Abstract
We demonstrated previously that antenatal glucocorticoid treatment (AGT, gestational days 16–19) altered the size and organization of the adult rat midbrain dopaminergic (DA) populations. Here we investigated the consequences of these AGT-induced cytoarchitectural disturbances on indices of DA function in adult rats. We show that in adulthood, enrichment of striatal DA fiber density paralleled AGT-induced increases in the numbers of midbrain DA neurons, which retained normal basal electrophysiological properties. This was co-incident with changes in (i) striatal D2-type receptor levels (increased, both sexes); (ii) D1-type receptor levels (males decreased; females increased); (iii) DA transporter levels (males increased; females decreased) in striatal regions; and (iv) amphetamine-induced mesolimbic DA release (males increased; females decreased). However, despite these profound, sexually dimorphic changes in markers of DA neurotransmission, in-utero glucocorticoid overexposure had a modest or no effect on a range of conditioned and unconditioned appetitive behaviors known to depend on mesolimbic DA activity. These findings provide empirical evidence for enduring AGT-induced adaptive mechanisms within the midbrain DA circuitry, which preserve some, but not all, functions, thereby casting further light on the vulnerability of these systems to environmental perturbations. Furthermore, they demonstrate these effects are achieved by different, often opponent, adaptive mechanisms in males and females, with translational implications for sex biases commonly found in midbrain DA-associated disorders.
Keywords: antenatal glucocorticoid treatment, dexamethasone, dopamine-dependent behaviors, midbrain dopaminergic systems, sex differences
INTRODUCTION
Midbrain dopaminergic (DA) systems are pivotal regulators of normal adaptive behavioral and cognitive functions (Williams and Goldman-Rakic, 1995; Robbins and Everitt, 1996), which are mediated by distinct projections from the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) to the striatum and the prefrontal cortex. Preclinical studies indicate that these DA systems are especially vulnerable to long-term disruption by perturbations in the in-utero environment (Gatzke-Kopp, 2010). Such neurobiological programming may also exist in the human population, where gestational stress is associated with increased risk of childhood behavioral problems (Talge et al, 2007) and the onset of neurological and neuropsychiatric disorders (Seckl and Holmes, 2007; Lupien et al, 2009). Many of these conditions not only involve midbrain DA systems, but also have a neurodevelopmental component, including schizophrenia, ADHD, autism and drug abuse (Lewis and Lieberman, 2000; Melichar et al, 2001; Boksa, 2004; Ben Amor et al, 2005; Szpir, 2006). The mechanisms underlying such neurobiological programming are unclear, but glucocorticoid hormones released in response to stress from the maternal/fetal adrenals are emerging as key factors in brain programming in humans and other animals (Seckl and Holmes, 2007), although the precise nature of changes in specific neuronal phenotypes is poorly characterized.
In previous studies we demonstrated that antenatal glucocorticoid treatment (AGT) with the synthetic glucocorticoid, dexamethasone, during late gestation increased the population size and altered the topographic organization of the midbrain DA neurons within the adult rat VTA and SNc (McArthur et al, 2005; McArthur et al, 2007a). This provided direct evidence that these systems are targets for neurobiological programming by glucocorticoids, on which we base our hypothesis that in-utero programming of midbrain DA systems by inappropriate exposure to glucocorticoids may represent a mechanism linking the early environment to the development of adult psychopathology. The importance of understanding this link from a clinical perspective is further highlighted by the association of childhood behavioral deficits with repeated courses of synthetic glucocorticoids that are commonly prescribed to accelerate lung maturation in the management of women at risk of preterm delivery, despite a lack of systematic investigations of their potential impact on the developing brain, and neurological and behavioral performance (French et al, 2004; Baud and Sola, 2007). Therefore, a central aim of the current study was to evaluate whether the AGT-induced cytoarchitectural disturbances in the VTA and SNc are sufficient to disrupt behaviors modulated by the midbrain DA networks. We used neuroanatomical and neurochemical studies to provide neurobiological indicators of DA transmission, which were complemented with a battery of behavioral tests known to depend on midbrain DA circuitry. These included tests of psychomotor activation and reinforcement, assessed through systemic administration of amphetamine (Kelly et al, 1975), and the acquisition and maintenance of cocaine self-administration (Roberts et al, 1977), which depend on the mesolimbic DA system. We also assessed spontaneous locomotor activity in a novel environment, known to depend on mesolimbic and nigrostriatal DA systems (Jones and Robbins, 1992), as well as prepulse inhibition (PPI) of the startle reflex (Humby et al, 1996; Swerdlow et al, 2001) and appetitive Pavlovian learning (Robbins and Everitt, 2002; Yin and Knowlton, 2006) as behavioral assays of DA function in the nucleus accumbens (NAc). Biological programming of several systems, including the brain, by in-utero overexposure to glucocorticoids via exogenous administration or stress-induced endogenous elevations, has been reported to be sex-specific (Seckl and Holmes, 2007; Brummelte et al, 2012), and the commonest disorders involving midbrain DA circuitry show a sex bias (Aleman et al, 2003; Becker and Hu, 2008; Gillies and McArthur, 2010). Therefore, we also investigated whether AGT programming of midbrain DA systems differs in male and female rats.
MATERIALS AND METHODS
Full details are provided in Supplementary Materials and Methods.
Animals and AGT
All procedures were conducted under license in accordance with the UK Animals (Scientific Procedures) Act of 1986. Animals were maintained under a 12/12-h reversed light/dark cycle (lights off at 0700 h) and controlled temperature (21–23 °C) and humidity (63%), with standard rat chow and drinking water available ad libitum. After timed matings, pregnant Sprague–Dawley rats (Harlan Olac, Beicester, UK) received AGT on gestational days (GDs) 16–19 via our established, non-invasive method of adding the synthetic glucocorticoid, dexamethasone (dexamethasone sodium phosphate; Fauliding Pharmaceuticals, Royal Leamington Spa, UK), to the drinking water at a concentration of 0.5 μg/ml; control dams continued to receive normal drinking water (McArthur et al, 2006; McArthur et al, 2007a). No more than two progeny per litter were included in each of the four experimental groups (male and female AGT and controls) in order to minimize potential effects of litter-of-origin. Group sizes of n=6 were used, except where otherwise stated (see figure legends). Separate cohorts of animals (controls and AGT groups at 3 months of age) were used for each experimental procedure, except the groups tested for amphetamine-stimulated behavior. As test-naive animals, this cohort first provided the data for the spontaneous locomotor activity study (day 1); they then underwent similar testing on day 2 in order to habituate the animals to the novel environment, thereby ensuring a stable baseline before testing amphetamine-induced activity.
Immunohistochemistry
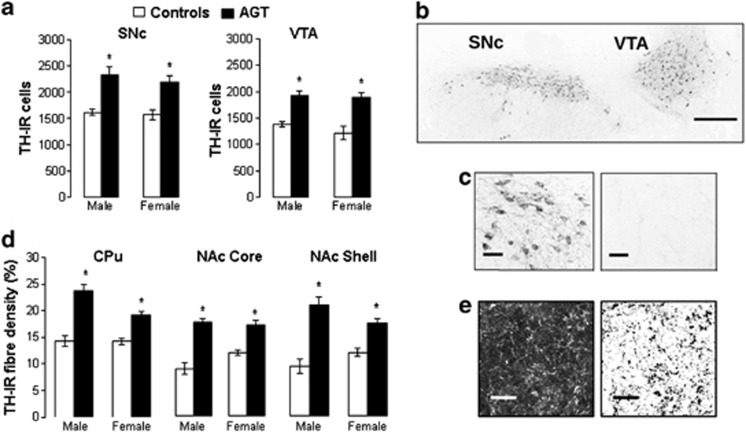
Animals were decapitated between 0900 and 1000 h. Brains were removed and processed for immunohistochemical identification of tyrosine hydroxylase immunorectivity (TH-IR) as a marker of DA cell bodies in the VTA and SNc, and their respective nerve terminals in the ventral striatum (NAc core and shell) and dorsal striatum (caudate putamen, CPu). Estimates of DA neuron numbers were based on protocols for measuring the effects of sex steroid hormones on cell numbers in specific brain nuclei (Balthazart et al, 2008; McArthur et al, 2011; Yamamura et al, 2011). In every second section between −5.1 mm and −5.4 mm, termed Level B (Carman et al, 1991; McArthur et al, 2005; McArthur et al, 2007a), SNc and VTA boundaries, defined by TH-IR cells (Figure 1b), were delineated using a CoolSNAP-Procf camera (Roper Scientific, Marlow, UK) attached to a NikonEclipse E800 microscope (Media Cybernetics, Finchampstead, UK) and an image analysis software package (Image ProPlus 4.5; Media Cybernetics). TH-IR cells (Figure 1c) were counted in squares (50 μm × 50 μm) within an overlay grid using a random systematic sampling protocol; the number of TH-IR cells per field, grid-square area and number of grid squares sampled were used to calculate cell densities. The level B volumes of the SNc and VTA were calculated according to the method of Cavalieri (Brodski et al, 2003; McArthur et al, 2007a). The total number of TH-IR neurons was estimated by relating their numerical cell density to the volume, and was used to calculate the group average (n=6 rats per group). Striatal TH-IR fiber density was analyzed in 1-μm optical sections captured using a Leica SP5 confocal microscope ( × 63 oil immersion objective). Threshold masks were applied using ImageJ software, at a standardized intensity for all samples (Figure 1e). Percentage area cover was then measured by systematic random sampling of matrix regions of the CPu and NAc, allowing for background correction, in at least six different sections per animal. The average fiber density for each region per animal was used to calculate the group average (n=6 rats per group).
Figure 1.
Exposure to antenatal glucocorticoid treatment (AGT; dexamethasone, 0.5 μg/ml, in dams' drinking water on gestational days 16–19) alters the neuroanatomy of the adult nigrostriatal and mesolimbic dopaminergic (DA) pathways compared with controls (dams received normal drinking water). (a) Tyrosine hydroxylase immunoreactive (TH-IR) cell counts in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA; region −5.1 mm to −5.4 mm relative to bregma of male and female rats exposed to AGT (solid histograms) were significantly greater (p<0.05) than in controls (open histograms). (b) Immunofluorescent visualization of TH-IR cells in representative coronal sections at low power (bar represents 100 μm) illustrating SNc and VTA boundaries; for clarity, images are presented in reverse greyscale. (c) Immunofluorescent visualization of TH-IR cells and control (no primary anti-TH antibody) in representative coronal sections of the SNc at high power (reverse greyscale images; bar represents 15 μm). (d) TH-IR fiber density (expressed as percentage area coverage) in the dorsal striatum (caudate putamen, CPu) and ventral striatum (nucleus accumbens (NAc) core and shell) of adult male and female rats exposed to AGT (solid histograms) was significantly greater (p<0.05) than in controls (open histograms). (e) TH-IR fibers in a representative confocal microscopic 1 μm optical coronal section (left-hand panel) and the overlay mask for calculating percentage area cover (right-hand panel) through the dorsal striatum. Bars represent 10 μm. Data are represented as means±SEM, n=6, *p<0.05 vs relative control group.
Autoradiography
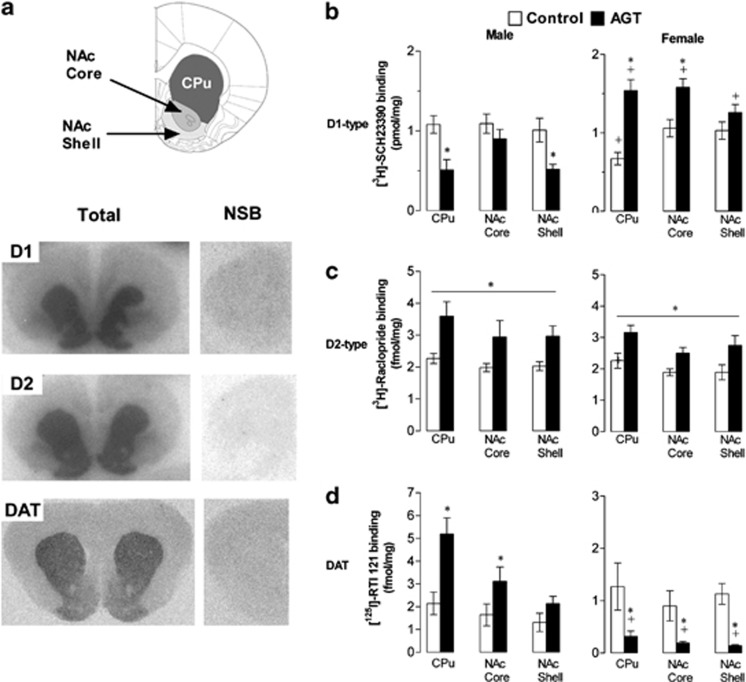
The DA transporter (DAT) and D1-type (D1) and D2-type (D2) receptors were analyzed as pre- and postsynaptic markers of DA function in the CPu, NAc (core and shell). Animals were decapitated between 0900 and 1000 h. Brains were removed and processed for quantification of DAT, D1 and D2 levels using specific binding of the highly selective ligands [125I]-RTI 121, [3H]-SCH23390 and [3H]-raclopride, respectively, following protocols previously described (McArthur et al, 2007b), with examples shown in Figure 2a. Films were developed and digitized using an MCID Core system attached to a CoolSNAP-Procf camera (Interfocus Imaging, Cambridge, UK). Densitometric analysis was performed by systematic random sampling, using NIH ImageJ, within the defined brain regions (Paxinos and Watson, 1998). Average values from six different sections per region per animal were used to calculate the group means (n=6 per group).
Figure 2.
Sexually dimorphic changes in the expression of dopaminergic (DA) signaling proteins after antenatal glucocorticoid treatment (AGT). (a) Top panel schematically illustrates regions of interest, namely the dorsal striatum (caudate putamen, CPu), nucleus accumbens (NAc) core, and NAc shell based on the rat brain atlas, and a coronal section +2.2 mm relative to bregma (Paxinos and Watson, 1998). Serial sections were collected between +1.7 mm and +2.2 mm relative to bregma for the NAc core and shell, whereas for the CPu serial sections were collected between −0.2 and +1.6 mm relative to bregma. Lower panels are representative autoradiographic images of total (left-hand panels) and non-specific (right-hand panels) ligand binding (NSB) to D1-type receptors (D1), D2-type receptors (D2) and the dopamine reuptake transporter (DA transporter, DAT). (b–d) Quantitative comparison of radioligand binding to D1 (b) D2 (c) and DAT (d) in CPu and NAc core, and shell of male (left-hand panels) and female (right-hand panels) rats exposed to AGT (dexamethasone, 0.5 μg/ml, in drinking water on gestational days 16–19; closed histograms) or untreated controls (open histograms). Note the use of different scales for male and female DAT binding. Data are mean±SEM, n=6, *p<0.05 (post-hoc analysis, (b, d)) vs relative control group, and p<0.001 (c) main effect of AGT and region;+p<0.05 vs corresponding male group, indicating a significant sex difference.
In-Vivo Microdialysis
Extracellular levels of DA and its metabolites were assessed by in-vivo microdialysis coupled with electrochemical detection using commercially available microdialysis probes (MAB 6 15 kDa cut-off PES; Microbiotech AB, Sweden) implanted stereotactically into the NAc core. After equilibration (3 h) and collection of three 20-min (20 μl) baseline dialysate samples, rats were injected with amphetamine (0.8 mg/kg i.p) and sample collection continued for an additional 2 h. Samples were stored at −70 °C until analysis. Probe placement was verified post mortem.
Behavioral Analysis
Male and female adult progeny of control and AGT dams were tested during the dark phase of the light–dark cycle.
Amphetamine-induced locomotor activity
Following habituation to the testing environment, locomotor activity induced by intraperitoneal (i.p.) injections of d-amphetamine (0.2, 0.4, 0.8 and 1.2 mg/kg) or vehicle (0.9% saline), using a Latin square design, was recorded over 120 min in photocell testing chambers.
Cocaine self-administration
A fixed-ratio schedule of reinforcement with ascending doses of cocaine (MacFarlan Smith, Edinburgh; 0.25, 0.5 and 1 mg/kg per 100 μl) was used to train rats to self-administer the drug. Operant chambers were equipped with an active lever, which activated an infusion pump to deliver cocaine intravenously, and an inactive lever. Lever presses were recorded during training sessions carried out for 7 consecutive days at each dose: sessions were terminated after a maximum of 50 self-administered cocaine infusions or a period of 2 h, whichever came first.
Spontaneous open-field locomotor activity was monitored in activity chambers (259 mm × 476 mm × 209 mm; Allentown) fitted with infrared photocell beams; beam breaks were recorded in bins of 5 min over a 90-min period.
PPI of Acoustic Startle
The efficacy of a weak sensory stimulus (prepulse) to inhibit a reflexive motor response to a subsequent intense sensory event or startle (pulse) was tested using Plexiglas startle chambers (Kinder Scientific, UK). The startle stimulus (120 dB, 40 ms) and four levels of prepulse (+3, +6, +12 and +16 dB above background for 20 ms) were tested alone and in combination (with 100 ms delay between onset of prepulse and pulse). PPI was calculated as the % inhibition of the startle response obtained in the pulse-alone trials at each of the four prepulse intensities, for each animal by the expression: PPI=100% × (1−(mean reactivity in prepulse–pulse trials/mean reactivity in pulse-alone trials)).
Pavlovian Appetitive Learning (Autoshaping)
In the autoshaping paradigm, learning results from an association of a conditioned stimulus (CS+), which predicts food delivery and is measured relative to a second identical stimulus (CS−) that is explicitly unpaired with food reward. With repeated exposures, animals acquire discriminated approach behavior to the CS+. Training sessions consisted of 50 trials (25CS+ 25CS−), and sessions were conducted daily for 7 consecutive days. For each trial, only the first contact with the CS, and not subsequent responses, was recorded as a lever press response, and contact with either lever was interpreted as approach behavior. Data were analyzed in blocks of 50 trials as mean CS+ and CS− approach scores. The mean difference between CS+ and CS− scores was taken as a measure of discriminative learning.
Data Analysis
Global analysis of variance (ANOVA) was carried out on all data, followed by the further analysis of significant main effects (p<0.05) using lower order ANOVAs and Tukey's HSD corrected pair-wise comparisons (p<0.05). Levene's test was used to confirm homogeneity of variance, except in experiments requiring repeated measures analysis, where data were examined for sphericity using Mauchly's test, and significant departures were adjusted using a Greenhouse–Geisser correction of degrees of freedom to provide more conservative probabilities.
Anatomical and radioligand binding studies
Data were analyzed using a general linear model with brain region, AGT and sex as between-subjects factors.
Behavioral analysis
Comparisons were made using repeated measures ANOVA with trial session, prepulse level or adult drug treatment as a within-subjects factor, and antenatal treatment (control or AGT) and sex as between-subjects factors.
Microdialysis
Cumulative DA efflux over a 2-h period was the dependable variable for statistical analysis, and was calculated by summation of DA levels minus baseline values in post-amphetamine samples. Post-hoc comparisons were carried out using independent samples two-tailed Student's t-tests. A significance level of p<0.05 was used for all analyses. All statistical analyses were performed using IBM SPSS statistical software (v19.0).
RESULTS
Enrichment of Striatal DA Fiber Density Accompanies Expansion of SNc and VTA DA Populations in Progeny of Dams Exposed to AGT
In progeny of both sexes, AGT increased DA cell counts in the SNc and VTA compared with controls (Figure 1a; main effects of AGT F1,20=79.24, p<0.001, and region F1,20=19.8, p<0.001), without affecting the reference volume containing the cells in males (controls, 51.52±4.41 × 106 μm3; AGT, 61.45±1.38 × 106 μm3) or females (controls, 60.83±4.63 × 106 μm3; AGT, 60.16±5.6 × 106 μm3). In addition, AGT increased fiber density in the striatum (main effects of treatment F1,48=140.7, p<0.001 and region F2,48=15.3, p<0.001; Figure 1d and e), with a significantly greater effect in males than in females (sex × treatment interaction F1,48=13.7, p=0.001). AGT failed to alter basal firing activity of confirmed TH-positive cells in the VTA (Supplementary Figure S1 and protocol) and had no effect on the expression of the transcription factor and DA phenotypic marker, Pitx3 (Supplementary Table S1).
Sexually Dimorphic Changes in Mesocorticolimbic and Nigrostriatal DA Receptor and Transporter Levels in Progeny of Dams Exposed to AGT
Binding densities in control animals for D1, D2 and DAT (open histograms, Figure 2b, c and d) were the same in both sexes for all regions, except for D1 receptors in the CPu, where binding density was greater in males compared with that in females (Figure 2b, p<0.05). AGT influences on D1 binding were region- and sex-specific (Figure 2b; region × sex × AGT interaction, F(2,40)=4.77, p=0.014), with a reduction in binding density in the CPu and NAc shell of males, whereas binding was increased in the CPu and NAc core of females (p<0.05) compared with same-sex controls. In both sexes, D2 binding was increased in all three striatal regions (Figure 2c; main effect of AGT, F(1,20)=15.07, p=0.001, and region, F(2,40)=13.34, p<0.0001, but not sex; p<0.05 by post-hoc analysis for CPu, NAc core, and shell). For DAT, AGT influences were again region- and sex-specific (Figure 2d; region × sex × AGT interaction (F(2,40)=6.67, p=0.003), with an increase in binding density in the CPu and NAc core of males, but a decrease in females in all three regions (p<0.05) compared with same-sex controls. Male–female comparisons showed that significant sex differences emerged for D1 and DAT binding densities across all regions studied after AGT (Figure 2b and d; p<0.05).
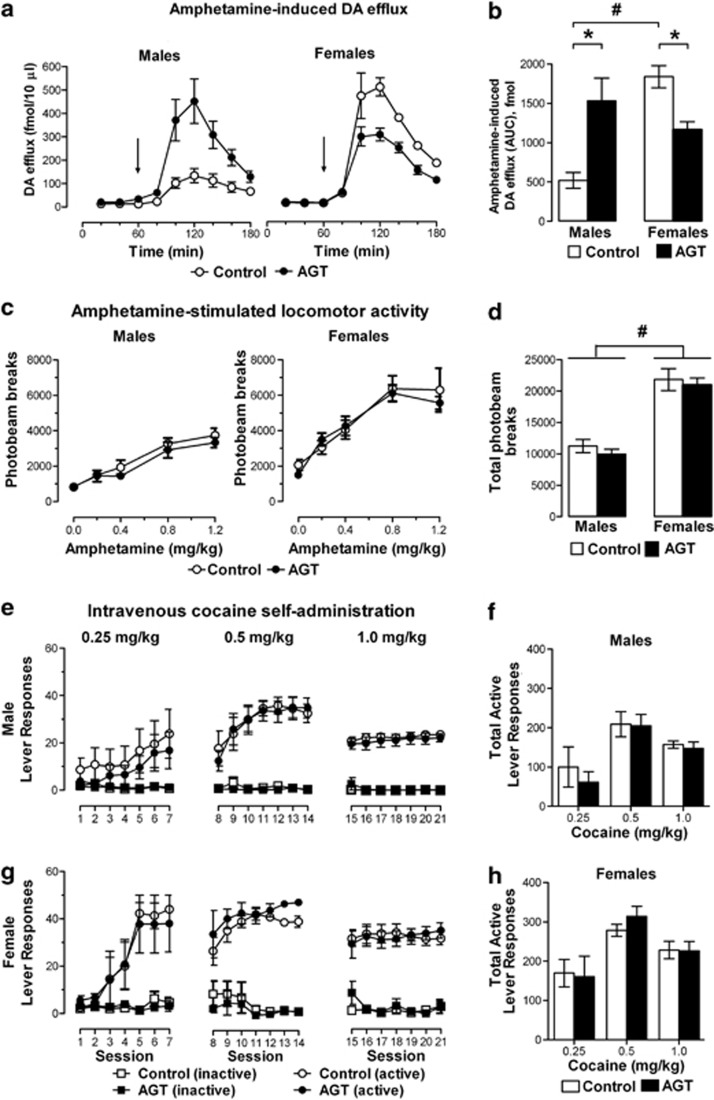
Sexually Dimorphic Effects of AGT on the Neurochemical Response to Amphetamine
Under baseline conditions, in-vivo microdialysis studies found no significant differences in DA levels in both sexes of control (male, 12.93±1.87 fmol/10 μl; female, 13.74±1.74 fmol/10 μl) and AGT-exposed (male, 24.73±5.03 fmol/10 μl; female, 15.66±0.65 fmol/10 μl) animals (Figure3a), whereas amphetamine-induced DA efflux was influenced by both sex and AGT (Figure3b; time × sex × AGT interaction, F(5,80)=8.08; p<0.001). Thus, in controls the amphetamine response was ∼3.5 times greater in females compared with that in males, as determined by summation of DA levels above baseline over the 2-h period following amphetamine injection (p<0.001 by independent samples two-tailed Student's t-test); after exposure to AGT, the amphetamine response increased threefold in males (p=0.014), but decreased in females (p=0.005).
Figure 3.
Impact of antenatal glucocorticoid treatment (AGT) on neurochemical and behavioral effects of psychostimulant drugs. Male and female rats exposed to AGT (dexamethasone, 0.5 μg/ml, in drinking water on gestational days 16–19; closed symbols in the line plots and solid bars in the bar plots) and controls (dams received normal drinking water; open symbols in the line plots and open bars in the bar plots) were tested in adulthood. Top panel (a, b): Amphetamine-induced dopaminergic (DA) efflux in the nucleus accumbens (NAc) is greatly enhanced in male progeny of dams exposed to AGT, but reduced in female progeny. Extracellular levels of DA in the NAc were assessed by in-vivo microdialysis coupled with electrochemical detection. Microdialysis samples were collected every 20 min for 3 h, and after the first three fractions (baseline) amphetamine was administered (0.8 mg/kg i.p.). (a) The line plots depict DA levels in each 20-min fraction and the arrow indicates the point of amphetamine administration. (b) The bar plot shows cumulative DA release above baseline (area under the curve, AUC). Data were used only from those animals where placement of the dialysis probe in the core of the NAc was confirmed post mortem; statistical analyses were adjusted accordingly for differences in group sizes. Values represent means±SEM for control males (n=5), AGT males (n=6), control females (n=4) and AGT females (n=5). #p<0.05, indicating a significant sex difference; *p<0.05 indicating a significant effect of AGT. Second panel (c, d): Amphetamine-stimulated locomotor activity in both male and female progeny is unaffected by AGT. After 2 consecutive days of spontaneous locomotor testing in a novel, open-field environment to habituate the animals to the activity boxes, animals were used to test the behavioral activating effects of amphetamine. Line plots (c), representing the total photobeam interruptions over the 2-h period immediately following i.p. administration of 0 (control, 0.9% NaCl), 0.2, 0.4, 0.8, and 1.2 mg d-amphetamine/kg, and histograms (d), representing the summation of photobeam interruptions for all five adult treatment groups, demonstrate significantly greater activity in females compared with males (#p<0.05), but no significant effect of prenatal treatment. Data are mean±SEM, n=6. Lower two panels (e, f, g, h): Cocaine self-administration in both male and female progeny is unaffected by AGT. Active (open/closed circles) and inactive (open/closed squares) lever responses were recorded at ascending doses of cocaine (0.25, 0.5 and 1.0 mg/kg/intravenous infusion) for adult male (e) and female (g) offspring. Active lever responses resulted in an intravenous infusion of cocaine; these are expressed as mean number of infusions in daily sessions of fixed-ratio schedule reinforcement, with each dose being allowed to be administered for seven daily acquisition training sessions. Responses on the inactive lever had no programmed consequences. Panels f and h depict the total number of active lever responses for males and females, respectively, over the seven acquisition training sessions for each dose of cocaine. Data are mean±SEM, n=6.
Lack of Effect of AGT on Psychostimulant Behaviors
Amphetamine-induced locomotor activity
Systemic administration of amphetamine increased locomotor activity in both sexes (Figure 3c), which was significantly greater in females than in males (dose F(4,60)=34.95, p<0.001; sex F(1,15)=39.57, p<0.001; Figure 3d). However, these responses were unaffected by AGT.
Cocaine self-administration
Figure 3e and h shows rates of responding for intravenous cocaine self-administration. ANOVA revealed significant main effects of cocaine dose (F(2,30)=26.33, p<0.001) and session (F(6,90)=47.69, p<0.001), and significant interactions between cocaine dose and sex (F(12,180)=9.67, p<0.001), and cocaine dose, session and sex (F(12,180)=4.02, p<0.01). Irrespective of dose, significantly higher rates of cocaine self-administration were achieved by females (sex: F(1,15)=10.87, p=0.005), but there was no main effect of AGT, nor any interaction between sex and AGT. The cumulative responses in adult animals, when collapsed across the seven training sessions for each dose confirms that there were no significant differences between control and AGT animals of either sex (Figure 3f and h).
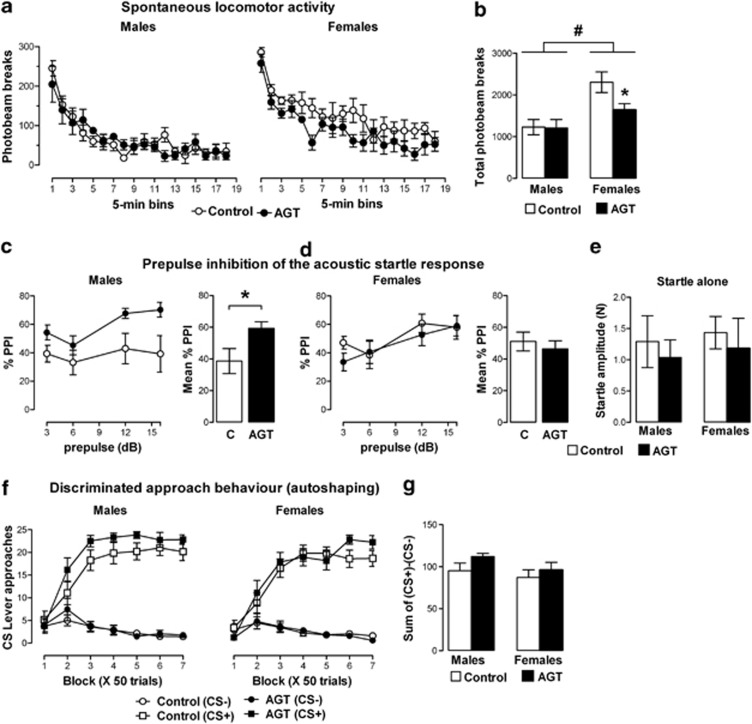
Sex-Specific Effects of AGT on Open-Field Locomotor Activity, But not Appetitive Pavlovian Conditioning
Spontaneous locomotor activity
Locomotor behavior in a novel, open-field environment decreased with time for all groups and was significantly higher in control females (Figure 4a: time F(17,340)=32.96, p<0.001; sex F(1,20)=14.52, p<0.001). As AGT programming effects on locomotor activity have been reported to be sex-specific (Kreider et al, 2005), we used a planned comparison of male and female responses separately, which showed that AGT suppressed activity in females (F(110)=5.24, p=0.045), whereas males were unaffected (Figure 4b).
Figure 4.
Impact of antenatal glucocorticoid treatment (AGT) on unconditioned and conditioned behaviors. Male and female rats exposed to AGT (dexamethasone, 0.5 μg/ml, in drinking water on gestational days 16–19; closed symbols in the line plots and solid bars in the bar plots) and controls (dams received normal drinking water; open symbols in the line plots and open bars in the bar plots) were tested in adulthood. Top panel (a, b): Spontaneous locomotor activity is suppressed in adult female, but not in male, progeny. Test-naive adult offspring were tested at 12 weeks of age for spontaneous locomotor activity in a novel, open-field environment (Day 1). Line plots (a) represent photobeam interruptions in 5 min time bins over a 90-min period, and histograms (b) represent the total number of beam breaks over the whole of the 90-min period for males and females. Activity was significantly attenuated by AGT in females, but not in males. Data are mean±SEM, n=6. #main effect of sex, p=0.001; *p<0.05 for AGT vs relative control group. Middle panel (c, d and e): Sensorimotor gating is significantly modulated after exposure to AGT in adult male, not female, progeny. The paradigm of prepulse inhibition (PPI) of the startle response was used to investigate sensorimotor gating in young adult offspring. For male (c) and female (d) groups, the line plots represent %PPI at each of the four prepulse intensities (3, 6, 12, and 16 dB above background white noise of 65 dB for 20 ms preceding the startle pulse of 120 dB for 40 ms); the adjacent histograms depict the mean %PPI across all four prepulse intensities. %PPI was calculated as 100% × (1−(mean reactivity in prepulse–pulse trials/mean reactivity in pulse-alone trials)). Data in e confirms that in both males and females, the startle response to the startle pulse alone (120 dB) was not significantly affected by AGT compared with controls. AGT significantly enhanced %PPI in adult males, but not in females. Data are mean±SEM, n=8; *p<0.05 for AGT vs vehicle. Bottom panel (f, g): Acquisition of discriminated approach behavior in adult male and female progeny is not affected by AGT. Autoshaping was conducted over 7 consecutive days with each daily session consisting of 50 trials (25 CS− and 25 CS+). (f) Approach responses to either reward-paired stimulus (CS+ square symbols) or unpaired stimulus (CS− circle symbols) in adult rats were measured, and (g) as an index of discriminative approach learning, the average difference score between approaches to CS+ and CS− were analyzed. Data are mean±s.e.m, n=14 (control males, control and AGT females), or n=12 (AGT males).
Sensorimotor gating
In both sexes, presentation of a prepulse stimulus inhibited the response to the 120-dB startle stimulus (Figure 4c and d; main effect of prepulse, F(3,84)=9.493, p<0.001). AGT enhanced PPI in males (sex × treatment interaction, (F(1,28)=4.612, p=0.041; sex: (F(1,14)=5.48, p=0.035; post-hoc analysis p<0.05), but females were unaffected.
Pavlovian appetitive learning
All groups acquired discriminated approach behavior to the reward-predictive CS (Figure 4f), with no differences between the sexes, or between controls and AGT groups (CS: F(1,50)=543.4, p<0.001; training session: F(6,300)=39.5, p<0.001; CS × training session interaction: F(6,300)=100.0, p<0.001). Analysis of different scores between CS+ and CS− responses over block confirmed discriminative learning (F(6, 300 )=100.0, p<0.001) and a lack of effect of sex and AGT (Figure 4g).
Discussion
This study replicated our earlier observation that late-gestational exposure to glucocorticoids increased the adult numbers of midbrain DA neurons (McArthur et al, 2005; McArthur et al, 2007a), and extends our understanding of neurobiological programming of the ascending DA systems in a number of important ways. Expansion of the SNc and VTA DA populations was accompanied by a substantial increase in DA innervation density in the CPu and NAc (core and shell). This did not alter basal extracellular NAc DA levels in either sex, but was co-incident with profound changes in levels of D1 and D2 receptors and DAT in striatal regions, as well as amphetamine-induced accumbal DA release. Most notably, apart from D2 levels, AGT-induced changes in these synaptic markers of DA transmission were qualitatively diametrically opposite in males and females. Remarkably, these robust sexually dimorphic changes were not accompanied in either sex by altered psychomotor and appetitive behaviors that are sensitive to the midbrain DA transmission, suggesting that they represent a broad spectrum of enduring adaptive mechanisms, which underpin apparent resilience to early environmental perturbations. Nevertheless, evidence of significant behavioral changes was seen in terms of the locomotor response in a novel environment (suppressed in females) and sensorimotor gating (enhanced in males). These findings provide empirical evidence that midbrain DA systems are neural substrates for glucocorticoid-induced neurobiological programming, which proceeds via different, often opponent, mechanisms in males and females.
Neurobiological Findings
Cellular, molecular and neurochemical markers of AGT-induced changes in striatal DA neurotransmission
Measures of DA cell counts in midbrain regions have been hampered by their heterogeneous distribution and poor delineation of the sub-nuclei at certain coronal levels (Guillery and Herrup, 1997; Alonso-Vanegas et al, 1999). Moreover, by subdividing the nuclei into 300 μm levels spanning the rostrocaudal extent of the SNc (levels A–D) and VTA (levels B–D) (Carman et al, 1991; McArthur et al, 2007a), we have shown that AGT-induced changes in numerical density of TH-IR cells at different levels is also heterogeneous, despite an overall effect to increase cell counts by at least 30% in the SNc and 50% in the VTA (McArthur et al, 2007a). This is due, in part, to concomitant effects on the regional volumes of the structures containing the cells, which may be increased, decreased or remain unchanged, depending on level, as well as an apparent redistribution of cells within the nuclei. Identification of this enduring effect on the 3D cytoarchitectural organization of midbrain DA neurons provided the basis for our hypothesis that inappropriate prenatal exposure to glucocorticoids would affect DA function. In the present study, we first sought to confirm AGT effects on cell counts by focusing on level B, where the SNc and VTA are particularly well delineated, and we have consistently demonstrated that AGT increases cell counts without affecting regional reference volume or perikarya size (McArthur et al, 2005, 2007a), thereby minimizing recognized sources of counting errors and bias (West, 2012). The current study further identifies late gestation as a critical developmental period when modification of the hormonal environment has enduring influences not only on DA neuronal survival but also target innervation in both sexes. Our methods do not allow us to determine the cells of origin in the increased striatal terminals, but as the vast majority of terminals in the dorsal and ventral striatum arise from SNc and VTA perikarya, these two effects are most likely connected.
Extracellular recordings from individual DA neurons in vivo suggested that basal activity in the expanded population in AGT-exposed offspring was unaffected. Expression of Pitx3, an exclusive marker of SN and VTA DA neurons required for phenotype survival and maintenance (Smidt and Burbach, 2007), was also unchanged after AGT. Thus, the additional TH-IR cells have the characteristics of typical DA neurons, with the potential to create a hyper-DA state. However, our microdialysis study demonstrated that in both sexes, more, apparently normal, cells and terminals did not equate with increased baseline extracellular DA levels, which were unperturbed in male and female control and AGT groups. This lack of effect of AGT on basal DA efflux could be explained by the increase in striatal D2 presynaptic, auto-inhibitory receptors, which paralleled the increase in terminal density in AGT-exposed males and females. In addition to their presynaptic location, D2 receptors are also postsynaptic markers in the dorsal and ventral striatum, where they distinguish the D2-expressing subpopulation of GABA-ergic medium spiny output neurons (MSNs) from the functionally distinct D1-expressing MSNs (Lobo and Nestler, 2011). As our autoradiographic study cannot distinguish the pre/postsynaptic D2 location, our interpretation requires caution. However, our related work (unpublished) demonstrated that AGT did not affect D2-binding density in male and female prefrontal cortical regions, which are devoid of presynaptic D2 receptors (Chiodo et al, 1984), thereby lending credence to the view that the striatal changes are a presynaptic adaptation, which stabilizes baseline extracellular DA levels in the face of an AGT-expanded population.
The DAT protein is another presynaptic marker, which is a key regulator of synaptic DA, and has been used as an indicator of terminal density. Although terminal density and DAT binding in the CPu, NAc core, and shell were similar in control males and females, and DAT binding increased in line with terminal density in males after AGT (both in the range 62–140%, depending on region), in AGT-exposed females DAT binding fell dramatically (by at least 80%) despite an increase in fiber density (∼40%). Consequently, DAT levels differed by an order of magnitude in males and females after AGT. However, these dramatic sex differences appeared not to affect baseline extracellular DA levels, potentially because of a dominant influence of the D2 auto-inhibitory receptors on tonic, baseline activity.
Contrasting with the constancy of baseline extracellular DA levels, our amphetamine study revealed striking sex dimorphisms in control animals and in the impact of AGT. DA releasing effects of amphetamine arise primarily from its ability to bind the nerve terminal protein, DAT (Fleckenstein et al, 2007), commonly regarded as a primary indicator of DA tone. As our control group showed no significant sex differences in striatal DAT binding, it was remarkable that amphetamine-induced efflux in males was only 25% of that in females, suggesting marked sex differences in intrinsic mesolimbic DA terminal dynamics. These findings support and extend other evidence of inherent sex dimorphisms in the ascending DA system (Becker, 1999). Our data do not allow us to deduce what consequences these activity differences (males<females) may have for basal ganglia output. Notably, however, control male D1 receptor binding in the CPu was almost double that of females, raising the possibility that natural sex differences in D1 receptor expression may counter differences in neuronal dynamics and have a key role in preserving excitability in D1–MSNs and basal ganglia output in males and females. This observation has the caveat that we do not know the relationship between dorsal striatal receptor changes and NAc DA efflux. AGT dramatically enhanced the effects of amphetamine on DA efflux in males, consistent with enhanced striatal DAT levels. The co-incident downregulation of postsynaptic D1 receptors may represent compensatory changes to preserve DA transmission. In contrast, AGT blunted amphetamine-induced DA efflux in females, consistent with reduced DAT levels, and the associated upregulation of postsynaptic D1 receptors may serve to preserve basal ganglia output. These observations indicate marked changes in intrinsic activity of midbrain DA neurons after AGT and highlight their extreme sex dimorphisms.
Behavioral Findings
Taken individually, one would predict that the substantial neurobiological changes that we have identified in the mesocorticolimbic and nigrostriatal systems after AGT would affect behavior. However, amphetamine-induced locomotor activity, cocaine self-administration, and learning in response to appetitive cues predictive of food, which are established tests of mesolimbic DA transmission (Jones and Robbins, 1992; Campbell et al, 1997; Roberts et al, 1977; Parkinson et al, 2002; Dalley et al, 2005) were unaffected. At face value, this does not support our hypothesis that in-utero programming of midbrain DA systems by inappropriate exposure to glucocorticoids may be a mechanism linking the early environment with the development of adult psychopathology. However, our neurobiological findings serve to demonstrate that apparent behavioral normality in AGT animals is achieved by the midbrain network operating outside its normal limits, and is, therefore, in a state of allostasis (‘stability through change' McEwen, 1998; Beauchaine et al, 2011). Moreover, these operational changes invoke different mechanisms in males and females. Hence, expansion of the DA system in males is accompanied by an increased intrinsic activity and downregulation of postsynaptic D1 receptors, whereas females show a decreased intrinsic activity and upregulation of D1 receptors, which, we propose, are compensatory changes serving to preserve DA transmission, thereby limiting behavioral consequences and contributing to resilience. In this study we aimed to investigate animals under basal conditions, which are thought to probe intrinsic functional connectivity (Van den Heuvel and Pasterkamp, 2008). In contrast, studies investigating the effects of AGT regimes similar to ours (except for using parenteral administration) have reported significant behavioral effects, especially in tests of anxiety and depression-like behaviors (Welberg and Seckl, 2001; Oliveira et al, 2006; Hauser et al, 2009) in progeny after stress exposure in adulthood. Although these other studies did not assess AGT influences under non-stressed conditions, it is concievable that, in extremity, the apparently protective adaptations that we report here may ultimately contribute to the allostatic burden. Further studies will determine whether the compensatory mechanisms represent a robust resilience or a predisposition to (psycho)pathology (McEwen, 1998).
In support of our hypothesis, however, we showed that not all behaviors were protected. Hence, locomotor response to novelty (an indicator of motivational arousal dependent on mesolimbic and nigrostriatal DA systems (Jones and Robbins, 1992)) was attenuated by AGT in female progeny, which would be compatible with the co-incident reduction in intrinsic mesolimbic activity. However, males were unaffected, possibly because the increased mesolimbic activity was co-incident with an increase in striatal DAT and a reduction in striatal D1 receptors, which may have ameliorated neurochemical and behavioral consequences. In contrast, AGT had no effect on PPI in females, whereas it exaggerated the ability of prepulses to inhibit startle in males (indicative of enhanced pre-attentional processing). Microdialysis studies in male rats have demonstrated that a weak stimulus (prepulse) before the startle stimulus prevents the fall in NAc DA levels associated with the startle (Humby et al, 1996). If the same holds true in females, a blunting or enhancement of mesolimbic DA activity in females and males, respectively, could explain such sex-specific effects of AGT on PPI, which would be compatible with our microdialysis data.
Collectively, our behavioral studies demonstrate that the enduring behavioral effects of AGT depend both on sex and the specific behavior tested. As the AGT-induced profile of neurobiological change was sex-specific, our findings clearly show that the mechanisms that confer enduring behavioral resilience or susceptibility to early environmental challenge occur via sexually dimorphic capacities for molecular adaptations within midbrain DA systems.
Implications and Conclusions
Synthetic glucocorticoids, such as dexamethasone, are widely used to accelerate lung maturation in threatened preterm birth (Liggins, 1994) but, until their potential impact on neurological development has been fully established, there is concern about their overuse in perinatal medicine (Finer et al, 2000; Baud and Sola, 2007). The present study, using a dose of dexamethasone (∼0.075 mg/kg/day (McArthur et al, 2006, 2007a)) in the low clinical range or below (∼0.2 mg/kg/day (Ballard and Ballard, 1995)), highlights the sensitivity of the developing midbrain DA systems to glucocorticoids and establishes the validity our model for detecting neuro(patho)physiological changes after AGT. Numerous reports suggest that neurodevelopmental programming by late-gestational stress can be reproduced by AGT, with a prime role for GRs in mediating these effects (Seckl and Holmes, 2007; Mesquita et al, 2009). The growing notion that such mechanisms underpin early-life stress programming is supported by the widespread expression of GRs in the rat brain throughout the third week of gestation, whereas expression of the mineralocorticoid receptor (MR, activated by glucocorticoids at a lower kd than GR) does not rise until E19.5 (Diaz et al, 1998). However, certain considerations currently preclude the conclusion that stress-induced elevations of endogenous glucocorticoids will target midbrain DA systems in the manner we have reported for our AGT regimen. Specifically, GR-selective synthetic glucocorticoids, such as dexamethasone, exert negative feedback effects to suppress baseline levels of endogenous corticosterone (rodents) or cortisol (humans), which have both GR and MR activity. In rodents, this was marked at pharmacological doses (0.8 mg/kg/day, i.m., GDs 18–20) (Samtani et al, 2006b), with a lesser effect at optimal doses for inducing lung maturation (0.288 mg/kg/day, i.m., GDs 18–20) (Samtani et al, 2006a), which normally depend on natural elevations in glucocorticoid/GR activity just before term. Measurements in human cord blood also indicate a 50% suppression of baseline cortisol levels 6 h after AGT, although cortisol response to stress in newborns appeared normal (Ballard and Ballard, 1995). AGT effects could, therefore, be attributable to reduced activation of MRs, as well as effects at GRs. Although relatively moderate adrenosuppression might be predicted with our low-dose dexamethasone regimen, further investigations are needed to establish this, along with the concomitant, relative activation of brain GR/MR in our model.
We have identified sex-specific mechanisms of glucocorticoid neurobiological programming in the midbrain DA systems that differentially affect specific male and female behaviors. These relate to female behaviors (motivational arousal), which are altered in depression (more prevalent in women (Kessler, 2003)) and male behaviors (pre-attentional processing/PPI), which are affected in male, but not in female, schizophrenic subjects (Kumari et al, 2008). These results add to the evidence that susceptibility of the relevant circuitry to environmental challenge is sexually dimorphic in a psychopathological context (Bale, 2006). Elucidation of the mechanisms promoting these dimorphisms is an important future challenge. Epigenetic programming is emerging as a critical factor in brain sexual differentiation, which is driven, to a large extent, by a surge in testosterone production by the testes, occurring between GDs 17 and postnatal day 10 in the male rat (McCarthy et al, 2009). Prenatal synthetic glucocorticoid treatment can also permanently modify the epigenome (Crudo et al, 2013) and produce endocrine and behavioral effects, which may be qualitatively and quantitatively different in males and females (Seckl and Holmes, 2007; Kapoor et al, 2008; Brummelte et al, 2012). Differential interactions of glucocorticoids with the sex-specific gonadal steroid environment at the level of epigenetic markers in the developing male and female midbrain DA systems therefore offers a compelling explanation that might account for their sexually dimorphic programming by AGT.
FUNDING AND DISCLOSURE
TWR consults for Cambridge Cognition, Lilly, Merck Sharpe and Dohme, Lundbeck, Teva, Shire, and Chempartners, and has held recent research grants with Lilly, GSK, and Lundbeck. All other authors declare no conflict of interest.
Acknowledgments
We thank David Theobald for technical support. This work was supported by The Wellcome Trust (grant number 086871/Z/08/Z), by a joint award from the MRC and Wellcome Trust in support of the Behavioral and Clinical Neuroscience Institute at Cambridge University, and by the UK Medical Research Council (U120085816) and a University Research Fellowship from the Royal Society to MAU. The work was completed within the MRC Imperial College-Cambridge University-Manchester University (ICCAM) strategic addiction cluster.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Author Contributions
JWD, TWR, MAU, and GEG designed the research; KV, SM, FB, and DC performed the research; KV, SM, and FB analyzed the data; and SM, KV, MAU, TWR, JWD, and GEG wrote the paper.
Supplementary Material
References
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Alonso-Vanegas MA, Fawcett JP, Causing CG, Miller FD, Sadikot AF. Characterization of dopaminergic midbrain neurons in a DBH:BDNF transgenic mouse. J Comp Neurol. 1999;413:449–462. doi: 10.1002/(sici)1096-9861(19991025)413:3<449::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Boseret G, Konkle AT, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci. 2008;27:801–817. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- Baud O, Sola A. Corticosteroids in perinatal medicine: how to improve outcomes without affecting the developing brain. Semin Fetal Neonatal Med. 2007;12:273–279. doi: 10.1016/j.siny.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Zalewski M, Crowell SE, Potapova N. The effects of allostatic load on neural systems subserving motivation, mood regulation, and social affiliation. Dev Psychopathol. 2011;23:975–999. doi: 10.1017/S0954579411000459. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amor L, Grizenko N, Schwartz G, Lageix P, Baron C, Ter-Stepanian M, et al. Perinatal complications in children with attention-deficit hyperactivity disorder and their unaffected siblings. J Psychiatry Neurosci. 2005;30:120–126. [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Animal models of obstetric complications in relation to schizophrenia. Brain Res Brain Res Rev. 2004;45:1–17. doi: 10.1016/j.brainresrev.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Brodski C, Weisenhorn DM, Signore M, Sillaber I, Oesterheld M, Broccoli V, et al. Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. J Neurosci. 2003;23:4199–4207. doi: 10.1523/JNEUROSCI.23-10-04199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Lieblich SE, Galea LA. Gestational and postpartum corticosterone exposure to the dam affects behavioral and endocrine outcome of the offspring in a sexually-dimorphic manner. Neuropharmacology. 2012;62:406–418. doi: 10.1016/j.neuropharm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Campbell A, Villavicencio AT, Yeghiayan SK, Balikian R, Baldessarini RJ. Mapping of locomotor behavioral arousal induced by microinjections of dopamine within nucleus accumbens septi of rat forebrain. Brain Res. 1997;771:55–62. doi: 10.1016/s0006-8993(97)00777-4. [DOI] [PubMed] [Google Scholar]
- Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra: relation between extent of lesion and rotational behavior. Brain Res. 1991;553:275–283. doi: 10.1016/0006-8993(91)90835-j. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Crudo A, Suderman M, Moisiadis VG, Petropoulos S, Kostaki A, Hallett M, et al. Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology. 2013;154:1168–1180. doi: 10.1210/en.2012-1980. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, et al. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R, Brown RW, Seckl JR. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci. 1998;18:2570–2580. doi: 10.1523/JNEUROSCI.18-07-02570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer NN, Craft A, Vaucher YE, Clark RH, Sola A. Postnatal steroids: short-term gain, long-term pain. J Pediatr. 2000;137:9–13. doi: 10.1067/mpd.2000.107799. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM. The canary in the coalmine: the sensitivity of mesolimbic dopamine to environmental adversity during development. Neurosci Biobehav Rev. 2010;35:794–803. doi: 10.1016/j.neubiorev.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hauser J, Feldon J, Pryce CR. Direct and dam-mediated effects of prenatal dexamethasone on emotionality, cognition and HPA axis in adult Wistar rats. Horm Behav. 2009;56:364–375. doi: 10.1016/j.yhbeh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson LS, Robbins TW, Geyer MA. Prepulses inhibit startle-induced reductions of extracellular dopamine in the nucleus accumbens of rat. J Neurosci. 1996;16:2149–2156. doi: 10.1523/JNEUROSCI.16-06-02149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH, Robbins TW. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav. 1992;43:887–895. doi: 10.1016/0091-3057(92)90422-c. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res Rev. 2008;57:586–595. doi: 10.1016/j.brainresrev.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Levin ED, Seidler FJ, Slotkin TA. Gestational dexamethasone treatment elicits sex-dependent alterations in locomotor activity, reward-based memory and hippocampal cholinergic function in adolescent and adult rats. Neuropsychopharmacology. 2005;30:1617–1623. doi: 10.1038/sj.npp.1300716. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA. Prepulse inhibition and ‘psychosis-proneness' in healthy individuals: an fMRI study. Eur Psychiatry. 2008;23:274–280. doi: 10.1016/j.eurpsy.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev. 1994;6:141–150. doi: 10.1071/rd9940141. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology. 2007a;32:1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- McArthur S, Robinson IC, Gillies GE. Novel ontogenetic patterns of sexual differentiation in arcuate nucleus GHRH neurons revealed in GHRH-enhanced green fluorescent protein transgenic mice. Endocrinology. 2011;152:607–617. doi: 10.1210/en.2010-0798. [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE. Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol. 2005;17:475–482. doi: 10.1111/j.1365-2826.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem. 2007b;100:678–692. doi: 10.1111/j.1471-4159.2006.04226.x. [DOI] [PubMed] [Google Scholar]
- McArthur S, Siddique ZL, Christian HC, Capone G, Theogaraj E, John CD, et al. Perinatal glucocorticoid treatment disrupts the hypothalamo-lactotroph axis in adult female, but not male, rats. Endocrinology. 2006;147:1904–1915. doi: 10.1210/en.2005-1496. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Melichar JK, Daglish MR, Nutt DJ. Addiction and withdrawal—current views. Curr Opin Pharmacol. 2001;1:84–90. doi: 10.1016/s1471-4892(01)00011-x. [DOI] [PubMed] [Google Scholar]
- Mesquita AR, Wegerich Y, Patchev AV, Oliveira M, Leao P, Sousa N, et al. Glucocorticoids and neuro- and behavioural development. Semin Fetal Neonatal Med. 2009;14:130–135. doi: 10.1016/j.siny.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Bessa JM, Mesquita A, Tavares H, Cavalho A, Silva R, et al. Induction of a hyperanxious state by antenatal dexamethasone: a case for less detrimental corticosteroids. Biol Psych. 2006;59:844–852. doi: 10.1016/j.biopsych.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, et al. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.1998The Rat Brain in Stereotaxic Co-Ordinates6th edn.Academic press: San Diego, CA [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Samtani MN, Pyszczynski NA, Dubois DC, Almon RR, Jusko WJ. Modeling glucocorticoid-mediated fetal lung maturation: II. Temporal patterns of gene expression in fetal rat lung. J Pharmacol Exp Ther. 2006a;317:127–138. doi: 10.1124/jpet.105.095869. [DOI] [PubMed] [Google Scholar]
- Samtani MN, Pyszczynski NA, Dubois DC, Almon RR, Jusko WJ. Modeling glucocorticoid-mediated fetal lung maturation: I. Temporal patterns of corticosteroids in rat pregnancy. J Pharmacol Exp Ther. 2006b;317:117–126. doi: 10.1124/jpet.105.095851. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Szpir M. Tracing the origins of autism: a spectrum of new studies. Environ Health Perspect. 2006;114:A412–A418. doi: 10.1289/ehp.114-a412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why. J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel DM, Pasterkamp RJ. Getting connected in the dopamine system. Prog Neurobiol. 2008;85:75–93. doi: 10.1016/j.pneurobio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinology. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- West MJ.2012Introduction to sterology Cold Spring Harb Protoc 2012doi: 10.1101/pdb.top070623 [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Barker JM, Balthazart J, Ball GF. Androgens and estrogens synergistically regulate the expression of doublecortin and enhance neuronal recruitment in the song system of adult female canaries. J Neurosci. 2011;31:9649–9657. doi: 10.1523/JNEUROSCI.0088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.