Significance
Cell differentiation in the embryo is regulated by diffusible substances called “morphogens,” but these have never been directly visualized as endogenous components of the extracellular space. Chordin is an antagonist of the bone morphogenetic protein (BMP) pathway copiously secreted by a dorsal region of the Xenopus embryo called “Spemann’s organizer” that has potent tissue-inducing activity. We report that Chordin protein forms a dorsal-to-ventral gradient in the embryo. This gradient is located in a narrow space containing extracellular matrix (ECM) that separates the ectoderm from the endomesoderm, which seems to serve as a highway for the diffusion of Chordin–BMP complexes over very long distances (2 mm) in the embryo. All vertebrate embryos have a similar ECM between ectoderm and mesoderm during gastrulation.
Abstract
The vertebrate body plan follows stereotypical dorsal–ventral (D-V) tissue differentiation controlled by bone morphogenetic proteins (BMPs) and secreted BMP antagonists, such as Chordin. The three germ layers—ectoderm, mesoderm, and endoderm—are affected coordinately by the Chordin–BMP morphogen system. However, extracellular morphogen gradients of endogenous proteins have not been directly visualized in vertebrate embryos to date. In this study, we improved immunolocalization methods in Xenopus embryos and analyzed the distribution of endogenous Chordin using a specific antibody. Chordin protein secreted by the dorsal Spemann organizer was found to diffuse along a narrow region that separates the ectoderm from the anterior endoderm and mesoderm. This Fibronectin-rich extracellular matrix is called “Brachet’s cleft” in the Xenopus gastrula and is present in all vertebrate embryos. Chordin protein formed a smooth gradient that encircled the embryo, reaching the ventral-most Brachet cleft. Depletion with morpholino oligos showed that this extracellular gradient was regulated by the Chordin protease Tolloid and its inhibitor Sizzled. The Chordin gradient, as well as the BMP signaling gradient, was self-regulating and, importantly, was able to rescale in dorsal half-embryos. Transplantation of Spemann organizer tissue showed that Chordin diffused over long distances along this signaling highway between the ectoderm and mesoderm. Chordin protein must reach very high concentrations in this narrow region. We suggest that as ectoderm and mesoderm undergo morphogenetic movements during gastrulation, cells in both germ layers read their positional information coordinately from a single morphogen gradient located in Brachet’s cleft.
The orchestration of tissue differentiation in the embryo to form a perfect individual time-after-time is a fascinating problem in developmental biology. The three germ layers—ectoderm, mesoderm and endoderm—are coordinately regulated to generate a well-organized body plan in which the various organs of the body develop. An experiment that opened the way for understanding embryonic cell differentiation was carried out in 1924 by Spemann and Mangold (1). They transplanted the dorsal side of the blastopore lip of an amphibian embryo, the region in which the involution of the mesoderm starts, into the ventral side of a host embryo and obtained Siamese twins. Their key discovery was the phenomenon of embryonic induction, in which the transplanted cells induced new cell fates on their neighbors, causing, for example, differentiation of central nervous system (CNS), somites, and kidneys. The inducing tissue was called the “organizer” because it induced the surrounding tissue to form a perfectly arranged secondary embryo. Spemann received the 1935 Nobel Prize in Physiology or Medicine for this work (reviewed in refs. 2 and 3).
Once molecular cloning became practical, a number of genes specifically expressed in Spemann’s organizer were isolated, starting with the homeobox gene goosecoid (4). Through the work of several laboratories, it was found that organizer cells secrete a mixture of growth factor antagonists among which the bone morphogenetic protein (BMP) antagonists Noggin, Follistatin, and Chordin are prominent (reviewed in refs. 5 and 6). BMPs are secreted growth factors of the TGF-β superfamily, first discovered by Urist at the University of California, Los Angeles as bone-inducing factors in decalcified bone matrix extracts (7). A morphogen gradient of BMP signaling plays the key role in the differentiation of cells into dorsal–ventral (D-V) tissue types in vertebrates and Drosophila (8, 9).
Embryos of the frog Xenopus laevis provide an excellent system to study the self-organizing properties of D-V patterning. When a blastula embryo is bisected, the ventral half forms a sphere consisting of ventral tissues (called a “belly-piece” by Spemann, ref. 1), whereas the dorsal half forms a well-proportioned embryo scaled to size. When the embryos are cut so that dorsal organizer tissue is present in both fragments, at low frequency, identical twins can be generated (10). These self-organizing properties of the embryo imply that cells can communicate with each other over very long distances. At the gastrula stage, the Xenopus embryo is 1.3 mm in diameter and consists of a single morphogenetic field of about 10,000 cells.
In the ectoderm, BMP signaling inhibition causes differentiation of the CNS, high levels of BMP induce epidermis, and intermediate concentrations induce neural crest. In the mesoderm, at low levels of BMP, signaling notochord is formed, and at progressively higher levels, kidney, lateral plate mesoderm (LPM), and blood tissues are induced (11, 12). Thus, histotypic differentiation depends on the graded activity of BMP signaling. Remarkably, the three germ layers respond coordinately to changes in BMP signaling, which can cause dorsalization or ventralization of many tissues in the embryo (13). A key question investigated here, is whether a single signaling gradient or multiple ones are used to pattern D-V differentiation of the different germ layers.
BMP binding to its cell-surface Serine/Threonine kinase receptors leads to the phosphorylation of two carboxyl-terminal serines in the related transcription factors Smad1/5/8, causing them to translocate into the nucleus. The activation of these three proteins can be detected by a phosphospecific antibody (14, 15). In Xenopus, nuclei in the ventral side of the early gastrula are enriched for pSmad1/5/8 (16, 17). The transparency of the zebrafish embryo enabled the visualization of a gradient of BMP activity (18, 19). Despite the very extensive morphogenetic movements that take place during gastrulation (such as involution, epiboly, and convergence–extension), a surprisingly stable gradient of pSmad1/5/8 is maintained in the ventral side of the zebrafish early embryo (18).
The Chordin biochemical pathway plays a fundamental role in the formation of the self-organizing gradient of BMP activity in Xenopus (20). Chordin is a BMP antagonist secreted by Spemann organizer tissue (21, 22) that is produced in great excess: if uniformly distributed throughout the embryo, it would reach levels of 33 nM in the extracellular space during gastrulation, whereas BMPs would be present in the picomolar range (23). In the dorsal side, where it is produced, Chordin should reach much higher levels. Transplanted organizers depleted of Chordin with antisense morpholino oligos (MOs) lose all tissue-inductive power (24), and in the triple depletion of Chordin, Noggin, and Follistatin, all dorsal tissues are lost in Xenopus embryos (25). When bound to Chordin, BMPs cannot signal through BMP receptors, but this inhibition can be reversed by cleavage of Chordin at two specific sites by Tolloid metalloproteinases (26).
The degradation of Chordin–BMP complexes by Tolloid activity is rate limiting and tightly regulated. On the ventral side of the embryo, BMP signaling activates Smad1/5/8 through phosphorylation, causing the transcription of a secreted Frizzled-related protein (sFRP) called “Sizzled” that lacks Wnt-inhibiting activity (27). Sizzled is a competitive inhibitor of Tolloid activity, which binds to the enzyme active site and prevents the cleavage of Chordin (23, 28, 29). In addition, Tolloid protease activity is inhibited noncompetitively by direct binding of BMP proteins to its CUB (Complement 1r/s, Uegf, Bmp1) domains (30).
The opposite transcriptional control of D-V genes is a key feature of self-regulation. Ventral genes are activated by pSmad1/5/8, whereas dorsal components are transcribed when BMP signaling is low (31). The dorsal organizer secretes Chordin, two BMPs [called “BMP2” and “ADMP” (anti-dorsalizing morphogenetic protein)], the Olfactomedin-related Ont1 adaptor protein that bridges the binding of Chordin to Tolloid (32), and a close homolog of Sizzled called “Crescent” that inhibits both Tolloid and Wnt (33). The ventral side expresses BMP4 and BMP7, Sizzled, and Crossveinless 2 (CV2, also known as Bmper). CV2 binds BMPs and Chordin and has Cysteine-rich BMP-binding domains similar to those of Chordin but remains tethered to the surface of cells in which it is synthesized (34, 35).
Proteins of similar biochemical activities at opposite poles of the embryo can compensate for each other. For example, only when all four BMPs are depleted simultaneously does the D-V gradient collapse, turning the entire ectoderm into the CNS (31). In a similar way, Chordin and CV2, or Crescent and Sizzled, which are under reciprocal transcriptional regulation, compensate for the loss of each other (33, 35). Embryological experiments and mathematical modeling in Xenopus have led to the proposal that a D-V flux of BMPs bound to Chordin may increase the robustness of the self-organizing Chordin–BMP–Tolloid extracellular pathway (8, 31, 36). However, none of the endogenous proteins of this morphogen pathway have ever been directly visualized in vertebrate embryos.
In the present study, we developed an improved immunolocalization method for Xenopus embryos and unexpectedly observed diffusion of overexpressed BMPs in a very narrow region of the gastrula embryo called “Brachet’s cleft.” This region of extracellular matrix (ECM) is rich in Fibronectin (37), and is well known in Xenopus (38) because it is used for the dissection of Keller mesodermal explants (39). An equivalent of Brachet’s cleft is found in all vertebrate embryos, for this space corresponds to the ECM that separates the ectoderm from the endomesoderm. Using an affinity-purified Chordin antibody, we report here that endogenous Chordin protein forms a D-V gradient in the Brachet’s cleft of gastrula embryos that extends to the ventral-most regions. The Chordin gradient was modified by depletion of components of the system such as Tolloid and Sizzled, and was complementary to the endogenous pSmad1/5/8-signaling gradient. Importantly, the gradient of Chordin protein was rescaled in dorsal half-embryos, and a second gradient formed in embryos with Spemann organizer transplants. The existence of a self-organizing Chordin gradient in Brachet’s cleft suggests a unique mechanism by which an extracellular morphogen can coordinately regulate patterning in the ectoderm and endomesoderm during morphogenesis.
Results
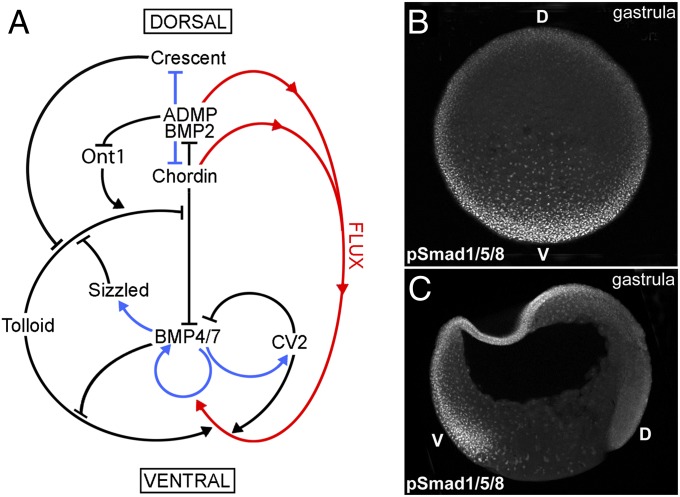
Spemann organizer tissue secretes multiple growth factor antagonists such as the BMP antagonists Noggin, Follistatin, and Chordin; the Wnt antagonists Dkk1, Frzb, Crescent, and sFRP2; and the Nodal, Wnt and BMP antagonist Cerberus (8). Among these, Chordin plays an essential role, and a conserved biochemical pathway of Chordin-interacting extracellular proteins has been elucidated from studies in Xenopus (20), zebrafish (12), and Drosophila (9). As shown in Fig. 1A, a system of extracellular BMP regulators (black lines) is transcribed ventrally when BMP signaling is high and dorsally when it is low (blue lines), and a flux of BMPs bound to Chordin (facilitated by proteolysis of Chordin by Tolloid) has been proposed (red lines) (8). The Chordin–BMP–Tolloid system is proposed to mediate cell–cell communication over long distances, so that for every action in the dorsal side there is a reaction in the ventral side of the gastrula. Despite extensive studies in Xenopus D-V patterning, the visualization of the endogenous distribution of any of the secreted proteins shown in Fig. 1A has not, to date, been possible.
Fig. 1.
A gradient of BMP activity patterns the D-V axis of the Xenopus gastrula. (A) BMP activity along the D-V axis results from a series of direct protein–protein interactions between Chordin and other partners (black arrows), transcriptional regulation (blue arrows), and protein flux (red arrows). The entire embryo participates in forming the BMP gradient, which results from the dueling activities of the dorsal and ventral signaling centers. (B) Transverse optical section at gastrula (stage 11) showing a ventral- (V) to-dorsal (D) gradient of BMP activity using anti-pSmad1/5/8 antibody as readout. (C) Gastrula (stage 11) embryo sectioned sagittally, showing higher BMP activity in the ventral animal cap and marginal zone nuclei as assessed by pSmad1/5/8 immunostaining.
Immunostaining the pSmad1/5/8 Gradient.
We developed an improved protocol for immunostaining whole-mount Xenopus embryos, described in detail in SI Materials and Methods. Using this unique method, phosphorylated Smad1/5/8 was visualized as a continuous D-V gradient of nuclear protein in transverse optical sections (perpendicular to the animal–vegetal axis) of Xenopus embryos at mid- and late gastrula stages (Fig. 1B). In sagittal sections, nuclear pSmad1/5/8 was observed in the ventral marginal zone (future mesoderm) and animal cap (future epidermis), but only at low levels in the dorsal side (Fig. 1C). As expected, ventral BMP signaling was increased by the depletion of Chordin, a key BMP antagonist in Xenopus (Fig. S1 A and B). These results are in agreement with previous work in zebrafish showing a remarkably stable gradient of BMP signaling and maximal in the ventral side that is maintained throughout gastrulation (18).
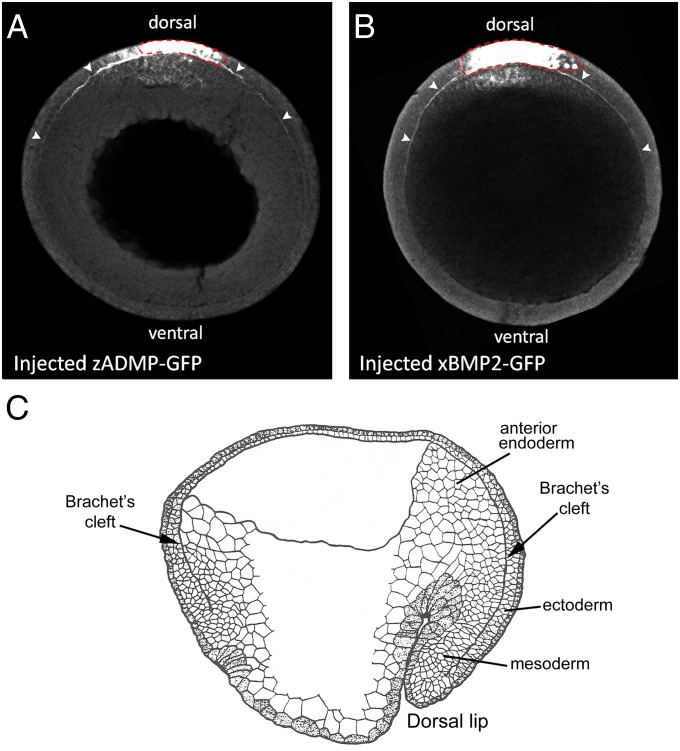
Overexpressed BMPs Diffuse Along Brachet’s Cleft.
We next investigated whether overexpressed BMPs could diffuse through the embryo. We chose BMP2 and ADMP because these dorsally secreted BMPs (32, 40, 41) have been proposed to be transported by facilitated diffusion to the ventral side of the embryo bound to Chordin, where they are released by the cleavage of Chordin by Tolloid (Fig. 1A; ref. 31). Messenger RNAs for zADMP (42) and xBMP2 were tagged with GFP Venus protein and microinjected into a dorsal B1 blastomere at the 32-cell stage. At midgastrula, embryos targeted to dorsal ectoderm expressed high levels of protein in injected cells, and lower levels were found in nearby endomesoderm, as well (Fig. 2 A and B). Unexpectedly, we noticed that the injected fusion proteins also diffused along a narrow region of the embryo, indicated by arrowheads in Fig. 2 A and B. This space corresponded to Brachet’s cleft (38), the thin extracellular region that separates the ectoderm from the anterior endoderm and mesoderm (Fig. 2C). Only BMP fusions were transported in this ECM, for a secreted construct of GFP alone (Materials and Methods) remained localized at the site of injection (Fig. S1C).
Fig. 2.
Overexpressed BMP-GFP fusion proteins diffuse in Brachet’s cleft. (A and B) Dorsally expressed zADMP-GFP and xBMP2-GFP fusion proteins diffuse within the narrow confines of Brachet’s cleft (marked by arrowheads) away from the point of mRNA injection in ectodermal cells (indicated by a red dotted line) (n = 7 and n = 6, respectively). GFP fusion proteins were detected by GFP immunostainings of transverse optical sections of stage-12 embryos through the animal–vegetal axis. (C) Diagram of stage-12 sagittal section of a Xenopus embryo (after P. Nieuwkoop, ref. 38). Brachet’s cleft is the narrow cavity that separates the mesodermal and anterior endodermal layers from the ectoderm and encircles the entire D-V axis.
The diffusion of these secreted proteins specifically in Brachet’s cleft suggested that the main morphogenetic gradient might be formed in the ECM between the ectoderm and the mesoderm, raising the possibility that a common gradient of growth factor activity may coordinately pattern two germ layers. During gastrulation, cells undergo extensive morphogenetic movements, and we propose that as they come in proximity to the ECM in Brachet’s cleft, they read from it positional signals required for proper cell differentiation.
A Gradient of Chordin in Brachet’s Cleft.
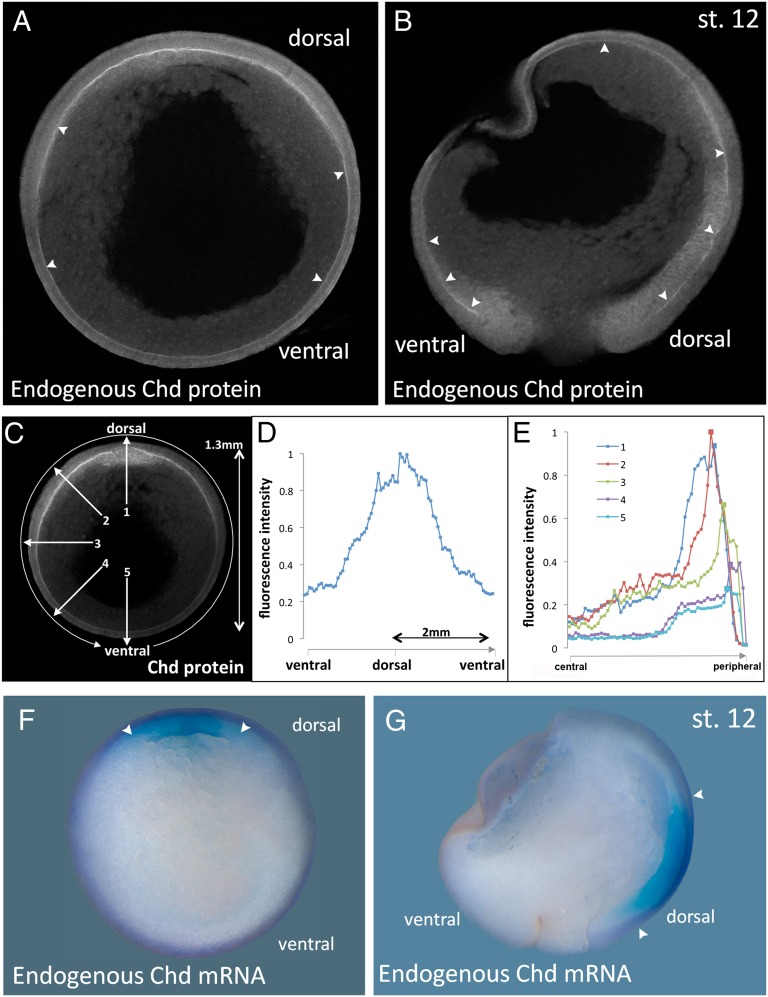
We next asked whether endogenous components of the BMP signaling pathway could be detected in the ECM between the ectoderm and mesoderm. Chordin protein is very abundantly secreted (23), facilitating the study of its endogenous distribution in the embryo. A polyclonal antibody raised against the amino terminus of Chordin (26) was affinity purified and used to stain bisected Xenopus gastrulae (between stages 11 and 12). As shown in Fig. 3A, Chd staining was observed in dorsal endomesoderm (roof of the archenteron) and in Brachet’s cleft. In the latter, a narrow line of Chordin staining, indicated by arrowheads, formed a gradient that extended ventrally. The Chordin signal was not limited to the narrow extracellular cleft and could be seen also staining, in a diffuse way, the adjoining ectoderm and mesoderm (Fig. 3 A and C). Although weak, fluorescence intensity in tissues next to Brachet’s cleft could be quantified with ImageJ (http://rsbweb.nih.gov/ij/) in the radial direction (Fig. 3E), particularly compared with Chordin-depleted embryos (see Fig. S3). This orthogonal diffusion of Chordin–BMP may account for the uniformity of the pSmad1/5/8 gradient in cells that do not come in direct contact with Brachet’s cleft. In sagittal sections, the extracellular staining was seen in dorsal mesoderm and in Brachet’s cleft, which encircles the embryo, reaching its ventral-most side (arrowheads in Fig. 3B; anatomical structures shown in Fig. S2).
Fig. 3.
Endogenous Chordin protein diffusion within Brachet’s cleft compared with the expression of chordin mRNA. (A and B) Immunostainings of Chordin protein in late gastrula (stage 12) embryos in transverse (n = 16) or sagittal (n = 15) sections, respectively, using an affinity-purified Chordin antibody. Chordin protein is detected throughout the entire length of Brachet’s cleft (arrowheads) forming a gradient from dorsal toward ventral. Chd, Chordin. (C) Imaging of the Chordin gradient following the entire circumference in a clockwise manner (circular arrow) or in a radial manner (numbered arrows). (D) Measurement of the Chordin gradient of the embryo seen in C. Note that the gradient forms over a very long distance of almost 2 mm; similar profiles were obtained in the four embryos analyzed. (E) Intensity of fluorescence plotted along five radial lines from central to peripheral. Line 1 is dorsal and shows Chordin protein peaks in organizer mesoderm and in Brachet’s cleft. In the other lines a peak is seen in Brachet’s cleft, even in the ventral-most region. (F and G) In situ hybridization of stage-12 embryo in transverse or sagittal section showing that chordin mRNA is transcribed only in the dorsal side (arrowheads); compare with the panels (A and B) showing Chordin protein localization at the same stage.
The gradient in Brachet’s cleft could be traced around the entire circumference of the embryo using image analysis (Fig. 3C). Remarkably, the gradient extends over a long distance of about 2 mm, as indicated in Fig. 3D. When the Chordin signal was measured from the center toward the periphery (in the radial direction), peaks of Chordin protein could be detected in Brachet’s cleft, progressively decreasing in the ventral direction but still clearly present even in the ventral-most cleft (Fig. 3 C and E). In contrast, chordin mRNA is transcribed only in dorsal regions (Fig. 3 F and G). These results indicate that Chordin protein is able to diffuse long range in the gastrula into regions that are distant from where it is produced.
Chordin staining in the ECM was entirely specific, for it was eliminated by depletion of Chordin with antisense MOs, as shown in Fig. S3. We conclude from these results that the Chordin antibody is specific, and that it detects an endogenous gradient of Chordin protein in Brachet’s cleft that extends from the dorsal to the ventral side of the embryo. Chordin was also released orthogonally from Brachet’s cleft into neighboring tissues, probably explaining the smoothness of the nuclear pSmad1/5/8-signaling gradient.
Regulation of the Chordin and BMP Activity Gradients.
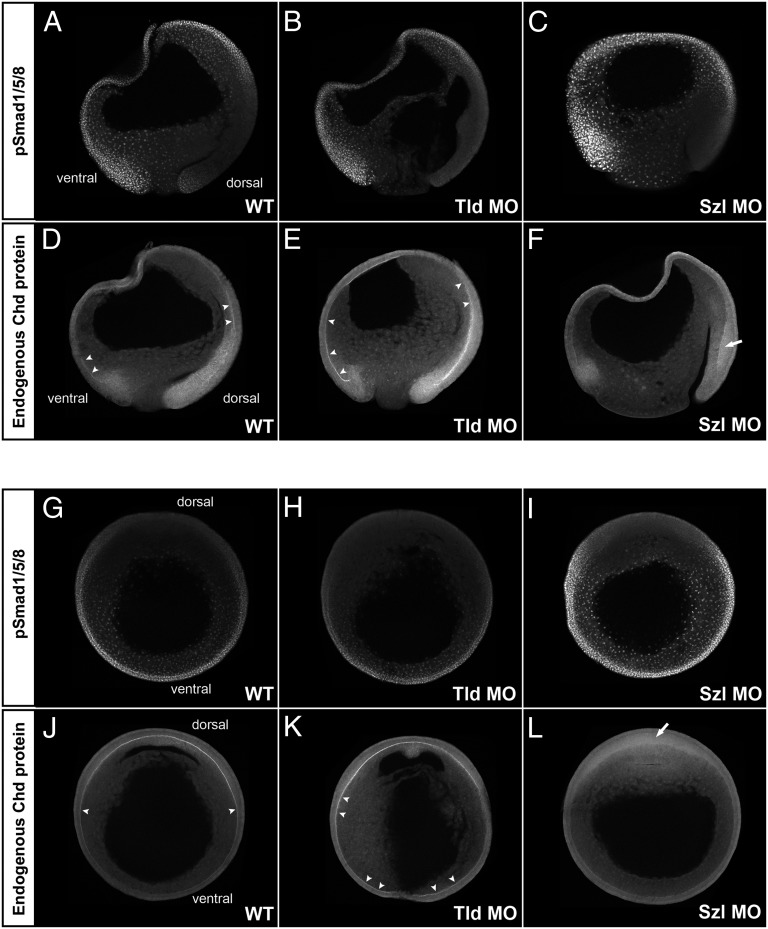
We next tested how the D-V gradient was affected by depletion of regulators of Chordin stability. The Chordin protein gradient was reciprocal to the pSmad1/5/8 gradient both in sagittal (Fig. 4 A and D) and transverse (compare Fig. 4 G with J) optical sections. This was expected, as Chordin is a BMP signaling antagonist.
Fig. 4.
Analysis of BMP signaling and Chordin protein localization in embryos depleted of Tolloid (Tld MO) or Sizzled (Szl MO). All embryos were siblings allowed to develop for the same period; all images were processed identically. (A–F) pSmad1/5/8 (Upper) and Chordin (Lower) immunostainings of sagittal optical sections. The gradient of BMP activity was complementary to that of Chordin localization in WT embryos (Left, n = 6 and n = 4, respectively). When translation of the Chordin-degrading enzyme Tolloid was inhibited in Tld MO-injected embryos (Center, n = 5 and n = 3, respectively), BMP activity was decreased on the dorsal side and increased accumulation of Chordin was observed in Brachet’s cleft (arrowheads). Conversely, when Sizzled was depleted (Szl MO), BMP activity (nuclear pSmad1/5/8) was greatly increased in the embryo, and Chordin failed to accumulate in the Brachet’s cleft (Right, n = 3). Diffuse accumulation of Chordin in dorsal ectoderm is indicated by the arrow. (G–L) pSmad1/5/8 (Upper) and Chordin (Lower) immunostainings of embryos sectioned transversely through the animal–vegetal axis at late gastrula (stage 12). pSmad1/5/8 staining was decreased in Tld MO embryos (H, n = 6) and increased in Szl MO embryos (I, n = 4) compared with WT (G, n = 6). Tld MO increased (K, n = 7) and Szl MO decreased (L, n = 5) Chordin staining in Brachet’s cleft compared with WT (J, n = 7).
When Xolloid-related and BMP1, two of the Tolloid metalloproteinases that digest Chordin, were depleted with MOs, dorsalized (low-BMP) phenotypes were obtained (32). Tolloid depletion (Tld MO) resulted in decreased nuclear pSmad1/5/8 signal in the dorsal side (compare Fig. 4 A with B and G with H), and increased Chordin protein staining in Brachet’s cleft (compare Fig. 4 D with E and J with K). Chordin stabilization was particularly striking in the ventral part of Brachet’s cleft (indicated with arrowheads in Fig. 4 E and K) upon Tolloid knockdown.
Sizzled is an inhibitor of Tolloid activity expressed at high BMP-signaling levels in the ventral side. When Sizzled is depleted, Tolloid proteases become more active, causing Chordin degradation and ventralized (high-BMP) phenotypes (23). Sizzled depletion resulted in a strong increase in nuclear pSmad1/5/8 accumulation, particularly in the ventral side of the embryo (compare Fig. 4 A with C and G with I), whereas Chordin protein was reduced in Brachet’s cleft (but increased in dorsal ectoderm) (Fig. 4 F and L). Taken together, these results suggest that the depletion of genes that degrade (Tolloid) or stabilize (Sizzled) Chordin have strong effects on the levels of Chordin in Brachet’s cleft and on the pSmad1/5/8-activity gradient.
The Chordin Gradient Rescales in Half-Embryos and Organizer Grafts.
A fundamental question is whether the observed gradient of Chordin protein is able to self-organize after bisection of embryos, rescaling in the same way embryonic patterning does. In addition, some have expressed doubts that the D-V signaling gradient is self-regulating (43). An obstacle in carrying out half-embryo experiments was that our method for the visualization of the Chordin gradient requires embryos devoid of pigment. However, the darker pigmentation of the ventral side is what enables one to distinguish the dorsal from the ventral side to perform bisections at blastula stage 9, before the dorsal blastopore lip appears. We noticed that at the eight-cell stage, ventral blastomeres are not only darker, but adopt a unique butterfly-like pattern extending toward the sides, whereas the dorsal blastomeres extend toward the equator (Fig. S4A). These peculiar shapes are observed only in regularly cleaving embryos (44) and, importantly, can be seen very distinctly in albino embryos (Fig. S4B). This allowed us to mark the ventral side by microinjection of a lineage tracer, such as Nile Blue, to carry out D-V dissections as accurately as in pigmented embryos (Fig. S4). We think that this simple observation, which allows one to reliably identify D-V polarity at a very early stage, will be of considerable benefit to the Xenopus field.
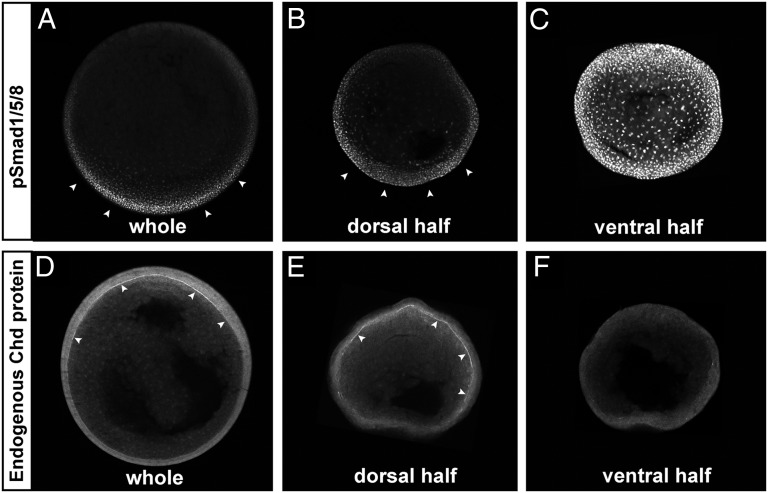
We examined endogenous pSmad1/5/8 and Chordin protein distribution using this method. Embryos were bisected at the blastula and allowed to develop until whole control embryos reached stage 12. As can be seen in Fig. 5 A and B, the gradient of pSmad1/5/8 was regenerated in dorsal halves. In ventral halves, which form a belly-piece consisting exclusively of ventral tissues, BMP signaling was uniform and unrestrained due to the lack of organizer signals, with nuclear pSmad1/5/8 reaching much higher levels (Fig. 5C) than those seen in normal embryos. Chordin protein formed a reciprocal gradient in Brachet’s cleft of dorsal half-embryos (although not as uniform as in whole embryos) (Fig. 5E), whereas only background staining was seen in ventral halves (Fig. 5F). We conclude from these experiments that the pSmad1/5/8 and Chordin gradients can be rescaled according to embryonic size.
Fig. 5.
The Chordin and pSmad1/5/8 gradients self-regulate in dorsal half-embryos. The ventral side of albino embryos was marked at the 8-cell stage using the method described in Fig. S4. Embryos were bisected at stage 9, cultured until stage 12, fixed, and sectioned transversely. All embryos were siblings and images were processed identically. (A–C) pSmad1/5/8 immunostaining of whole embryo (A, n = 3), dorsal (B, n = 6) or ventral (C, n = 3 of 4 showing the phenotype) half-embryos. Note that the BMP gradient was reestablished in the dorsal half-embryo, while very strong uniform pSmad1/5/8 was present in the ventral half-embryo. (D–F) Chordin immunostaining of whole embryo (D, n = 3), dorsal (E, n = 10) or ventral (F, n = 7) half-embryo. The Chordin gradient was regenerated in the Brachet’s cleft of dorsal half-embryos (in 8 of 10), while no Chordin expression was detected in the ECM of the ventral half-embryo (in 6 of 7). Similar results were obtained in two independent experiments.
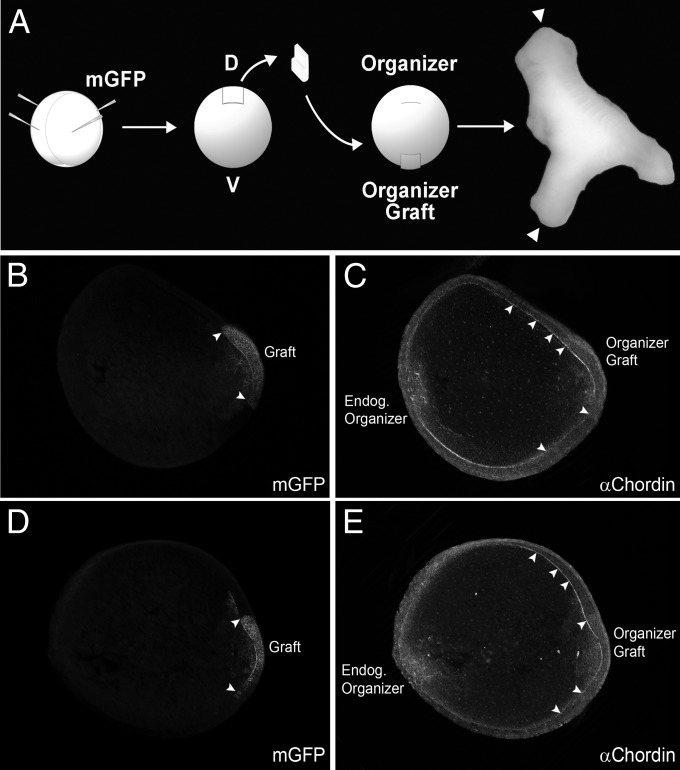
We next tested whether Chordin diffusion could be detected in embryos receiving Spemann organizer transplants (Fig. 6). To distinguish the grafted organizer, albino donor embryos were microinjected with mRNA encoding a GFP targeted to the cell membrane via a palmitoylation signal (membrane GFP or mGFP) (45). Albino embryos received the transplant in the ventral side at stage 10, as soon as the blastopore lip became distinguishable. Immunostainings of transversely bisected embryos were performed at late gastrula stage 12. The Chordin signal was detected in Brachet’s cleft diffusing long-range from the GFP-positive grafted tissue, of which two examples are shown (Fig. 6 B–E, arrowheads) (n = 19). The results suggest that the Brachet cleft ECM provides a highway for the long-distance-facilitated diffusion of Chordin from ventrally transplanted organizer tissue.
Fig. 6.
Chordin protein diffuses long-range in Brachet’s cleft of embryos transplanted with Spemann organizer tissue. (A) Diagram of experimental procedure. mGFP was injected in the donor embryo (four injections at the four-cell stage) to trace the lineage of grafted tissue. Secondary axes were complete (with heads, indicated with arrowheads in this photograph) in 75% of the cases (n = 6/8). (B–E) Two embryos transplanted at stage 10 and fixed at stage 12 stained with GFP and Chordin antibodies are shown in optical transverse sections. B and D show localization of the grafted organizer tagged with mGFP; borders are indicated by arrowheads. In C and E, a second gradient of Chordin was observed, with Chordin protein staining in Brachet’s cleft extending a considerable distance from the graft (arrowheads) (n = 19). This indicates that Chordin protein diffuses from the graft through Brachet’s cleft.
Discussion
Localization of the Chordin Gradient in Vivo.
A gradient of BMP signaling patterns the embryo in many species (15, 20), and it has been proposed that Chordin facilitates the D-V flux of BMPs in a Tolloid-regulated way (8, 9). However, the physical location of endogenous gradient-forming signaling components remains unknown. In this study we developed a unique method that improved immunolocalization of proteins in Xenopus. When the distribution of microinjected BMP2-GFP and ADMP-GFP was examined with anti-GFP antibodies, we were surprised to detect long-range diffusion of these proteins in Brachet’s cleft, the ECM that separates the ectoderm from the mesoderm and anterior endoderm (Fig. 2C). Using an affinity-purified polyclonal antibody against Chordin, we showed that an endogenous gradient of Chordin protein formed in Brachet’s cleft at mid- and late gastrula, extending from dorsal to the most ventral regions of the embryo. The endogenous gradient of Chordin was the mirror image of the pSmad1/5/8 gradient of BMP activity. The Chordin staining was specific because it was absent from embryos depleted of Chordin.
Depletion of components of the Chordin biochemical pathway modified the gradients in the ways predicted by the pathway in Fig. 1A. Tolloid is an enzyme that degrades Chordin (26), and its depletion by Tld MOs (32) resulted in a shallower pSmad1/5/8 gradient. This was accompanied by the accumulation of endogenous Chordin protein in Brachet’s cleft, which formed a more intense gradient that extended to the ventral-most ECM. Conversely, depletion of Sizzled (an inhibitor of Tolloid; ref. 23) increased pSmad1/5/8 signaling and decreased Chordin staining in Brachet’s cleft. In an elegant recent study, Inomata et al. showed that a microinjected Chordin-GFP fusion was able to rapidly diffuse between neighboring cells in Xenopus ectodermal animal cap explants (29). Using fluorescence recovery after photobleaching, they showed that when Chordin is stabilized by the overexpression of Sizzled, it is able to rapidly diffuse. A similar diffusion constant was measured for Sizzled-GFP (29). Thus, these key proteins can efficiently diffuse in-between cells. Like Chordin, Sizzled is very abundantly secreted at gastrula stages (23), and it would be interesting to determine in the future the endogenous distribution of this ventrally produced protein.
A Self-Organizing Chordin Gradient.
Since the classical work of Spemann, we know that the amphibian embryo has the remarkable property of a self-organizing pattern, one of the most mysterious properties of living organisms (2). The dorsal half of a bisected blastula embryo gives rise to embryos containing the normal allocations of tissue differentiations but scaled to a smaller size. We found that dorsal halves regenerated a Chordin protein gradient in Brachet’s cleft, as well as a ventral gradient of pSmad1/5/8 BMP signaling. Ventral half-embryos, which lack a Spemann’s organizer and differentiate into ventral tissues (31), contained very high, uniform levels of pSmad1/5/8 signaling, and did not stain with Chordin antibody. In embryos transplanted with Spemann organizer tissue in the ventral side, a second gradient of Chordin was formed in Brachet’s cleft. The Chordin protein diffused a long distance from the lineage-marked organizer graft. The Chordin gradient observed in ECM is self-regulating and resilient after experimental manipulations. In the future, it will be interesting to manipulate the formation of the cleft, by direct microinjection of proteins into Brachet’s cleft or by depletion of components required for its formation, such as Frizzled-7, PAPC, and RhoA (46).
Chordin is secreted in large amounts (23), so it must reach extremely high concentrations within the narrow confines of Brachet’s cleft. This unexpected endogenous graded localization could have important consequences for how we view embryonic patterning. The gradient of BMP activity that patterns both the ectoderm and the mesoderm may result from a single Chordin gradient, helping explain how dorsalizing or ventralizing treatments can affect both germ layers coordinately (13). It is possible that ectodermal and mesodermal cells in contact or in proximity to the Chordin/BMP gradient in the ECM receive Chordin/BMP signals released orthogonally in both directions, enabling cells to read their D-V positional cues. During gastrulation, the germ layers become patterned while simultaneously undergoing very extensive and rapid morphogenetic movements. Perhaps embryonic cells sense their positional information as they move along the ECM surface instead of depending solely on signals relayed in a “bucket-brigade” fashion from cell to cell.
Comparison with Other Systems.
There is a precedent in the literature in which the ECM has been considered as a highway for long-range facilitated diffusion of growth factors. Studying left–right asymmetry in Xenopus embryos, Marjoram and Wright discovered that the ECM flanking the LPM provides a principal surface for the accumulation and facilitated transport of Nodal and its antagonist Lefty at the tailbud stage (47). Using animal cap grafts injected with tagged mRNAs, they demonstrated long-range diffusion of both Nodal and Lefty along the ECMs that separate the ectoderm and endoderm from the LPM. It was found that Nodal moved rostral-ward through the ECM highway, whereas the inhibitor Lefty diffused faster in ECM and also orthogonally into deep adjacent tissue, eventually reaching the opposite side of the embryo. The ECM regions flanking LPM contain Fibronectin and heparan sulfate proteoglycans (HSPGs) (47). Brachet’s cleft is the direct precursor to the ECM that separates the epidermis from the LPM at tailbud stages. In our own study, at the neurula stage, Chordin protein was also detected in the space between the presomitic mesoderm/LPM and the endoderm. Thus, the long-range diffusion of morphogens along the ECM separating cell layers might be a general phenomenon in growth-factor signaling in embryonic development. Syndecan-1, a transmembrane HSPG that has been shown to bind Chordin (48), is expressed in the inner layer of the ectoderm immediately adjacent to Brachet’s cleft at the gastrula stage in Xenopus (49) and could regulate diffusion.
The Chordin/BMP pathway is ancestral and conserved in organisms as diverse as Drosophila, spiders, amphioxus, hemicordates, and vertebrates (50). The Drosophila homolog of Chordin is called “Short gastrulation” (Sog) and is expressed in the ventral side (low-BMP, where the CNS is formed), for the D-V axis was inverted in the course of evolution. Sog is required to shuttle BMPs (called “Dpp” and “Screw”) to the dorsal side to achieve maximal signaling (51, 52). Endogenous Sog protein has been shown to diffuse at a distance from its source during Drosophila D-V patterning (53). However, Sog protein was only detected in dorsal blastoderm cells after its endocytosis, as the method used did not allow direct staining in the outer ECM itself.
Facilitated diffusion, driven by Tolloid proteolysis, during D-V patterning may take place in the perivitelline space separating the embryonic membrane from the innermost layer of the egg shell. Indeed, Sog protein is enriched in the apical side (toward the outside) of the cells that secrete it in the blastoderm embryo (53). In another study, anti-GFP antibodies microinjected into the perivitelline space were shown to be transported together with Dpp-GFP and concentrated on BMP receptors in the dorsal-most cells of the embryo (54). The requirement for a functional ECM during D-V patterning has been demonstrated in Drosophila, as Type IV Collagen is necessary for patterning by Sog/Dpp (55). However, because Type IV Collagen accumulates in both the basal (basal lamina) and apical (perivitelline) surfaces of the blastoderm epithelium, it is unclear where the gradient is actually formed. It will be important in the future to determine directly whether a Sog gradient diffuses in the perivitelline ECM or is generated by a cell-to-cell relay mechanism. If the Sog gradient were formed in the perivitelline ECM, this would raise the possibility that this region is the topological homolog of Brachet’s cleft.
All vertebrate embryos contain an ECM equivalent to Brachet’s cleft in the space between the ectoderm and the endomesoderm; perhaps a Chordin/BMP gradient is formed in this region in other embryos such as zebrafish, chick, and mouse. We have analyzed here the primary embryonic morphogenetic field, yet there are many other later “secondary” self-organizing fields that are formed during organogenesis (e.g., eye, olfactory, pituitary, and limb fields) and regeneration (2, 56). Perhaps long-range-signaling gradients of growth factors and their regulators, emanating from opposite poles of the fields and facilitated by diffusion in the ECM, might be worthwhile investigating in these systems, as well.
Materials and Methods
Antibodies.
An anti-Chordin rabbit polyclonal antiserum raised against the 19 amino-terminal residues of Xenopus Chordin (22) was affinity-purified as described in SI Materials and Methods and used at 1:100. Other antibodies used were rabbit polyclonal anti-pSmad1/5/8 (Cell Signaling 9511L, 1:100), rabbit anti-GFP (Molecular Probes 1:200), and mouse anti-GFP (Santa Cruz Biotechnology 9996, 1:100). Cy3-conjugated goat anti-rabbit antibody (Jackson Immunoresearch, 111–166-003) was used at 1:500 for Chordin staining. For lineage tracing of Spemann’s grafts injected with mGFP mRNA, mouse anti-GFP was used and visualized using Dylight 488-conjugated donkey anti-mouse as the secondary antibody (Jackson Immunoresearch 715–485-150, 1:500).
Immunostaining and in Situ Hybridization.
A step-by-step protocol of the immunostaining procedure is provided in SI Materials and Methods. Some of the steps used to decrease nonspecific background in optical sections included bisection of fixed embryos, treatment of albino embryos in methanol/H2O2 to reduce autofluorescence, Guanidinium⋅HCl for antigen retrieval, reduction of disulfide bonds with DTT followed by blocking of SH groups with iodoacetate to increase signals of extracellular proteins, treatment with sodium borohydride to reduce aldehydes and ketones to alcohols to further decrease autofluorescence, and use of Murray’s solution (benzyl alcohol/benzyl benzoate) to render embryos transparent. Chordin and pSmad1/5/8 were visualized using a Cy3-conjugated secondary antibody (the red channel has lower autofluorescence in Xenopus) and Dylight 488-conjugated secondary antibody was used for GFP staining. Images were acquired and processed identically with an Apotome or a confocal microscope (Zeiss) using a 5× objective and AxioVision 4.8 software (Zeiss). The gradient was quantified using ImageJ, as explained in SI Materials and Methods. For in situ hybridizations, see www.hhmi.ucla.edu/derobertis/index.html.
Embryological Methods.
Antisense MOs used for knockdown of Chordin (24), Tolloids (32), and Sizzled (27) were as described. The zADMP and xBMP2-GFP constructs are described in SI Materials and Methods. For DNA injections, 25 pg of plasmid were injected in a single animal cell into the B1 dorsal blastomere at the 32-cell stage in albino embryos. The dorsal location of the injected cell in ectoderm was confirmed by fluorescent confocal imaging after immunostaining. Marking of the presumptive dorsal or ventral side of the embryo was done by subcortical injection of Nile Blue dye into a single cell at the 8-cell stage using the morphological recognition criteria as described in the legend to Fig. S4. Bisection experiments were done at the blastula stage as described (31), and half-embryos were allowed to develop until control siblings reached stage 12. Spemann grafts were performed as described (24), except that transplantation was performed in 1x Steinberg solution and grafted embryos were transferred after 1 h into 0.1x Steinberg for culture until stage 12. Research using Xenopus embryos has been approved by the University of California, Los Angeles Office of Animal Research Oversight and the Chancellor's Animal Research Committee.
Supplementary Material
Acknowledgments
We thank N. Peyriéras and Y. Sasai for gifts of zebrafish ADMP and Xenopus BMP2, respectively, and members of the E.M.D.R. laboratory for discussions and critical reading of the manuscript. Y.M. was supported by a fellowship from the Japan Society for the Promotion of Science. This work was made possible by long-term support from National Institutes of Health Grant HD21502-26, the Norman Sprague Endowment, and the Howard Hughes Medical Institute, of which E.M.D.R. is an Investigator.
Footnotes
The authors declare no conflict of interest.
See Profile on page 20349.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319745110/-/DCSupplemental.
References
- 1.Spemann H, Mangold H. (1924) Induction of embryonic primordial by implantation of organizers from a different species. Roux Arch Entw Mech 100:599–638. [Reprinted and translated in De Robertis EM, Arechaga J, eds. (2001) Int J Dev Biol 45(1):13–31] [PubMed]
- 2.Spemann H. (1938) Embryonic Development and Induction (Yale Univ Press, New Haven, CT). [Reprinted (1962) (Hafner, New York)]
- 3.Hamburger V. The Heritage of Experimental Embryology: Hans Spemann and the Organizer. Oxford: Oxford Univ Press; 1988. [Google Scholar]
- 4.Cho KWY, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: The role of the Xenopus homeobox gene goosecoid. Cell. 1991;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 6.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urist MR. Bone: Formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 8.De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 2009;126(11-12):925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136(22):3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7(4):296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heasman J. Patterning the early Xenopus embryo. Development. 2006;133(7):1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- 12.Langdon YG, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet. 2011;45:357–377. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- 13.Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988;127(1):64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- 14.Massagué J. TGFβ signaling in context. Nature. 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramel MC, Hill CS. Spatial regulation of BMP activity. FEBS Lett. 2012;586(14):1929–1941. doi: 10.1016/j.febslet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Faure S, Lee MA, Keller T, ten Dijke P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development. 2000;127(13):2917–2931. doi: 10.1242/dev.127.13.2917. [DOI] [PubMed] [Google Scholar]
- 17.Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129(1):37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- 18.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14(1):108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashiguchi M, Mullins MC. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development. 2013;140(9):1970–1980. doi: 10.1242/dev.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plouhinec JL, De Robertis EM. Systems biology of the self-regulating morphogenetic gradient of the Xenopus gastrula. Cold Spring Harb Perspect Biol. 2009;1(2):a001701. doi: 10.1101/cshperspect.a001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasai Y, et al. Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79(5):779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86(4):589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: Secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124(1):147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell. 2003;4(2):219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- 25.Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8(3):401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Piccolo S, et al. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91(3):407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130(4):805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- 28.Muraoka O, et al. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8(4):329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- 29.Inomata H, Shibata T, Haraguchi T, Sasai Y. Scaling of dorsal-ventral patterning by embryo size-dependent degradation of Spemann’s organizer signals. Cell. 2013;153(6):1296–1311. doi: 10.1016/j.cell.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Lee HX, Mendes FA, Plouhinec JL, De Robertis EM. Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev. 2009;23(21):2551–2562. doi: 10.1101/gad.1839309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123(6):1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inomata H, Haraguchi T, Sasai Y. Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell. 2008;134(5):854–865. doi: 10.1016/j.cell.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Ploper D, Lee HX, De Robertis EM. Dorsal-ventral patterning: Crescent is a dorsally secreted Frizzled-related protein that competitively inhibits Tolloid proteases. Dev Biol. 2011;352(2):317–328. doi: 10.1016/j.ydbio.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serpe M, et al. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14(6):940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambrosio AL, et al. Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell. 2008;15(2):248–260. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453(7199):1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 37.Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: Novel roles for integrin and fibronectin. Development. 2001;128(18):3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- 38.Nieuwkoop PD, Florschütz PA. Quelques caractères spéciaux de la gastrulation et de la neurulation de l'oeuf de Xenopus laevis, Daud. et de quelques autres Anoures. Arch Biol (Liege) 1950;61:113–150. [Google Scholar]
- 39.Keller R. Early embryonic development of Xenopus laevis. Methods Cell Biol. 1991;36:61–113. doi: 10.1016/s0091-679x(08)60273-3. [DOI] [PubMed] [Google Scholar]
- 40.Moos M, Jr, Wang S, Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development. 1995;121(12):4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- 41.Inui M, et al. Self-regulation of the head-inducing properties of the Spemann organizer. Proc Natl Acad Sci USA. 2012;109(38):15354–15359. doi: 10.1073/pnas.1203000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willot V, et al. Cooperative action of ADMP- and BMP-mediated pathways in regulating cell fates in the zebrafish gastrula. Dev Biol. 2002;241(1):59–78. doi: 10.1006/dbio.2001.0494. [DOI] [PubMed] [Google Scholar]
- 43.François P, Vonica A, Brivanlou AH, Siggia ED. Scaling of BMP gradients in Xenopus embryos. Nature. 2009;461(7260):E1. doi: 10.1038/nature08305. discussion E2. [DOI] [PubMed] [Google Scholar]
- 44.Klein SL. The first cleavage furrow demarcates the dorsal-ventral axis in Xenopus embryos. Dev Biol. 1987;120(1):299–304. doi: 10.1016/0012-1606(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 45.Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125(23):4681–4690. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- 46.Gorny AK, Steinbeisser H. Brachet’s cleft: A model for the analysis of tissue separation in Xenopus. Wiley Interdiscip Rev Dev Biol. 2012;1(2):294–300. doi: 10.1002/wdev.24. [DOI] [PubMed] [Google Scholar]
- 47.Marjoram L, Wright C. Rapid differential transport of Nodal and Lefty on sulfated proteoglycan-rich extracellular matrix regulates left-right asymmetry in Xenopus. Development. 2011;138(3):475–485. doi: 10.1242/dev.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jasuja R, Allen BL, Pappano WN, Rapraeger AC, Greenspan DS. Cell-surface heparan sulfate proteoglycans potentiate chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of chordin. J Biol Chem. 2004;279(49):51289–51297. doi: 10.1074/jbc.M408129200. [DOI] [PubMed] [Google Scholar]
- 49.Teel AL, Yost HJ. Embryonic expression patterns of Xenopus syndecans. Mech Dev. 1996;59(2):115–127. doi: 10.1016/0925-4773(96)00584-9. [DOI] [PubMed] [Google Scholar]
- 50.De Robertis EM. Evo-devo: Variations on ancestral themes. Cell. 2008;132(2):185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holley SA, et al. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86(4):607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 52.Ashe HL, Levine M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature. 1999;398(6726):427–431. doi: 10.1038/18892. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasan S, Rashka KE, Bier E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev Cell. 2002;2(1):91–101. doi: 10.1016/s1534-5807(01)00097-1. [DOI] [PubMed] [Google Scholar]
- 54.Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434(7030):229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455(7209):72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 56.De Robertis EM, Morita EA, Cho KWY. Gradient fields and homeobox genes. Development. 1991;112(3):669–678. doi: 10.1242/dev.112.3.669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.