Significance
Inhibitory synaptic transmission controls neuronal network activity, and its aberrant function is involved in many brain disorders, including schizophrenia, epilepsy, or mental retardation. We identified an as yet unknown mechanism by which the small GTPase TC10 determines the strength of inhibitory neurotransmission by activating collybistin, which in turn recruits the scaffold protein gephyrin and GABAA receptors to inhibitory postsynapses.
Keywords: RhoQ, Cdc42, postsynaptic scaffold, synaptogenesis, neuroligin
Abstract
In many brain regions, gephyrin and GABAA receptor clustering at developing inhibitory synapses depends on the guanine nucleotide exchange factor collybistin (Cb). The vast majority of Cb splice variants contain an autoinhibitory src homology 3 domain, and several synaptic proteins are known to bind to this SH3 domain and to thereby activate gephyrin clustering. However, many functional GABAergic synapses form independently of the known Cb-activating proteins, indicating that additional Cb activators must exist. Here we show that the small Rho-like GTPase TC10 stimulates Cb-dependent gephyrin clustering by binding in its active, GTP-bound state to the pleckstrin homology domain of Cb. Overexpression of a constitutively active TC10 variant in neurons causes an increase in the density of synaptic gephyrin clusters and mean miniature inhibitory postsynaptic current amplitudes, whereas a dominant negative TC10 variant has opposite effects. The enhancement of Cb-induced gephyrin clustering by GTP-TC10 does not depend on the guanine nucleotide exchange activity of Cb but involves an interaction that resembles reported interactions of other small GTPases with their effectors. Our data indicate that GTP-TC10 activates the major src homology 3 domain-containing Cb variants by relieving autoinhibition and thus define an alternative GTPase-driven signaling pathway in the genesis of inhibitory synapses.
Chemical synaptic transmission between neurons requires the tight packing of ionotropic neurotransmitter receptors in the postsynaptic plasma membrane. Core components of many inhibitory GABAergic postsynapses are the cell adhesion protein neuroligin 2 (NL2), the scaffolding protein gephyrin, the guanine nucleotide exchange factor (GEF) collybistin (Cb), and GABAA receptors (GABAARs) (1, 2). The assembly of such GABAergic postsynapses is triggered by the interaction of NL2 with the src homology 3 (SH3) domain of Cb. This leads to the activation of Cb, which is otherwise autoinhibited by intramolecular interactions of its SH3 domain with the Dbl homology (DH) and pleckstrin homology (PH) domains, followed by membrane recruitment of Cb and synaptic accumulation of gephyrin and GABAARs (3). However, this NL2/Cb/gephyrin/GABAAR interaction cascade cannot account for the formation of all GABAergic synapses, because in the hippocampus of NL2 KO mice gephyrin and GABAAR clusters are lost only from perisomatic regions of CA1 pyramidal neurons (3). In contrast, deletion of Cb leads to a loss of gephyrin from both perisomatic and dendritic postsynapses (4). Thus, the formation of a substantial subset of GABAergic postsynapses must be regulated by Cb-interacting proteins other than NL2.
Another class of Cb interaction partners with a potential role in gephyrin clustering are small Rho-like GTPases. They regulate many fundamental cellular processes, including actin cytoskeleton rearrangements (5), and the actin cytoskeleton plays an important role in the formation of inhibitory postsynapses, particularly at early stages of synapse formation (6, 7). The small GTPase Cdc42 is an established Cb substrate (8–10), and a recent analysis of 12 Rho-like GTPases identified Cdc42 as the only family member that can be activated in vitro by the human Cb ortholog hPem2 (11). However, Cdc42 expression is not required for gephyrin and GABAAR clustering at postsynapses, indicating that Cb may regulate cytoskeleton remodeling by activating other Rho-like GTPases (10). The small GTPase most closely related to Cdc42 is TC10. Its sequence [67.4% amino acid identity (12)] and structure (13) are similar to those of Cdc42, it shares common cellular functions and effectors with Cdc42 (14), and profilin, an actin and gephyrin binding protein (15, 16), is an effector of TC10 (14). In contrast to Cdc42, which is ubiquitously expressed in the mammalian brain, the expression of TC10 is limited to specific areas, including the CA1 region of the hippocampus (17), where the most prominent reduction in gephyrin and GABAAR clustering is observed in Cb KO mice (4). Here we provide evidence for an effector-type binding of GTP-TC10 to the PH domain of Cb that results in Cb activation, triggers synaptic gephyrin clustering, and enhances GABAergic neurotransmission.
Results
TC10 Stimulates Cb-Dependent Gephyrin Redistribution in COS-7 Cells.
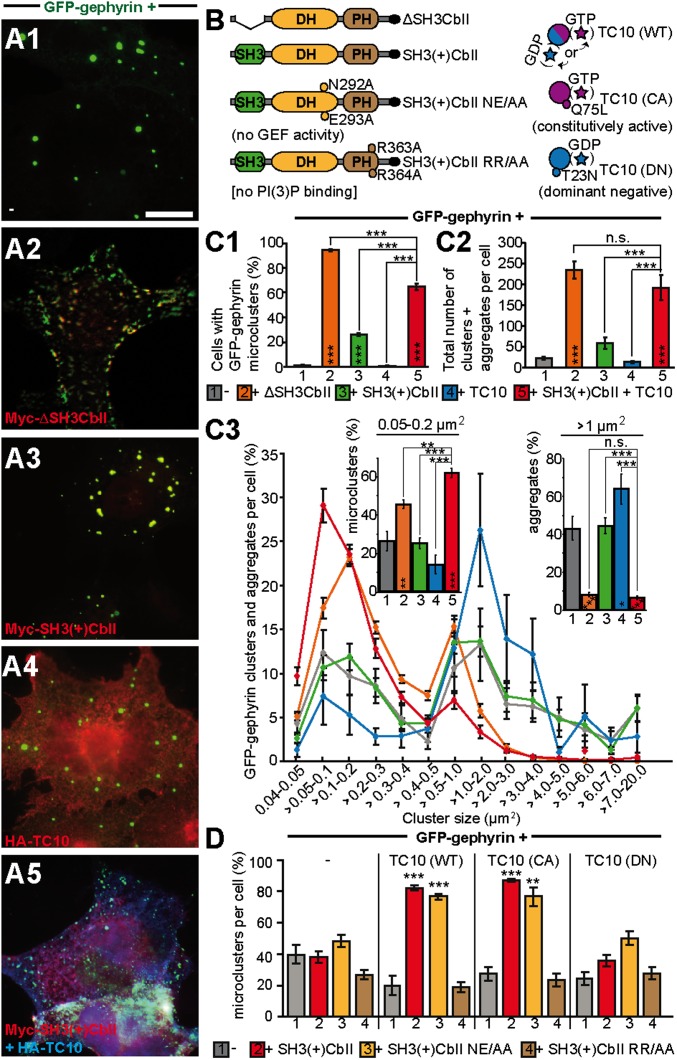
To test whether TC10 functions in Cb activation and gephyrin clustering, we first used COS-7 cells (18), in which recombinant gephyrin forms large intracellular aggregates that are redistributed into membrane-associated microclusters upon coexpression of a constitutively active Cb-splice variant lacking the N-terminal SH3 domain (ΔSH3CbII; Fig. 1B). However, mammalian isoforms of Cb detected in vivo contain an SH3 domain that renders the protein inactive in this assay, resulting in the preferential accumulation of gephyrin in cytoplasmic aggregates (18, 19). Different Cb-interacting proteins, such as NL2, NL4, or the α2-subunit of GABAARs, activate the intrinsically inactive Cb-splice variant SH3(+)CbII and enhance the formation of gephyrin microclusters at the plasma membrane (3, 20, 21). Cotransfection of GFP-gephyrin together with Myc-SH3(+)CbII and HA-TC10 in COS-7 cells also generated gephyrin microclusters (Fig. 1 A5), similar to cells expressing ΔSH3CbII and GFP-gephyrin (Fig. 1 A2). In contrast, GFP-gephyrin alone or together with either TC10 or SH3(+)CbII produced predominantly intracellular gephyrin aggregates (Fig. 1 A1, A3, and A4). 3D reconstructions of image stacks of cells coexpressing TC10, GFP-gephyrin, and SH3(+)CbII confirmed that most of the gephyrin microclusters formed were located at the plasma membrane (Fig. S1). The fraction of cells displaying GFP-gephyrin microclusters (>50 GFP-positive puncta per cell) upon coexpression of SH3(+)CbII was significantly higher in the presence of TC10 (64.8% ± 2.7%) compared with cells expressing either GFP-gephyrin alone (1.08% ± 0.54%), only GFP-gephyrin and TC10 (0.56% ± 0.28%), or only GFP-gephyrin and SH3(+)CbII (26.17% ± 1.3%) (Fig. 1 C1). In cells expressing the ΔSH3CbII variant, almost all cells contained peripheral GFP-gephyrin microclusters (94.35% ± 0.72%). The mean total number of gephyrin clusters and aggregates per cell did not differ significantly between cells expressing GFP-gephyrin and ΔSH3CbII (234.57 ± 20.87) and cells expressing GFP-gephyrin, SH3(+)CbII, and TC10 (192.37 ± 30.24), whereas it was much lower for cells coexpressing GFP-gephyrin and SH3(+)CbII only (58.2 ± 13.08) (Fig. 1 C2). Additionally, the distribution of cluster sizes per cell was similar in cells double-transfected with GFP-gephyrin and ΔSH3CbII and cells triple-transfected with GFP-gephyrin, SH3(+)CbII, and TC10, with the majority of clusters ranging between 0.04 and 0.4 µm2 (Fig. 1 C3). In contrast, in GFP-gephyrin, GFP-gephyrin plus SH3(+)CbII, and GFP-gephyrin plus TC10 transfected cells, aggregates (>1 µm2) consistently outnumbered microclusters (≤0.4 µm2; Fig. 1 C3). Quantification of the relative fractions of aggregates and small (0.05–0.2 µm2) microclusters confirmed that TC10 coexpression shifts the size of gephyrin clusters toward lower values (Fig. 1 C3, Insets). These results indicate that TC10 potently stimulates gephyrin recruitment to submembraneous microclusters, likely by activating SH3(+)CbII.
Fig. 1.
TC10 stimulates Cb-dependent gephyrin microcluster formation in COS-7 cells. (A1–A5) Images of COS-7 cells transfected as indicated. GFP-gephyrin accumulates in large cytoplasmic aggregates when expressed alone (A1) or together with Myc-SH3(+)CbII (A3) or HA-TC10 (A4). In the presence of Myc-ΔSH3CbII (A2), GFP-gephyrin forms microclusters at the plasma membrane. Similarly, HA-TC10 and Myc-SH3(+)CbII jointly trigger GFP-gephyrin microcluster formation (A5). (Scale bar, 10 µm.) (B) Schematic representation of the Cb splice variants and mutants, as well as of the TC10 WT and mutants, used in this study. (C1) Percentages of GFP-gephyrin (co)-transfected cells classified as displaying GFP-gephyrin microclusters (>50 puncta per cell; n = 3 independent transfections, n = 321–428 cells). Significance levels compared with cells transfected with GFP-gephyrin only (gray bar) are shown within the bars. (C2) Total numbers of GFP-gephyrin clusters and aggregates from images of transfected COS-7 cells (n = 14–34 transfected cells per transfection condition). Significance indicated as in C1. (C3) Gephyrin puncta counted in C2 were binned according to their size (n = 14–34 cells). (Insets) Relative fractions of small microclusters (0.05–0.2 µm2; Left) and aggregates (>1 µm2; Right). (D) Percentages of microclusters (0.04–0.4 µm2) per cell in COS-7 cells transfected as indicated (n = 6–34 cells). Statistical significance was tested between the conditions without coexpression of HA-TC10 (first four columns) and those in the presence of TC10 (WT, CA, or DN).
It has been previously shown that a ΔSH3CbII NE232-233AA mutant lacks catalytic activity toward Cdc42 (10). N232 within the DH domain of ΔSH3CbII forms a hydrogen bond with D65 of Cdc42 (9) located within the highly conserved YDRLRPL switch II-α2 motif (13). The corresponding residue of TC10 within its YDRLRPL motif is D79. To test whether TC10 stimulation of SH3(+)CbII-mediated gephyrin clustering requires GEF activity, we coexpressed the corresponding SH3(+)CbII NE292-293AA (NE/AA; Fig. 1B) with TC10 and GFP-gephyrin. This resulted in a significantly higher fraction of cells displaying gephyrin microclusters compared with cells lacking recombinant TC10 (76.98% ± 1.87% vs. 48.65% ± 3.9%, respectively; Fig. 1D and Fig. S2A). In contrast, a PH domain mutant unable to bind phosphatidyl-inositol-3-phosphate [PI(3)P], SH3(+)CbII RR363-364AA [RR/AA; Fig. 1B; RR303-304AA in ΔSH3CbII (10)], failed to generate microclusters upon TC10 coexpression (Fig. 1D and Fig. S2A). These results corroborate previous findings that Cb binding to PI(3)P is essential for gephyrin microcluster formation, whereas its GEF activity is dispensable (10, 22).
Enhanced microcluster formation by the SH3(+)CbII isoform or its GEF-deficient NE/AA mutant, but not by the RR/AA mutant, was also observed when Cdc42 instead of TC10 was coexpressed with GFP-gephyrin in COS-7 cells (Fig. S3). This is consistent with a previous report showing a positive effect of Cdc42 on gephyrin clustering (23) and further supports our conclusion that the effects of Cb on gephyrin redistribution do not depend on its GEF activity but involve interactions with a GTPase and PI(3)P.
GTP-TC10 Is Required for the Enhancement of Cb-Dependent Gephyrin Microcluster Formation.
To study how TC10 stimulates Cb-mediated gephyrin microcluster formation in COS-7 cells, we coexpressed GFP-gephyrin and SH3(+)CbII together with either the constitutively active GTP-bound TC10 Q75L mutant (TC10 CA; Fig. 1B) or the dominant-negative GDP-bound T23N mutant (TC10 DN; Fig. 1B) (24). TC10 CA increased the fraction of cells with gephyrin microclusters (87.53% ± 1.24%; Fig. 1D and Fig. S2B), whereas TC10 DN had no effect (35.74% ± 3.64%) compared with control cells expressing only GFP-gephyrin and SH3(+)CbII (37.89% ± 3.77%). Thus, GTP-bound TC10 is required to enhance Cb-dependent gephyrin recruitment to the plasma membrane.
Cb Induces GTP-TC10 Accumulation in Vivo.
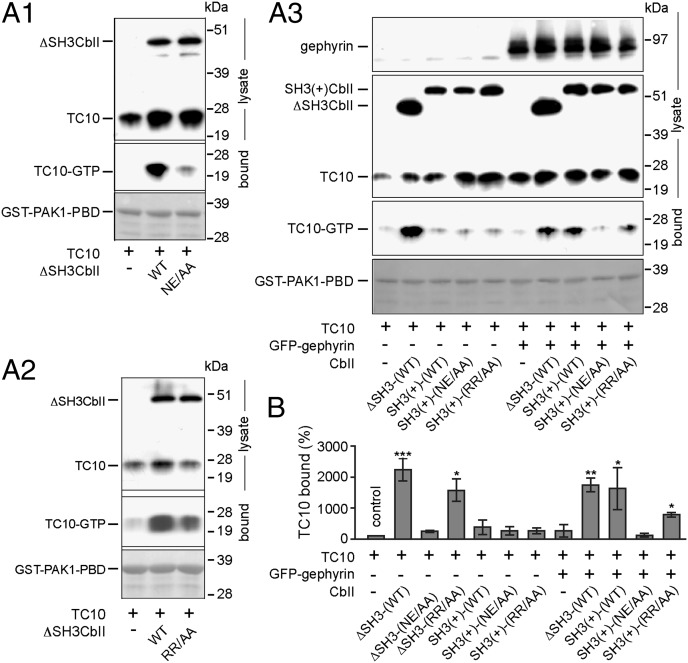
Because our results indicated a potential interaction of TC10 with Cb, we tested whether TC10 can be activated by Cb using an in vivo assay described for Cb-mediated Cdc42 activation (10). TC10 was expressed either alone or together with ΔSH3CbII or SH3(+)CbII in HEK 293 cells, and after cell lysis the amount of activated TC10 was determined by cosedimentation with immobilized GST-PAK1-PBD, a known effector that specifically binds GTP-bound GTPases (12). GTP-TC10 binding to PAK1-PBD was markedly increased in extracts prepared from ΔSH3CbII-coexpressing cells compared with cells expressing TC10 alone (Fig. 2 A1 and B). Coexpression of the GEF-deficient ΔSH3CbII NE/AA mutant strongly reduced the amount of GTP-TC10 bound compared with WT ΔSH3CbII (Fig. 2 A1 and B), showing that the effector cosedimentation assay is effective in detecting a Cb-induced generation of GTP-TC10. Notably, GTP-TC10 levels did not differ significantly between extracts from cells coexpressing either ΔSH3CbII WT or the ΔSH3CbII RR/AA mutant (Fig. 2 A2 and B). Thus, binding of Cb to lipids is not a prerequisite for GTP-TC10 formation.
Fig. 2.
ΔSH3CbII and SH3(+)CbII differentially activate TC10 in nonneuronal cells. (A1–A3) HEK 293 cells were transfected with Myc-TC10 either alone (-) or together with the indicated HA- (A1) or Myc-tagged (A2 and A3) Cb constructs in the absence (A1 and A2) or presence (A3, Top, last 5 lanes) of GFP-gephyrin. Cell lysates were used for cosedimentation with immobilized GST-PAK1-PBD. GTP-bound TC10 was detected by Western blotting with an anti-Myc antibody. MemCode staining (Bottom) was used to confirm that equal amounts of GST-PAK1-PBD had been added to each lysate. (B) Relative band intensities of TC10 bound to GST-PAK1-PBD (n = 3–4 independent experiments). Statistical significance was compared with Myc-TC10 expressed alone (first bar).
Previous studies have shown that SH3(+)CbII and ΔSH3CbII activate Cdc42 in cells, indicating that the difference in gephyrin clustering activity observed between these isoforms is unrelated to their enzymatic activity (ref. 23 and Fig. S4 A1 and A2). However, in our GST-PAK1-PBD cosedimentation experiments coexpression of SH3(+)CbII WT or its NE/AA and RR/AA mutants with TC10 did not significantly increase GTP-TC10 levels compared with control expression of TC10 alone (Fig. 2 A3 and B). Because in TC10-expressing COS-7 cells SH3(+)CbII had enhanced GFP-gephyrin clustering activity (Fig. 1), we triple-transfected cells with Myc-TC10, HA-CbII, and GFP-gephyrin cDNAs. In cells coexpressing GFP-gephyrin, GTP-TC10 was accumulated by both Cb isoforms (SH3+ and ΔSH3) and the SH3(+)CbII RR/AA mutant, but not by the NE/AA mutant (Fig. 2 A3 and B). These results show that the SH3 domain prevents the activation of Cb by TC10 only under specific cellular conditions.
We next used an in vitro method (11) to determine the GEF activities of ΔSH3CbII and SH3(+)CbII toward TC10. Using purified proteins, we spectroscopically determined the rate of nucleotide exchange of GTPase-bound fluorescent methylanthraniloyl-GDP (mant-GDP) by unlabeled GDP in the presence or absence of ΔSH3CbII or SH3(+)CbII. Confirming results reported for human Cb (11), both CbII isoforms acted as GEFs for Cdc42 but not for TC10 (Fig. S4B). SDS/PAGE of samples taken at the end of the in vitro exchange reaction showed that the GEF activity of SH3(+)CbII toward Cdc42 was not due to proteolytic cleavage of the SH3 domain during incubation (Fig. S4C). Thus, the SH3 domain does not affect the GEF activity of Cb toward Cdc42. In agreement with this result, both Cb isoforms activated Cdc42 in vivo to similar extents and in the absence of recombinant GFP-gephyrin (Fig. S4 A1 and A2).
GTP-TC10 Binds Preferentially to the PH Domain of Cb.
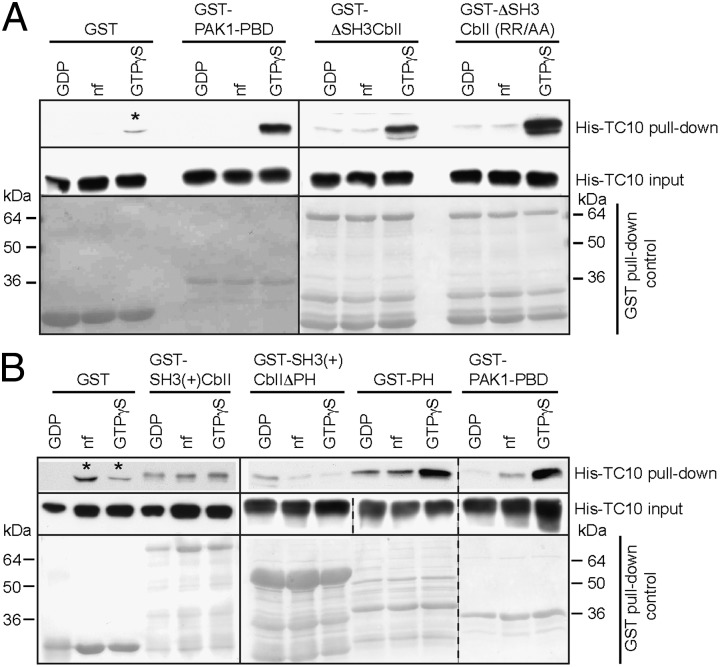
Because the SH3(+)CbII NE/AA GEF-deficient mutant generated gephyrin microclusters in the presence of TC10, TC10 seems to mediate gephyrin clustering through interactions that are unrelated to the catalytic activity of Cb. In binding assays with purified recombinant proteins [GST-CbII coupled to Sepharose beads and His-tagged TC10 preloaded with GDP, preloaded with GTPγS or trapped in its nucleotide-free (nf) state], we found a strong interaction of ΔSH3CbII with GTPγS-bound TC10, and only weak interactions with the GDP-bound or nf forms of the GTPase (Fig. 3A). Control incubations with immobilized GST-PAK1-PBD and GST confirmed the specificity of this binding (Fig. 3A). The ΔSH3CbII RR/AA mutant displayed similar binding to GTPγS-TC10 as the WT protein, indicating that the two arginine residues in the PH domain of Cb required for membrane recruitment are not involved in TC10 binding (Fig. 3A).
Fig. 3.
GTP-TC10 interacts with the PH domain of Cb. (A) Purified His-TC10, either nf or preloaded with GDP or GTPγS, was incubated with the indicated recombinant proteins bound to glutathione-Sepharose beads. Bound His-TC10 was detected by Western blotting. Note that both GST-ΔSH3CbII WT and the RR/AA mutant interacted preferentially with GTPγS-preloaded TC10, as did GST-PAK1-PBD but not GST (Top). (Middle) Similar amounts of His-TC10 were included in all incubations; (Bottom) respective MemCode stainings indicating comparable amounts of the GST-tagged bait proteins used. (B) His-TC10 was incubated with the indicated recombinant proteins bound to glutathione-Sepharose beads. Western blot analysis of the proteins bound revealed a faint interaction of SH3(+)CbII with TC10 that was similar for GDP, GTPγS, or nf conditions (Top). Deletion of the PH domain [GST-SH3(+)CbIIΔPH] strongly reduced the interaction with GTPγS-TC10, whereas the isolated PH domain (GST-PH) displayed preferential binding to TC10 in its GTP-bound state, as did GST-PAK1-PBD but not GST (Top). Asterisks in A and B indicate nonspecifically stained bands with a different migration pattern.
To map the TC10 binding site on Cb, we performed binding assays with the SH3(+)CbII variant and truncated proteins. GST-SH3(+)CbII bound to TC10, but with less preference for a specific nucleotide-loaded state of TC10 (Fig. 3B). A mutant lacking the PH domain [GST-SH3(+)CbIIΔPH] also bound TC10, but predominantly in its GDP-bound form. The PH domain alone (GST-PH) displayed a very strong interaction with GTPγS-TC10, comparable to that of GST-ΔSH3CbII (Fig. 3A) and PAK1-PBD (Fig. 3B). These results indicate that the GTP-TC10 binding site of Cb is localized within the PH domain.
GTP-TC10 Promotes Postsynaptic Gephyrin Clustering.
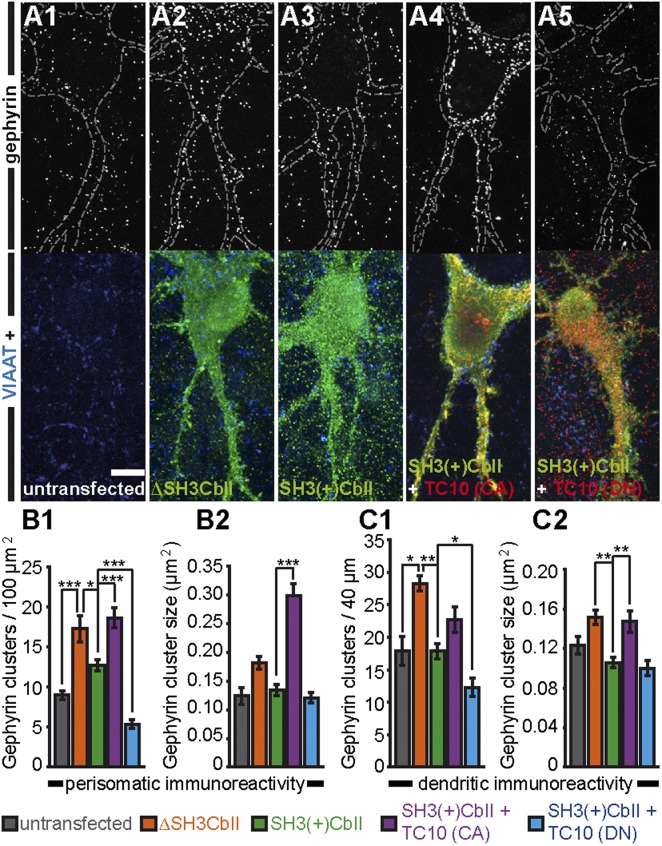
Expression of TC10 in neurons is restricted to some brain areas, including the hippocampus (ref. 17 and Fig. S5). To determine whether TC10 enhances Cb-dependent gephyrin clustering in neurons, we transfected cultured rat hippocampal neurons at days in vitro (DIV) 4 with Myc-tagged ΔSH3CbII or SH3(+)CbII either alone (Fig. 4 A2 and A3) or together with the HA-TC10 CA or DN mutants (Fig. 4 A4 and A5). Cells were fixed at DIV 14 and stained with gephyrin-, vesicular inhibitory amino acid transporter (VIAAT)-, and tag-specific antibodies (Fig. 4 A1–A5 and Fig. S6). The densities, but not sizes, of perisomatic and dendritic gephyrin clusters in ΔSH3CbII-expressing neurons were significantly increased compared with untransfected neurons (Fig. 4 A1, A2, and B1–C2). In contrast, the average cluster densities and sizes of gephyrin puncta in neurons expressing SH3(+)CbII were comparable to those of untransfected neurons (Fig. 4 A3 and B1–C2). Coexpression of SH3(+)CbII and TC10 CA led to significant increases in perisomatic gephyrin cluster densities and sizes, as well as in dendritic gephyrin cluster sizes, compared with neurons expressing SH3(+)CbII alone (Fig. 4 A3, A4, and B1–C2). In contrast, in TC10 DN and SH3(+)CbII cotransfected cells, both perisomatic and dendritic gephyrin cluster densities were significantly reduced (Fig. 4 A5 and B1–C2). Quantification of the densities of VIAAT immunoreactive puncta on the perisomatic or dendritic regions of neurons expressing SH3(+)CbII alone or together with TC10 CA or DN revealed no significant differences between groups (Fig. S6), indicating that the numbers of inhibitory nerve terminals were not changed upon TC10 overexpression. Thus, GTP-TC10 stimulates gephyrin clustering in neurons, likely by facilitating SH3(+)CbII-dependent gephyrin redistribution, whereas GDP-TC10 impairs gephyrin recruitment to inhibitory postsynaptic sites.
Fig. 4.
TC10 activity enhances SH3(+)CbII-mediated clustering of gephyrin in cultured hippocampal neurons. (A1–A5) Cultured rat hippocampal neurons were cotransfected at DIV 4 with the empty pcDNA3 vector and either the Myc-ΔSH3CbII (A2) or Myc-SH3(+)CbII (A3) cDNAs, or cotransfected with Myc-SH3(+)CbII and HA-TC10 CA (A4) or HA-TC10 DN (A5), respectively; untransfected cultures (A1) served as control. At DIV 14, the cells were fixed and immunostained for gephyrin, VIAAT, HA, and Myc. (Upper) Endogenous gephyrin immunoreactivity; (Lower) corresponding costainings. Note the increase in punctate gephyrin immunoreactivity in neurons cotransfected with TC10 CA (A4) and reduced gephyrin clustering in neurons coexpressing TC10 DN (A5) compared with neurons expressing Myc-SH3(+)CbII alone (A3). (Scale bar, 10 µm for A1–A5.) Dotted lines in A1–A5 indicate the borders of transfected cells. (B1–C2) Bar diagrams of (B1) perisomatic gephyrin cluster densities per 100 µm2 surface area and (B2) average sizes of perisomatic gephyrin clusters (n = 258–1,344 clusters analyzed), (C1) gephyrin immunoreactive clusters per 40 µm dendrite length and (C2) average sizes of dendritic gephyrin clusters (n = 179–590 clusters analyzed). Bars correspond to values obtained from the perisomatic surface area and one randomly selected second-order dendrite (60–100 µm distal to the soma) per neuron, respectively (n = 10–28 cells, n = 3 independent experiments).
TC10 Regulates GABAergic Postsynaptic Strength.
To determine the physiological consequences of TC10 and Cb coexpression on inhibitory neurotransmission, we recorded GABAergic miniature inhibitory postsynaptic currents (mIPSCs) in rat hippocampal neurons coexpressing GFP and Myc-SH3(+)CbII without or with HA-TC10 CA or DN, respectively. Mean mIPSC amplitudes were significantly (32.9%) increased in TC10 CA-coexpressing cells (79.5 ± 3.66 pA) compared with control cells expressing GFP and Myc-SH3(+)CbII only (53.36 ± 2.59 pA; Fig. 5 A1 and A2), whereas no significant change was found in TC10 DN-coexpressing cells (51.8 ± 2.75 pA). Conversely, the mean mIPSC frequency was reduced in TC10 DN- (33.6%; 2.87 ± 0.3 Hz vs. 4.32 ± 0.35 Hz in control cells) but not TC10 CA-coexpressing neurons (3.95 ± 0.45 Hz; Fig. 5 A1 and A3). The larger mean mIPSC amplitude found upon TC10 CA coexpression can be attributed to an increased number of functional postsynaptic GABAARs, whereas the reduction in mean mIPSC frequency seen in TC10 DN-expressing neurons may reflect a loss of inhibitory receptors at a subset of postsynapses. In line with this interpretation, the mean amplitudes of evoked IPSCs (eIPSCs) induced by rapidly raising the extracellular K+ concentration to 50 mM using our fast application system were significantly (38.2%) increased in TC10 CA-coexpressing neurons (8.45 ± 0.72 nA) compared with controls (6.2 ± 0.63 nA), whereas in TC10 DN-coexpressing cells they appeared mildly, albeit not significantly, reduced (5.2 ± 0.6 nA; Fig. 5 B1 and B2). Furthermore, exogenous application of GABA, which activates both synaptic and extrasynaptic GABAARs, shifted the distribution of GABA-induced current amplitudes toward larger values in TC10 CA-coexpressing neurons, whereas in TC10 DN-coexpressing neurons the corresponding amplitudes were reduced (Fig. S7).
Fig. 5.
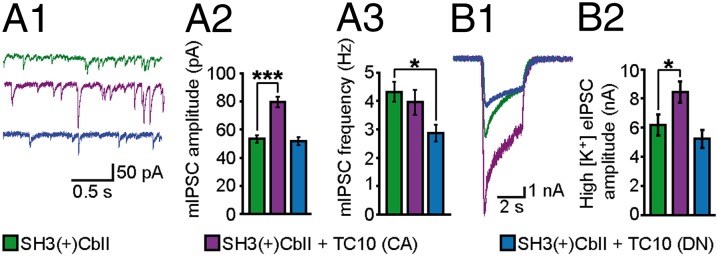
TC10 activity enhances GABAergic mIPSCs in cultured hippocampal neurons. (A1) Representative traces of mIPSCs recorded from neurons coexpressing GFP and Myc-SH3(+)CbII without (green) or together with HA-TC10 CA (purple) or HA-TC10 DN (blue), respectively. (A2 and A3) mIPSC mean amplitudes (A2) and frequencies (A3) of neurons transfected as described in A1. Note significant increase in mIPSC amplitude in neurons coexpressing TC10 CA compared with control cells. In contrast, coexpression of TC10 DN led to a significant decrease in the mean mIPSC frequency. (B1 and B2) Representative recordings (B1) and mean amplitudes (B2) of high [K+] eIPSCs from neurons transfected as described in A1. Data in A2 and A3 were obtained from n = 96–135 neurons, and in B2 from n = 41–55 neurons. n = 3–4 independent experiments.
Discussion
In the present study we identify the small Rho-like GTPase TC10 as a regulator of Cb-mediated gephyrin clustering and hippocampal GABAergic transmission. TC10 is highly homologous to the only Cb substrate known so far, Cdc42, and its expression in various brain regions may explain why postsynaptic gephyrin clustering proceeds normally in forebrain neurons of Cdc42 KO mice (10). By using an established gephyrin microcluster formation assay in nonneuronal cells, we found that TC10 activates the most prominent Cb isoforms in the mammalian brain (i.e., the SH3 domain-containing ones). A comparable stimulation of gephyrin clustering has been previously seen upon coexpression of SH3(+)CbII with NL2, NL4, or the α2-subunit of GABAARs (3, 20, 21). In analogy to the activation mechanism of Asef, the closest Cb homolog (25), binding of the cytoplasmic domains of these postsynaptic membrane proteins to the autoinhibitory SH3 domain of SH3(+)CbII is thought to relieve intramolecular interactions of the SH3 with the DH and PH domains, resulting in an open conformation that allows the PH domain to interact with PI(3)P-rich membrane regions (2).
We propose that GTP-TC10 binding to the PH domain of Cb induces a conformational change in SH3(+)CbII that is similar to the one triggered by binding of other activators to the SH3 domain of Cb, thereby exposing its lipid binding sites. This would result in membrane binding of Cb and subsequent gephyrin recruitment. Alternatively, TC10 might induce gephyrin clustering by a recently discovered mechanism involving cyclin-dependent kinase 5 (CDK5). Cb and CDK5 have been shown to regulate the phosphorylation of gephyrin at postsynaptic membrane specializations (26), and CDK5-dependent phosphorylation of TC10 is important for localizing TC10 to lipid rafts (27). There, TC10 disrupts cortical actin filaments upon GTP loading, whereas the subsequent dephosphorylation of TC10 through inactivation of CDK5 allows for F-actin reassembly. A similar interplay between CDK5-dependent phosphorylation of gephyrin and TC10 and the resulting effects on F-actin stability might constitute important determinants of Cb-induced gephyrin clustering at inhibitory postsynapses.
In agreement with a recent study on human Cb (11), our in vitro activation assays with recombinant proteins failed to detect an activation of TC10 by SH3(+)CbII and ΔSH3CbII, although both CbII isoforms activated Cdc42 to similar extents, albeit with very low rate constants. In contrast, in cells both GTPases were activated upon coexpression of ΔSH3CbII. This indicates that the GEF activity of Cb might be regulated in vivo by additional factors, as reported previously for several Dbl proteins (28). The cell-based assays also revealed important differences in the potential of the two CbII isoforms to activate TC10 and Cdc42. Whereas in agreement with previous work (23) both ΔSH3CbII and SH3(+)CbII activated Cdc42 in cells, TC10 was activated by SH3(+)CbII only upon coexpression of a neuronal gephyrin splice variant. This result suggests that gephyrin may regulate the activation potential of SH3(+)CbII at inhibitory postsynapses.
The notion that Cb-mediated gephyrin clustering involves a GEF-independent interaction between Cb and small GTPases is supported by our in vitro binding data. In agreement with previous results on Cdc42 (23), we found a strong interaction of ΔSH3CbII with GTPγS-loaded TC10, but only weak interactions with GDP-loaded or nf TC10. The strength of this interaction was comparable to that between TC10 and the PBD of PAK1, a known downstream effector of both TC10 and Cdc42, indicating that Cb binds tightly to the active forms of these two GTPases. This interaction between TC10 or Cdc42 and Cb resembles a GTPase–effector pair rather than a GTPase–GEF interaction. Similar scenarios apply to several other GEFs, where secondary noncatalytic sites bind to GTP-bound GTPases to regulate downstream signaling (29–31). Consistent with an autoinhibitory role of the SH3 domain, SH3(+)CbII bound much more weakly to TC10, and its binding was barely affected by GTP loading.
Our binding experiments with GST-SH3(+)CbIIΔPH or GST-PH revealed a specific interaction between the PH domain of Cb and GTP-TC10. Thus, besides the canonical binding site required for a typical GEF–GTPase interaction with GDP-TC10 that resides in the DH domain, we identified an unconventional interaction between GTP-TC10 and the PH domain of Cb, as previously described for other GEFs (30, 31). Interactions between the PH domains of GEFs and GTP-loaded GTPases are likely required for GEF recruitment to the plasma membrane by their interacting GTPases. In the case of SH3(+)Cb, binding of GTP-TC10 could perturb autoinhibitory intramolecular interactions and thereby induce an open conformation. Although overexpression of a GEF-deficient mutant of Cb does not seem to affect gephyrin cluster formation in nonneuronal cells (present study) and in WT neurons (10), we cannot exclude that the interaction of activated TC10 with the PH domain induces some GEF activity of endogenous Cb at postsynaptic sites through a positive feedback mechanism. Beyond this activation scenario, GTP-TC10 binding might influence the function of the PH domain of Cb. The PH domain of Cb binds to PI(3)P (22), and the disruption of this interaction causes strong defects in postsynaptic gephyrin clustering (10). Further, neither TC10 nor Cdc42 induced gephyrin targeting to the plasma membrane when coexpressed with the PI(3)P-binding deficient Cb mutant, SH3(+)CbII RR/AA. Thus, the interaction of the PH domain of Cb with PI(3)P is an essential step in the gephyrin clustering cascade that cannot be bypassed by GTPase overexpression. Interestingly, the RR/AA mutation did not affect binding of the PH domain to active TC10, as indicated by our in vitro binding assays. This indicates that GTP-TC10 and PI(3)P bind to different sites of the PH domain, leading to the interesting possibility that GTP-TC10 binding might not only affect the conformation of Cb but also its ability to bind membrane lipids.
A key finding of the present study is that the density and size of gephyrin clusters were increased at perisomatic and dendritic postsynapses when SH3(+)CbII was coexpressed with TC10 CA in dissociated rat hippocampal neurons, whereas coexpression of TC10 DN reduced gephyrin clustering compared with controls. In agreement with these results, coexpression of Cdc42 CA or DN with the SH3(+)CbII isoform affects the shape and localization of postsynaptic gephyrin clusters (23). In our study, the marked increase in gephyrin cluster density and size seen upon TC10 CA coexpression correlated with an increase in GABAergic inhibition, as illustrated by larger mIPSC and eIPSC amplitudes. In contrast, coexpression of TC10 DN led to significant reduction in mean mIPSC frequency, which likely reflects a major loss of gephyrin-dependent GABAARs (32) at a subset of inhibitory synapses, resulting in mIPSC amplitudes below threshold values. Together, our cell biological and electrophysiological analyses of hippocampal neurons indicate that GTP-TC10 promotes the recruitment of both gephyrin and functional GABAARs to inhibitory postsynaptic sites.
In summary, the present study identifies an alternative route of SH3(+)Cb activation in which GTP-TC10 binding to the PH domain of Cb interferes with autoinhibitory interactions between the PH and SH3 domains (Fig. S8), thereby enabling Cb to adopt an open conformation that is required for targeting to perisomatic and dendritic GABAergic postsynapses. This Cb activation pathway may operate in parallel with others, such as activation by NL2, NL4, and GABAARα2, and likely contributes to the genesis of inhibitory synapses that form independently of these synaptic proteins.
Materials and Methods
Antibodies and cDNA Constructs.
Details are provided in SI Materials and Methods.
Purification of GST- and His-Tagged Proteins.
GST-TC10 was expressed and purified as previously described (11), and GST-CbII and His-TC10 as detailed in SI Materials and Methods.
In Vitro Binding Assays.
For in vitro binding assays, TC10 was loaded with GDP or GTPγS (Millipore) at 120 µM in 50 mM Tris/HCl (pH 7.5), 1 mM DTT, 5 mM EDTA, and protease inhibitors for 20 min at 30 °C. The nf state was generated by incubating TC10 in this buffer without added nucleotide. Details are provided in SI Materials and Methods.
In Vivo TC10 and Cdc42 Activation Assays.
Assays were performed as previously described (10). Details are provided in SI Materials and Methods.
In Vitro Guanine Nucleotide Exchange.
Nucleotide exchange kinetics were assayed as previously described (11). Details are provided in SI Materials and Methods.
Transfection and Immunostaining of COS-7 Cells.
Details are provided in SI Materials and Methods.
Transfection and Immunostaining of Cultured Rat Hippocampal Neurons.
Rat hippocampal neurons were prepared from embryonic day 18 rats. Neurons were transfected at DIV 4 using the CalPhos mammalian transfection kit (Clontech). Details are provided in SI Materials and Methods.
Electrophysiological Recordings of GABAergic mIPSCs and eIPSCs on Dissociated Rat Hippocampal Neurons.
Cells were whole-cell voltage-clamped at −70 mV as described in SI Materials and Methods.
Quantifications and Statistics.
Experimental data were evaluated by investigators blind to experimental conditions. ImageJ (http://rsb.info.nih.gov/ij) was used to analyze immunostainings from images processed under standardized intensity thresholding. Statistical comparisons in this study were made using the one-way ANOVA variance test followed by a Tukey multiple comparison test, always applying a 95% confidence interval. All values represent means ± SEM. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, and ***P < 0.001); n.s. indicates no significant difference.
Supplementary Material
Acknowledgments
We thank Drs. Perihan Nalbant and Jeffrey E. Pessin for plasmids. This work was supported by the Max Planck Gesellschaft, the Center for Nanoscale Microscopy and Molecular Physiology of the Brain, and Deutsche Forschungsgemeinschaft (Grant PA 2087/1-1 to T.P. and Grant AH 92/5-1 to M.J. and M.R.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309078110/-/DCSupplemental.
References
- 1.Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70(3):385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulos T, Soykan T. The role of collybistin in gephyrin clustering at inhibitory synapses: Facts and open questions. Front Cell Neurosci. 2011;5:11. doi: 10.3389/fncel.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulopoulos A, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63(5):628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos T, et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26(17):3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J Neurosci. 1995;15(6):4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charrier C, Ehrensperger MV, Dahan M, Lévi S, Triller A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J Neurosci. 2006;26(33):8502–8511. doi: 10.1523/JNEUROSCI.1758-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid T, Bathoorn A, Ahmadian MR, Collard JG. Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. J Biol Chem. 1999;274(47):33587–33593. doi: 10.1074/jbc.274.47.33587. [DOI] [PubMed] [Google Scholar]
- 9.Xiang S, et al. The crystal structure of Cdc42 in complex with collybistin II, a gephyrin-interacting guanine nucleotide exchange factor. J Mol Biol. 2006;359(1):35–46. doi: 10.1016/j.jmb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Reddy-Alla S, et al. PH-domain-driven targeting of collybistin but not Cdc42 activation is required for synaptic gephyrin clustering. Eur J Neurosci. 2010;31(7):1173–1184. doi: 10.1111/j.1460-9568.2010.07149.x. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal M, Dvorsky R, Ahmadian MR. Deciphering the molecular and functional basis of Dbl family proteins: A novel systematic approach toward classification of selective activation of the Rho family proteins. J Biol Chem. 2013;288(6):4486–4500. doi: 10.1074/jbc.M112.429746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neudauer CL, Joberty G, Tatsis N, Macara IG. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr Biol. 1998;8(21):1151–1160. doi: 10.1016/s0960-9822(07)00486-1. [DOI] [PubMed] [Google Scholar]
- 13.Hemsath L, Dvorsky R, Fiegen D, Carlier MF, Ahmadian MR. An electrostatic steering mechanism of Cdc42 recognition by Wiskott-Aldrich syndrome proteins. Mol Cell. 2005;20(2):313–324. doi: 10.1016/j.molcel.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 14.Murphy GA, et al. Cellular functions of TC10, a Rho family GTPase: Regulation of morphology, signal transduction and cell growth. Oncogene. 1999;18(26):3831–3845. doi: 10.1038/sj.onc.1202758. [DOI] [PubMed] [Google Scholar]
- 15.Mammoto A, et al. Interactions of drebrin and gephyrin with profilin. Biochem Biophys Res Commun. 1998;243(1):86–89. doi: 10.1006/bbrc.1997.8068. [DOI] [PubMed] [Google Scholar]
- 16.Giesemann T, et al. Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: A possible link to the microfilament system. J Neurosci. 2003;23(23):8330–8339. doi: 10.1523/JNEUROSCI.23-23-08330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe K, et al. The small GTP-binding protein TC10 promotes nerve elongation in neuronal cells, and its expression is induced during nerve regeneration in rats. J Neurosci. 2000;20(11):4138–4144. doi: 10.1523/JNEUROSCI.20-11-04138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3(1):22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- 19.Harvey K, et al. The GDP-GTP exchange factor collybistin: An essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24(25):5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoon M, et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci USA. 2011;108(7):3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saiepour L, et al. Complex role of collybistin and gephyrin in GABAA receptor clustering. J Biol Chem. 2010;285(38):29623–29631. doi: 10.1074/jbc.M110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalscheuer VM, et al. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum Mutat. 2009;30(1):61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagarajan SK, Ghosh H, Harvey K, Fritschy JM. Collybistin splice variants differentially interact with gephyrin and Cdc42 to regulate gephyrin clustering at GABAergic synapses. J Cell Sci. 2011;124(Pt 16):2786–2796. doi: 10.1242/jcs.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang SH, et al. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature. 2001;410(6831):944–948. doi: 10.1038/35073608. [DOI] [PubMed] [Google Scholar]
- 25.Mitin N, et al. Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression. Nat Struct Mol Biol. 2007;14(9):814–823. doi: 10.1038/nsmb1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhse J, et al. Phosphorylation of gephyrin in hippocampal neurons by cyclin-dependent kinase CDK5 at Ser-270 is dependent on collybistin. J Biol Chem. 2012;287(37):30952–30966. doi: 10.1074/jbc.M112.349597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada S, et al. CDK5-dependent phosphorylation of the Rho family GTPase TC10(alpha) regulates insulin-stimulated GLUT4 translocation. J Biol Chem. 2008;283(51):35455–35463. doi: 10.1074/jbc.M806531200. [DOI] [PubMed] [Google Scholar]
- 28.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13(1):13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 29.Margarit SM, et al. Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112(5):685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, et al. Activated RhoA binds to the pleckstrin homology (PH) domain of PDZ-RhoGEF, a potential site for autoregulation. J Biol Chem. 2010;285(27):21070–21081. doi: 10.1074/jbc.M110.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen LA, et al. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell. 2007;18(6):2244–2253. doi: 10.1091/mbc.E06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kneussel M, et al. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19(21):9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.