movements and feeding-activity of zooplankton in St Georges Bay, NS, using net tows and a newly developed passive trap. Can J Fish Aquat Sci. 1986;43(5):952–967. [Google Scholar]
- 70.Pedersen SA, Hansen BH, Altin D, Olsen AJ. Chronic exposure of the North Atlantic copepod Calanus finmarchicus (Gunnerus 1770) to CO2-acidified seawater; effects on survival, growth and development. Biogeosciences Discussions. 2013;10:5273–5300. [Google Scholar]
- 71.Falkenhaug T, Tande KS, Semenova T. Diel, seasonal and ontogenetic variations in the vertical distributions of four marine copepods. Mar Ecol Prog Ser. 1997;149(1-3):105–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.