Abstract
We recently reported that the D2/D3 agonist pramipexole may have pro-cognitive effects in euthymic patients with bipolar disorder (BPD); however, the emergence of impulse-control disorders has been documented in Parkinson's disease (PD) after pramipexole treatment. Performance on reward-based tasks is altered in healthy subjects after a single dose of pramipexole, but its potential to induce abnormalities in BPD patients is unknown. We assessed reward-dependent decision making in euthymic BPD patients pre- and post 8 weeks of treatment with pramipexole or placebo by using the Iowa Gambling Task (IGT). The IGT requires subjects to choose among four card decks (two risky and two conservative) and is designed to promote learning to make advantageous (conservative) choices over time. Thirty-four BPD patients completed both assessments (18 placebo and 16 pramipexole). Baseline performance did not differ by treatment group (F=0.63; p=0.64); however, at week 8, BPD patients on pramipexole demonstrated a significantly greater tendency to make increasingly high-risk, high-reward choices across the five blocks, whereas the placebo group's pattern was similar to that reported in healthy individuals (treatment × time × block interaction, p<0.05). Analyses of choice strategy using the expectancy valence model revealed that after 8 weeks on pramipexole, BPD patients attended more readily to feedback related to gains than to losses, which could explain the impaired learning. There were no significant changes in mood symptoms over the 8 weeks, and no increased propensity toward manic-like behaviors were reported. Our results suggest that the enhancement of dopaminergic activity influences risk-associated decision-making performance in euthymic BPD. The clinical implications remain unknown.
Keywords: bipolar disorder, dopamine, pramipexole, decision-making
INTRODUCTION
Dopamine (DA) is critical in normal cognitive functioning and reward-based learning (Balleine et al, 2007; Schultz, 2006). Although several studies, in both animals and humans, have demonstrated beneficial cognitive effects of pro-dopaminergic agents on measures of attention and working memory (Costa et al, 2009; Granon et al, 2000; Mattay et al, 2000; Mehta et al, 2000), there have also been reports of rare but concerning side effects, including induced impulse-control disorders in individuals with no prior history of such difficulties (Dodd et al, 2005; Driver-Dunckley et al, 2007; Giovannoni et al, 2000; Klos et al, 2005; Madden et al, 2010; McKeon et al, 2007; Munhoz et al, 2009; Potenza et al, 2007; Riba et al, 2008; Voon et al, 2006b; Weintraub et al, 2006).
For example, the DA D2/D3 receptor agonist, pramipexole, as well as other DA receptor agonists have been implicated in the emergence of risk-seeking behaviors, such as pathological gambling in multiple case reports and cross-sectional studies in patients with Parkinson's disease (PD) (Bodi et al, 2009; Dodd et al, 2005; Lader, 2008; Pontone et al, 2006; Potenza et al, 2007; Voon et al, 2006a, 2006b Weintraub et al, 2006, 2010). A purported mechanism for this effect is related to pramipexole's high selective affinity for D3 receptors, which are primarily expressed in the mesocorticolimbic DA pathway—a circuitry that is active during impulsive decision making (Madden et al, 2010). Indeed, several studies that have used pramipexole in single-dose challenge paradigms have confirmed its actions on reward-related neural networks, primarily at low doses and in healthy individuals (Riba et al, 2008; Ye et al, 2011). Low doses of pramipexole (eg, 0.25–0.5 mg) are thought to influence reward via a paradoxical effect related to activation of the presynaptic D2 autoreceptor, resulting in a blockade of phasic DA release and a blunted response to rewarding stimuli (Riba et al, 2008). In contrast, higher doses of pramipexole, including those in the range used to treat PD, act as specific agonists both presynaptically and postsynaptically to enhance dopaminergic activity (Mierau et al, 1995), which may be more relevant to the potential to induce compulsive behaviors, such as pathological gambling, although the specific distinctions have not been directly demonstrated.
Although pramipexole has received indications for PD and restless legs syndrome, it has also been used off-label in several studies as an antidepressant in patients with major mood disorders (Aiken, 2007). Preliminary data suggest that pramipexole is safe and effective in treating depression when added to mood stabilizers in patients with bipolar disorder (BPD) (Goldberg et al, 2004; Zarate et al, 2004) at least in short-term trials; however, less is known over longer durations (Cassano et al, 2004). Given the data regarding pramipexole's effect on impulse-control and reward-based decision making in patients with PD, particular caution should be taken when using it in patients with BPD, who are predisposed to risky behaviors associated with impaired decision making (Lader, 2008).
Bipolar patients exhibit impulsive behaviors, such as increased spending and hypersexual activity during periods of manic exacerbations, and when tested in the laboratory they consistently demonstrate deficits on decision-making tasks compared with healthy controls (Adida et al, 2008, 2011; Jollant et al, 2007; Martino et al, 2011; Yechiam et al, 2008). Even during periods of remission, patients with BPD demonstrate decreased reward-based learning (Pizzagalli et al, 2008), which may be related to reduced sensitivity to emotional contexts that highlight rewards or punishments (Chandler et al, 2009). Convergent evidence from research on the behavioral activation system (BAS) suggests that patients with BPD report elevated sensitivity to reward-relevant stimuli, an abnormality which may also be related to risk for developing BPD (Johnson et al, 2012). More research is required to better understand the exact nature of reward-related abnormalities in BPD.
There is a paucity of data on the potential for pramipexole to cause impulse-control disorders in patients with BPD. A single case report described a 56-year-old male with bipolar II disorder, who developed pathological gambling after 44 weeks of treatment with pramipexole. Upon drug discontinuation, the gambling behavior was abruptly extinguished, implicating pramipexole as causative (Strejilevich et al, 2011). As there are no empirical data on the prevalence of pramipexole exposure in BD patients in the community, it is impossible to establish the rarity of such a case or to compare it with similar side effects associated with other medications. Nonetheless, given the convergent data in PD and in healthy control subjects alongside a predisposition toward impulsive behaviors in BPD (Adida et al, 2008, 2011; Jollant et al, 2007; Martino et al, 2011; Yechiam et al, 2008), it would be reasonable to predict that BPD patients might be especially susceptible to the development of impulse-control disorders or behaviors associated with poor emotional decision making when taking a DA agonist.
Therefore, in the context of an 8-week, double-blind, placebo-controlled trial of pramipexole for cognitive enhancement in BPD (Burdick et al, 2012), we measured emotional decision-making capacity before and after drug administration in a subset of euthymic patients with BPD, who completed the study using the Iowa Gambling Task (IGT). The aim of the study was to test for effects of pramipexole on decision making related to risk and reward outcomes. We hypothesized that pramipexole would have a deleterious effect on gambling task performance in patients with BPD.
MATERIALS AND METHODS
Patient Participants
Data were collected between July 2006 and April 2010, in a Stanley Medical Research Institute-funded, 8-week, double-blind, placebo-controlled, adjunctive trial of pramipexole. The study was approved by the North Shore-Long Island Jewish Health System (NSLIJHS) Institutional Review Board and was registered on clinicaltrials.gov (NCT00597896). All participants signed informed consent before any study procedure.
Fifty stable outpatients with a clinical diagnosis of bipolar I or bipolar II disorder were randomized to the placebo or the pramipexole condition. Forty-five subjects completed the trial and were included in the primary analyses investigating the effects of pramipexole on neurocognitive functioning (Burdick et al, 2012). During the study, we added a measure of emotional decision making (the IGT) to our battery to more comprehensively assess side effects associated with impulsive behaviors. The analyses of this task, therefore, included a cohort of 34 patients who completed both pre- and posttreatment assessments—details on the full sample are available in Burdick et al (2012). The IGT BPD sample had a mean age of 43.9±11.6 years, were 59% female, and 68% Caucasian. At baseline, the sample had an estimated premorbid IQ of 100.8±9.0, a mean Hamilton Depression Rating Scale (HAM-D) score of 5.1±2.9, and a mean Clinician Administered Rating Scale for Mania (CARS-M) of 2.9±2.3—indicative of euthymia. Again, at week 8 the BPD sample was euthymic with a mean HAM-D score of 3.9±3.6 and a mean CARS-M score of 2.8±3.9. There were no clinical or demographic differences between the IGT sample reported here and the full sample of BPD subjects reported in Burdick et al 2012 (data not shown).
All subjects were recruited from the Zucker Hillside Hospital (ZHH-NSLIJHS) outpatient division. Individuals who provided written informed consent were eligible to participate in the investigation if there was no history of CNS trauma, known neurological disorder, or learning disability. In addition, patients who received a DSM-IV diagnosis of current/recent substance abuse or dependence (ie, within the past month) were excluded from participation. Participants were also excluded if their premorbid IQ was estimated to be 80 or below on the WRAT Reading subtest.
Although medication status is an important factor, given the high prevalence of combination pharmacotherapy for BPD, it was not practical to exclude patients who were taking any medications. Rather, to help control for the effects of medication on cognition, patients were excluded from the analyses if they used a benzodiazepine, sedative, or sleeping pill within 6 h of neurocognitive testing. Furthermore, patients taking medications that are known to interact with pramipexole (eg, cimetidine) were excluded. We recognize the potential for lithium, anticonvulsants, antidepressants, and antipsychotic medications to influence cognitive performance, but it was not feasible to disallow these medications given their widespread use in mood stabilization. However, a stable regimen, for at least 4 weeks before randomization with no medication/dosage changes during the 8-week study period, was required for inclusion.
Measures
As part of their participation in the study, subjects were administered a comprehensive assessment battery, at baseline and at week 8, consisting of behavioral ratings, a structured diagnostic interview, and a neurocognitive battery. The full details on the neurocognitive assessment are provided in Burdick et al (2012); the current study focused on the single measure of emotional decision making, the IGT, which is described below.
DSM-IV-based diagnoses were determined using the Structured Clinical Interview for DSM-IV (SCID-IV). When available, the subjects' report was augmented by medical records and collateral information from family members, and the resulting data were presented to senior faculty, residents, fellows, and other trained SCID raters at a weekly diagnostic conference, to establish a consensus diagnosis. The current severity of affective symptoms was assessed weekly throughout the 8-week clinical trial using the HAM-D (Hamilton, 1960) and the CARS-M (Altman et al, 1994). ZHH raters were trained to a high inter-rater reliability (ICC>0.80) on the administration of these scales.
The IGT (Bechara et al, 1994) was used to assess emotional decision-making abilities. We chose this task over other reward-based measures because of its face validity in the context of pramipexole's potential to induced pathological gambling. It also represents a useful measure in that it entails both gains and losses, incorporating both positive and negative feedback. Specifically, in this task the subject is asked to choose cards from four decks (100 choices in total), resulting in monetary gains or losses. Decks A and B are disadvantageous (high risk–high reward) in that the subjects obtain large immediate wins, but they also attain intermittent large losses, resulting in a net overall loss. In contrast, decks C and D are advantageous (low risk–low reward) in that they result in smaller immediate gains but lower long-term penalties and net an overall win. Healthy volunteers are able to discern over 5 blocks (20 cards each) that choosing from decks C and D is most beneficial, and a normal learning curve is typical over the 5 blocks (Bechara et al, 1994).
In addition to learning curve analyses on the IGT, an expectancy valence (EV) model can be deployed for this task to determine card-by-card strategy (Busemeyer and Stout, 2002). This model results in three indices. Recency, the learning rate parameter, reflects a person's ability to use information from prior trials to form expectancies based upon probabilistic function. Healthy individuals typically begin with a random choice approach until expectancies develop, which can then be used to influence card selection. Higher scores on this parameter indicate better expectancy-based learning. Attention weight given to losses as opposed to gains (Attention Losses/Gains)determines to what degree a person uses feedback related to losing money versus feedback regarding wins (gains) over the course of the task to inform subsequent card choice. This is related to the emotional reaction a person has when he/she wins or loses (called a valence), from which is derived an attention weight parameter that reflects a person's relative attention to losses and wins. When equal weight is given, the value is 0.5; when losses are totally ignored and all choices are based on wins, the value is 0, and likewise when only information related to losses is attended to, the value is 1.0. Reliability, a marker of choice consistency or erratic, impulsive choice behavior is a measure of sensitivity. Increased sensitivity (higher scores) indicate more consistent, less random card choice. The current study utilized the learning curve and the three EV indices as primary outcome measures for the IGT. The description of this task as an ‘emotional' decision-making task is based upon this EV model; others have referred to this measure as a reward-based decision-making task.
Data Analysis
Treatment groups were compared on basic demographic and clinical variables using multivariate analysis of variance and χ2-test where appropriate.
To examine the effects of pramipexole on IGT performance, the data were analyzed in two different ways. First, NET scores were derived by subtracting the number of cards chosen from advantageous decks from the total chosen from the disadvantageous decks ((C+D)−(A+B)) over the course of five blocks (trials 1–20 (NET 1), 21–40 (NET 2), 41–60 (NET 3), 61–80 (NET 4), and 81–100 (NET 5)). A repeated-measures (RM) ANCOVA was conducted with Group (active drug vs placebo) serving as the between-subjects factor; Time (baseline, week 8) as a two-level within-subjects factor; and Block as a five-level within-subjects factor. Covariates included age, change in HAM-D ratings, and change in CARS-M ratings, and the main and interaction effects are described below. To better understand the pattern of performance that emerged, the card-by-card strategy implemented by the two patient groups' performance at baseline and at the conclusion of the 8-week trial was then assessed. A series of three, RM-ANCOVAs was conducted; Recency, Attention Losses/Gains, and Reliability (consistency of response) served as the dependent variables.
RESULTS
Treatment groups (placebo, n=18; pramipexole, n=16) did not differ in age, premorbid IQ, race, or sex distribution (Table 1). All subjects in the pramipexole arm reached the maximum daily dosage (1.5 mg/day) by week 4 and remained at this dose for the duration of the 8 weeks. There were no significant differences by treatment type with regard to symptoms of depression or mania at baseline or at week 8, and both groups were in the euthymic range at the time of both assessments. There were nonsignificant differences between groups with regard to the distribution of bipolar subtype (I vs II) and prevalence of prior substance-use history (no subject met current DSM-IV criteria for abuse or dependence). Moreover, there were no differences in the classes of psychotropic medications used by subjects assigned to pramipexole vs those assigned to placebo (Table 1). Finally, the change in depression ratings (HAM-D) from baseline to week 8 was comparable in the active (mean change of 1.6±3.7) and the placebo (mean change of 1.0±3.2) arms (F=0.23; df=1, 33; p=0.64), as was the change in mania symptoms (CARS-M) with a pramipexole group mean change of −0.13±4.0 vs the placebo group mean change of 0.44±3.1 (F=0.27; df=1, 33; p=0.64). These change scores represent clinically insignificant changes in affective symptoms over the 8-week study period; however, we included change scores as covariates in all models to ensure control for even a slight variance in mood.
Table 1. Characteristics of the Sample by Group.
| Placebo (n=18) | Pramipexole (n=16) | Stat | p-value | |
|---|---|---|---|---|
| Age (years) | 44.9 (12.9) | 42.7 (10.4) | F=0.31 | 0.58 |
| % Female | 61.1 | 56.3 | χ2=0.08 | 0.77 |
| % Caucasian | 61.1 | 75.0 | χ2=0.75 | 0.39 |
| Bipolar I/bipolar II | 13/5 | 11/5 | χ2=0.05 | 0.82 |
| % With past Substance use D/O | 50% | 50% | χ2=0.00 | 1.00 |
| Premorbid IQ | 100.8 (9.7) | 100.9 (8.5) | F<0.01 | 0.98 |
| Baseline HAM-D | 4.7 (2.8) | 5.6 (3.0) | F=0.85 | 0.37 |
| Baseline CARS-M | 2.3 (2.1) | 3.7 (2.4) | F=3.26 | 0.08 |
| Week-8 HAM-D | 3.7 (3.5) | 4.1 (3.9) | F=0.07 | 0.79 |
| Week-8 CARS-M | 1.8 (2.8) | 3.8 (4.7) | F=2.27 | 0.14 |
| % On antipsychotics | 66.7 | 62.5 | χ2=0.06 | 0.80 |
| % On antidepressants | 50.0 | 37.5 | χ2=0.54 | 0.43 |
| % On lithium | 38.9 | 50.0 | χ2=0.42 | 0.52 |
| % On anticonvulsants | 50.0 | 43.8 | χ2=0.13 | 0.72 |
IGT Analyses by Block
A RM-ANCOVA revealed a significant main effect of Time (F=4.20; df=1, 29; p=0.05); a significant two-way interaction of Time × Block (F=4.12; df=4, 26; p=0.004); and a significant three-way interaction for Time × Block × Group (F=3.49; df=4, 26; p=0.01). These results survive the Bonferroni correction for multiple testing corrected for the three factors included in the model. There were no other significant main effects or interactions (Table 2). Post-hoc tests of within-subject contrasts for the Time × Block × Group interaction were significant for a linear trend (F=8.45; df=1, 29; p=0.007).
Table 2. R-MANCOVA Results.
| df | F | p-value | |
|---|---|---|---|
| Time | 1, 29 | 4.20 | 0.05* |
| Time × age | 1, 29 | 2.70 | 0.11 |
| Time × HAM-D Δ | 1, 29 | 0.03 | 0.87 |
| Time × CARS-M Δ | 1, 29 | 0.10 | 0.75 |
| Time × Group | 1, 29 | 0.004 | 0.95 |
| Block | 4, 26 | 1.17 | 0.33 |
| Block × HAM-D Δ | 4, 26 | 0.60 | 0.67 |
| Block × Age | 4, 26 | 1.11 | 0.36 |
| Block × CARS-M Δ | 4, 26 | 0.86 | 0.49 |
| Block × Group | 4, 26 | 0.27 | 0.90 |
| Time × Block | 4, 26 | 4.12 | 0.004* |
| Time × Block × HAM-D Δ | 4, 26 | 0.90 | 0.47 |
| Time × Block × CARS-M Δ | 4, 26 | 0.81 | 0.52 |
| Time × Block × Group | 4, 26 | 3.49 | 0.01* |
p-value ⩽ 0.05
Covariates/other variables of interest
When the model was repeated without age and mood state change scores as covariates, all results remained significant. In addition, when sex was entered as a fixed factor, there were no significant main or interaction effects and the three-way interaction Time × Block × Group remained significant. Although groups did not differ on prior substance-use disorders, given its potential to influence results we re-ran the analysis including this as a factor and, again, the results remained significant. Finally, in an effort to test for the effects of concomitant medications on the results, we first dichotomized patients based on the presence/absence of lithium, antipsychotics, anticonvulsants, and antidepressants. Although this is not ideal, it is the best way to capture these variables in our data set. We ran the RM-ANOVA with medication class as the between-subjects factor and found no significant main effects of any of the medication classes.
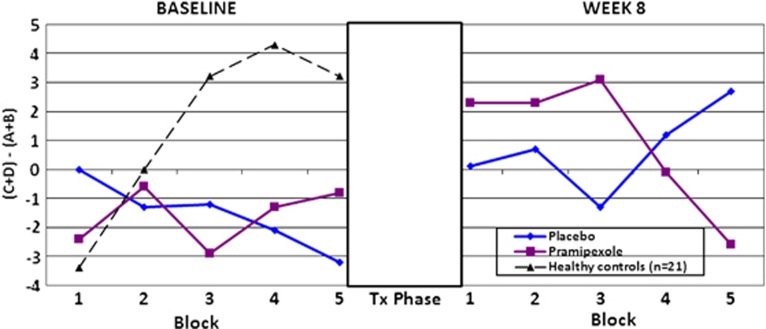
To explicate the three-way interaction, we conducted separate RM-ANCOVAs for baseline IGT performance and for week 8 performance. For baseline analyses, we included baseline ratings for depression (HAM-D) and mania (CARS-M) as covariates. There were no significant main effects and no significant interaction effects, indicating that IGT performance did not differ by treatment group at baseline (Block × Group interaction: F=0.63; df=4, 27; p=0.64; Figure 1 left panel), with both patient groups showing evidence of a blunted learning curve in comparison with what is typically reported in healthy controls (Bechara et al, 1994). For week 8 analyses, we included HAM-D and CARS-M scores at week 8 as covariates. After the 8-week treatment period, results indicate a differential pattern of performance in patients taking pramipexole vs those taking placebo (Group × Block interaction: F=2.57; df=4, 27; p=0.04; Figure 1, right panel). There were no other significant main or interaction effects at week 8. The pattern of performance after treatment indicated nonsignificant changes from baseline to week 8 in the placebo condition, albeit with some evidence of an improvement with a more normalized learning curve, which may represent practice effects.
Figure 1.
The Iowa Gambling Task (IGT) results by group pre- and post-treatment. Performance across five blocks on the IGT. The x-axis represents the five blocks of the task, with the left panel marking the performance at baseline and the right panel marking the performance at week 8. A gray-scale rectangle depicts the 8-week treatment trial, during which time no testing was conducted. The y-axis shows the NET score variable, which is calculated by subtracting the total number of cards chosen from the disadvantageous decks from the total number of cards chosen from the advantageous decks.
In contrast, patients on pramipexole evidenced a pattern of performance over the five blocks akin to a reverse learning curve. In other words, patients taking pramipexole chose increasingly more disadvantageous choices vs advantageous choices over the five blocks, a pattern that is opposite from what is normally seen in healthy individuals (Bechara et al, 1994).
A small sample of healthy controls (n=21) involved in a separate study are depicted in the figures as a reference sample for purposes of illustration. The healthy controls had a mean age of 26.9±8.5 years, were 19% female, and 57% Caucasian. These subjects were not well-matched to our BPD sample demographically and did not complete repeat testing on the IGT; therefore, although their data are provided graphically to depict ‘normal' IGT performance relative to BPD performance, they are not included in the statistical analyses.
Practice effects have been documented in healthy individuals on the IGT (Bechara et al, 2000) over at least three repeated administrations (improvements seen on each attempt). This is in contrast to what has been shown in individuals with VM prefrontal cortex (PFC) lesions/damage, where improvements are negligible (nonsignificant) over time and recovery (Waters-Wood et al, 2012). The presence of practice effects in healthy samples has made estimating reliability on this task difficult. One study published in Brazil specifically addressed a test-retest reliability of the IGT in healthy individuals and noted ‘moderately positive correlations' for Blocks 4 and 5 but weaker relationships in the earlier blocks (Cardoso et al, 2010). In an effort to address the issue of stability in our BD sample, we conducted secondary analyses restricted to the BD sample, to illustrate that as a group (all BD subjects) there is relative stability of performance on Blocks 4 and 5 of the IGT (ICC=0.49; p=0.03). We then split the sample based on treatment group and re-ran the reliability analyses and find that the placebo group shows high stability in Blocks 4 and 5 (ICC=0.57; p=0.04) but the subjects taking pramipexole are less reliable over time (ICC=0.36; p=0.20). Of note, despite higher stability of performance in the placebo condition, these subjects show statistically significant evidence of improvement (or practice effect) by Block 5 (as per paired Student's t-tests by block; t=3.39; df=17; p=0.003), whereas those subjects taking pramipexole not only failed to improve as expected by Block 5 (t=0.55; df =15; p=0.59), their performance actually worsened.
IGT Analyses of Card-by-Card Strategy
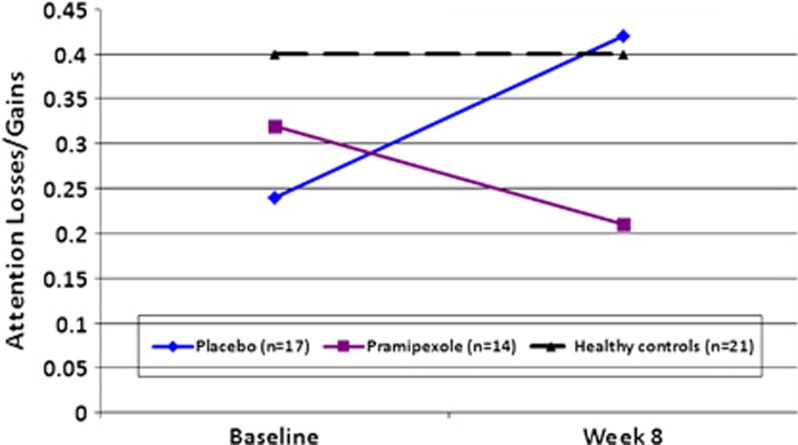
A series of RM-ANCOVAs were then conducted to ascertain whether pramipexole use affected the card-by-card strategy implemented. Recency, Attention Losses/Gains, and Reliability served as the two-level within-subjects-dependent variables in these analyses. When including age, HAM-D change score, and CARS-M change score as covariates in the model, there were no significant main or interaction effects for the Recency or the Reliability indices (data not shown). In contrast, the analyses for the Attention Losses/Gains parameter revealed a significant Time × Group interaction (F=5.08; df=1; p=0.03). There were no other significant main or interaction effects for this index. These results suggest a change from baseline choice strategy to week 8 choice strategy such that subjects taking placebo attended more to losses than to gains after the 8-week study, performing more like a healthy control cohort. In contrast, subjects taking pramipexole attended more to gains than to losses after treatment (Figure 2). Follow-up MANOVA indicated a significant group difference for change score on the Attention Losses/Gains index (baseline score−week 8 score) consistent with this pattern (F=6.01; df=1, 29; p=0.02; placebo mean change score +0.17±0.37; pramipexole mean change score −0.11±0.26). When using the healthy controls' performance as a reference group (black dotted line in Figure 2), the pattern of subjects taking placebo after 8 weeks appears normal, whereas the subjects taking pramipexole deviates further away from the normal pattern of performance. Again, secondary analyses indicated no main effects of concomitant medication on these results.
Figure 2.
Choice strategy: Attention to Losses vs Gains. The card-by-card selection strategy employed with scores from the Attention Losses/Gains index of the expectancy valence model. The x-axis is labeled for the two time points (baseline and week 8), with the y-axis representing the ratio of attention given to negative feedback information (losses) relative to positive feedback (wins). Higher scores represent more attention to losses than to gains.
DISCUSSION
To our knowledge, this is the first study to assess the effects of a DA receptor agonist on emotional decision making in patients with BPD. Our results suggest that after 8 weeks on pramipexole, BPD subjects demonstrate impaired performance on the IGT, consistent with prior reports of DA agonist-induced impulse-control disorders (eg, pathological gambling) in patients with PD (Dodd et al, 2005; Voon et al, 2006a, 2006b; Potenza et al, 2007; Lader, 2008; Bodi et al, 2009; Weintraub et al, 2006, 2010; Pontone et al, 2006). The pattern of performance noted in the pramipexole group is consistent with a reverse learning curve, indicating that over the five blocks of the IGT, patients on pramipexole increasingly chose cards from the disadvantageous decks, the opposite from the pattern expected in healthy individuals (Bechara et al, 1994). High-risk, high-reward choice preferences on a gambling task post-pramipexole treatment were noted, despite no exacerbation in manic symptoms and in the absence of any reported behavioral abnormalities.
Results from the EV model suggest that the poor performance in the pramipexole group at week 8 might be explained by the patients' over-reliance on feedback related to winning money (gains) when making card choices. Both patient groups showed a tendency to attend more readily to gains vs losses at baseline, which is consistent with a hypothesized reduced sensitivity to reward (Chandler et al, 2009). However, after treatment, BPD subjects who were assigned to the placebo condition attended more equally to positive and negative feedback, similar to patterns noted in healthy individuals. This improvement in the placebo condition is most likely reflective of practice effects related to a familiarity with the task. In contrast, BPD patients who had been on 8 weeks of pramipexole evidenced an exaggerated tendency to ignore loss information with a greater regard for feedback indicating wins.
These data are consistent with a substantial literature on the BAS and its relationship with mania and BPD (for review, see Johnson et al, 2012). In general, evidence suggests that patients with BPD are willing to expend more effort toward rewarding stimuli and increase goal pursuit following an initial reward. Experimental methods used to amplify activity in the incentive salience system, through knockout of the DA transporter gene or via administration of amphetamine, not only result in behavioral changes consistent with mania but also enhance an animal's willingness to expend effort toward reward without altering other aspects of learning (Berridge, 2007).
These results are also consistent with prior reports of compulsive gambling in patients with PD after clinical treatment with pramipexole and similar agents (Aiken, 2007). Several neuroimaging studies have attempted to elucidate the effect that pramipexole has on reward processing in the context of single-dose challenge paradigms in both PD patients and in healthy subjects. In a study of healthy males, Riba et al (2008) showed a blunted activation in the rostral basal ganglia and midbrain, following an unexpected monetary gain 2 h after administration of 0.5 mg of pramipexole vs a placebo. Similarly, a decrease in the correlation between reward prediction error and BOLD response was observed in the orbitofrontal cortex and striatum in early-stage PD patients ∼1–2 h after administration of 1.0 mg of pramipexole (van Eimeren et al, 2009), suggesting impairment in reward circuitry. A recent functional connectivity analysis of healthy males demonstrated that pramipexole increases activity in the nucleus accumbens and weakens connectivity between this structure and the PFC during reward anticipation (Ye et al, 2011). Taken together, these data suggest that pramipexole induces abnormal activation patterns and connectivity within the neural circuitry critical to reward-based learning, an action that is likely to explain the IGT data in our BPD patients. It remains possible, of course, that the changes noted on the IGT performance in our cohort were related to some unknown third variable and were not direct effects of the drug; however, given the matching of the treatment groups at both baseline and week 8, we believe that this is an unlikely explanation.
Pramipexole is a partial/full D2/D3 agonist with selective affinity for DA receptors of the D2 subfamily (Antonini and Calandrella, 2011). Unlike psychostimulant medications, it has an eightfold greater affinity for D3 over the D2 receptor (Mierau et al, 1995; Gerlach et al, 2003) and it acts on presynaptic and on postsynaptic receptors (Piercey, 1998). In intact dopaminergic systems and at lower doses, pramipexole primarily stimulates presynaptic autoreceptors of the D3 and D2 type, thereby reducing the synthesis and synaptic release of DA. Stimulation of D2 and D3 postsynaptic receptors is observed with reduced DA release due to loss or damage of the presynaptic terminations (Mierau et al, 1995). Specifically, in patients with PD, pramipexole imparts its therapeutic effects on motor deficits through the postsynaptic D2 and D3 receptors to correct an imbalance between the direct and the indirect striatofugal nerve tracts by enhancing the direct transmission (through D3 receptors) and reducing the indirect transmission (through D2 receptors; (Mierau et al, 1995)).
Pramipexole's relative specificity allows for a unique opportunity to enhance DA in phylogenetically older regions of the brain that are associated with emotion regulation and cognitive function (Camacho-Ochoa et al, 1995). Consistent with this mechanism of action, pramipexole has a direct antidepressant effect in patients with PD (Leentjens et al, 2009) above and beyond its efficacy for motor symptoms associated with the illness (Barone et al, 2010). This antidepressant effect has also been noted in mood-disordered patients (Aiken, 2007), including patients with BPD (Goldberg et al, 2004; Zarate et al, 2004). Of particular relevance, preclinical data suggest a neurotrophic effect of pramipexole in models of neurodegenerative disorders (Lauterbach et al, 2010), which is in part mediated by the anti-apoptotic protein bcl-2 (Takata et al, 2000). In line with these beneficial effects of pramipexole, but somewhat contrary to the IGT data described here, we recently reported an improvement on measures of attention and working memory in this same cohort after pramipexole treatment (Burdick et al, 2012), suggestive of a pro-cognitive effect of this agent in euthymic BPD patients.
One potential explanation for these contradictory effects might be related to the action of pramipexole at both the D2 and the D3 receptor sites. Although the primary binding site for pramipexole is the D3 receptor, its affinity to the D2 receptor remains high (Kvernmo et al, 2006). D2 receptors are localized to several brain regions that are critical to higher-order cognitive control (van Holstein et al, 2011), and D2 agonists such as bromocriptine have demonstrated pro-cognitive effects in healthy individuals (Kimberg et al, 1997; Luciana et al, 1998), particularly in cognitive domains linked to PFC functions. We would hypothesize that pramipexole's activity at the D2 receptors could readily explain enhancement on attention and working memory tasks that are closely linked with the availability of DA in the PFC (Badre, 2012). In contrast, D3 receptors are most widely distributed in regions involved in reward-based learning (Beninger and Banasikowski, 2008), which might be more directly relevant for task performance on emotional decision-making measures, such as the IGT. D3 receptors are primarily expressed in the mesocorticolimbic DA pathway, and structures in this circuitry show increased activation during impulsive decision making (see Madden et al, 2010 for review). D3 receptors are involved in phasic, not tonic, DA signaling, suggesting an important role for the D3 receptor in modulating the emotional experience of novelty, reward, and risk assessment (Kelley et al, 2012).
As this study was primarily focused on the potential cognitive enhancing properties of pramipexole and was not specifically designed to test for deleterious effects on emotional decision making, only a subset of our sample completed the IGT at both time points. This resulted in a smaller sample size with reduced power; nonetheless, even in a relatively small cohort, we were able to detect a significant three-way interaction effect, indicating an effect of pramipexole on reward-based learning in BPD. In addition, all of our BPD subjects were medicated at the time of assessment and most were taking more than one psychotropic medication, making stratification for concomitant medication status unfeasible. Future, larger-scale studies will be important in determining the effects of other medications on these results. Finally, we did not conduct any neuroimaging with these subjects and, therefore, cannot determine with any certainty the mechanism by which pramipexole may simultaneously act to enhance certain aspects of neurocognitive functions and to impair reward-based learning.
In summary, we report a novel finding of abnormal reward-related decision making after treatment with the D2/D3 agonist, pramipexole, in patients with BPD. This is the first study to describe the reward-based effects of pramipexole in the context of a controlled clinical trial; our results are consistent with prior work in single-dose studies of healthy subjects and patients with PD. Future studies should address the potential clinical implications of our findings over a longer duration. Although we found no evidence of increased mania or impulsive behaviors in our cohort, it is possible that the deficits noted on the IGT could serve as markers of impending affective instability that could not be fully captured in an 8-week study. Additional work in understanding the relative benefits and deleterious effects of dopaminergic agents in BPD will be important in guiding future treatment efforts targeting neurocognition as an outcome.
FUNDING AND DISCLOSURE
Financial Support for this work included grants from the National Institute of Mental Health (NIMH) to KEB (K23MH077807) and from the Stanley Medical Research Institute (05T-670) to AKM and KEB. Drs Burdick, Braga, and Gopin report no competing interests. Dr Malhotra has served as consultant or speaker for Bristol-Myers Squibb, Merck, Astra Zeneca, Vanda Pharmaceuticals and Clinical Data, and has received research support from Pfizer, Janssen Pharmaceuticals, Bristol-Myers Squibb, and Eli Lilly.
References
- Adida M, Clark L, Pomietto P, Kaladjian A, Besnier N, Azorin JM, et al. Lack of insight may predict impaired decision making in manic patients. Bipolar Disord. 2008;10:829–837. doi: 10.1111/j.1399-5618.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–365. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Aiken CB. Pramipexole in psychiatry: a systematic review of the literature. J Clin Psychiatry. 2007;68:1230–1236. [PubMed] [Google Scholar]
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Antonini A, Calandrella D. Pharmacokinetic evaluation of pramipexole. Expert Opin Drug Metab Toxicol. 2011;7:1307–1314. doi: 10.1517/17425255.2011.614232. [DOI] [PubMed] [Google Scholar]
- Badre D. Opening the gate to working memory. Proc Natl Acad Sci USA. 2012;109:19878–19879. doi: 10.1073/pnas.1216902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:573–580. doi: 10.1016/S1474-4422(10)70106-X. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Banasikowski TJ. Dopaminergic mechanism of reward-related incentive learning: focus on the dopamine D(3) receptor. Neurotox Res. 2008;14:57–70. doi: 10.1007/BF03033575. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bodi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N, et al. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Braga RJ, Nnadi CU, Shaya Y, Stearns WH, Malhotra AK. Placebo-controlled adjunctive trial of pramipexole in patients with bipolar disorder: targeting cognitive dysfunction. J Clin Psychiatry. 2012;73:103–112. doi: 10.4088/JCP.11m07299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment: decomposing performance on the Bechara gambling task. Psychol Assess. 2002;14:253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- Camacho-Ochoa M, Walker EL, Evans DL, Piercey MF. Rat brain binding sites for pramipexole, a clinically useful D3-preferring dopamine agonist. Neurosci Lett. 1995;196:97–100. doi: 10.1016/0304-3940(95)11857-s. [DOI] [PubMed] [Google Scholar]
- Cardoso OC, Carvalho JCN, Cotrena C, Schneider-Bakos DG, Kristensen HC, Fonseca RP. Estudo de fidedignidade do instrumento neuropsicológico Iowa Gambling Task. Jornal Brasileiro de Psiquiatria. 2010;59:279–285. [Google Scholar]
- Cassano P, Lattanzi L, Soldani F, Navari S, Battistini G, Gemignani A, et al. Pramipexole in treatment-resistant depression: an extended follow-up. Depress Anxiety. 2004;20:131–138. doi: 10.1002/da.20038. [DOI] [PubMed] [Google Scholar]
- Chandler RA, Wakeley J, Goodwin GM, Rogers RD. Altered risk-aversion and risk-seeking behavior in bipolar disorder. Biol Psychiatry. 2009;66:840–846. doi: 10.1016/j.biopsych.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Dell'Agnello G, Caltagirone C, Carlesimo GA. Dopamine and cognitive functioning in de novo subjects with Parkinson's disease: effects of pramipexole and pergolide on working memory. Neuropsychologia. 2009;47:1374–1381. doi: 10.1016/j.neuropsychologia.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley ED, Noble BN, Hentz JG, Evidente VG, Caviness JN, Parish J, et al. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol. 2007;30:249–255. doi: 10.1097/wnf.0b013e31804c780e. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P. Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J Neural Transm. 2003;110:1119–1127. doi: 10.1007/s00702-003-0027-5. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, O'Sullivan JD, Turner K, Manson AJ, Lees AJ. Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry. 2000;68:423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry. 2004;161:564–566. doi: 10.1176/appi.ajp.161.3.564. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Annu Rev Clin Psychol. 2012;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Guillaume S, Jaussent I, Bellivier F, Leboyer M, Castelnau D, et al. Psychiatric diagnoses and personality traits associated with disadvantageous decision-making. Eur Psychiatry. 2007;22:455–461. doi: 10.1016/j.eurpsy.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Duker AP, Chiu P. Dopamine agonists and pathologic behaviors. Parkinsons Dis. 2012;2012:603631. doi: 10.1155/2012/603631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, D'Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord. 2005;11:381–386. doi: 10.1016/j.parkreldis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kvernmo T, Hartter S, Burger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther. 2006;28:1065–1078. doi: 10.1016/j.clinthera.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lader M. Antiparkinsonian medication and pathological gambling. CNS Drugs. 2008;22:407–416. doi: 10.2165/00023210-200822050-00004. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC, Victoroff J, Coburn KL, Shillcutt SD, Doonan SM, Mendez MF. Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. J Neuropsychiatry Clin Neurosci. 2010;22:8–18. doi: 10.1176/jnp.2010.22.1.8. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Koester J, Fruh B, Shephard DT, Barone P, Houben JJ. The effect of pramipexole on mood and motivational symptoms in Parkinson's disease: a meta-analysis of placebo-controlled studies. Clin Ther. 2009;31:89–98. doi: 10.1016/j.clinthera.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS, Brewer AT, Pinkston JW, Fowler SC. Effects of pramipexole on impulsive choice in male wistar rats. Exp Clin Psychopharmacol. 2010;18:267–276. doi: 10.1037/a0019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Torralva T, Manes F. Decision making in euthymic bipolar I and bipolar II disorders. Psychol Med. 2011;41:1319–1327. doi: 10.1017/S0033291710001832. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, et al. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- McKeon A, Josephs KA, Klos KJ, Hecksel K, Bower JH, Michael BJ, et al. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord. 2007;13:516–519. doi: 10.1016/j.parkreldis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau J, Schneider FJ, Ensinger HA, et al. Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors. Eur J Pharmacol. 1995;290:29–36. doi: 10.1016/0922-4106(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Munhoz RP, Fabiani G, Becker N, Teive HA. Increased frequency and range of sexual behavior in a patient with Parkinson's disease after use of pramipexole: a case report. J Sex Med. 2009;6:1177–1180. doi: 10.1111/j.1743-6109.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- Piercey MF. Pharmacology of pramipexole, a dopamine D3-preferring agonist useful in treating Parkinson's disease. Clin Neuropharmacol. 1998;21:141–151. [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry. 2008;64:162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67:1258–1261. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Voon V, Weintraub D. Drug Insight: impulse control disorders and dopamine therapies in Parkinson's disease. Nat Clin Pract Neurol. 2007;3:664–672. doi: 10.1038/ncpneuro0680. [DOI] [PubMed] [Google Scholar]
- Riba J, Kramer UM, Heldmann M, Richter S, Munte TF. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS One. 2008;3:e2479. doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Strejilevich SA, Martino DJ, Igoa A, Manes F. Pathological gambling in a bipolar patient treated with pramipexole. J Neuropsychiatry Clin Neurosci. 2011;23:E2–E3. doi: 10.1176/jnp.23.1.jnpe2. [DOI] [PubMed] [Google Scholar]
- Takata K, Kitamura Y, Kakimura J, Kohno Y, Taniguchi T. Increase of bcl-2 protein in neuronal dendritic processes of cerebral cortex and hippocampus by the antiparkinsonian drugs, talipexole and pramipexole. Brain Res. 2000;872:236–241. doi: 10.1016/s0006-8993(00)02493-8. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson's disease. Neuropsychopharmacology. 2009;34:2758–2766. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holstein M, Aarts E, van der Schaaf ME, Geurts DE, Verkes RJ, Franke B, et al. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl) 2011;218:567–78. doi: 10.1007/s00213-011-2340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006a;67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, Duff-Canning S, de Souza M, Fox S, et al. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006b;66:1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- Waters-Wood SM, Xiao L, Denburg NL, Hernandez M, Bechara A. Failure to learn from repeated mistakes: persistent decision-making impairment as measured by the iowa gambling task in patients with ventromedial prefrontal cortex lesions. J Int Neuropsychol Soc. 2012;18:927–930. doi: 10.1017/S135561771200063X. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Hammer A, Camara E, Munte TF. Pramipexole modulates the neural network of reward anticipation. Hum Brain Mapp. 2011;32:800–811. doi: 10.1002/hbm.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Hayden EP, Bodkins M, O'Donnell BF, Hetrick WP. Decision making in bipolar disorder: a cognitive modeling approach. Psychiatry Res. 2008;161:142–152. doi: 10.1016/j.psychres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry. 2004;56:54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]