Abstract
In recent years, interactions between neurons and glia have been evaluated as mediators of neuropsychiatric diseases, including drug addiction. In particular, compounds that increase expression of the astroglial glutamate transporter GLT-1 (N-acetylcysteine and ceftriaxone) can decrease measures of drug seeking. However, it is unknown whether the compounds that influence broad measures of glial physiology can influence behavioral measures of drug relapse, nor is it clear whether the upregulated GLT-1 is functionally important for suppressing of drug seeking. To address these questions, we sought to determine whether the glial modulator and neuroprotective agent propentofylline (PPF) modifies drug seeking in rats using a reinstatement model of cocaine relapse. We found that 7 days of chronic (but not acute) administration of PPF significantly decreased both cue- and cocaine-induced reinstatement of cocaine seeking. We next determined whether the effect of systemic PPF on reinstatement depended upon its ability to restore expression of GLT-1 in the nucleus accumbens. PPF restored the cocaine-induced decrease in GLT-1 in the accumbens core; then, using an antisense strategy against glutamate transporter GLT-1, we found that restored transporter expression was necessary for PPF to inhibit cue-primed cocaine seeking. These findings indicate that modulating glial physiology with atypical xanthine derivatives like PPF is a potential avenue for developing new medications for cocaine abuse, and support the hypothesis that neuron–glial interactions contribute to mechanisms of psychostimulant addiction, particularly via expression and function of astroglial glutamate transporters.
Keywords: astrocyte, addiction, vivo-morpholino, nucleus accumbens, glutamate, GLT-1
INTRODUCTION
Important functional roles have been increasingly ascribed to the glial cells in both health and disease (Aguzzi et al, 2013; Barres, 2008). Beyond sustaining normal nervous system function, in many instances of injury or disease, the activation of both microglia and astrocytes is a fundamentally important process (Liu et al, 2011). Activation of astrocytes refers to a morphological and functional responsiveness to nervous system trauma including ischemia, disease, or injury, and is typically associated with increased expression and release of proinflammatory cytokines, and increased expression of cytoskeletal proteins including glial fibrillary acidic protein and vimentin (Pekny and Nilsson, 2005; Sofroniew, 2009). Reactive astrocytes are also frequently associated with decreased expression and function of glutamate transporters including GLT-1/EAAT2 and GLAST/EAAT1, which can exacerbate excitotoxicity (Binns et al, 2005; Cata et al, 2006; Pekny and Nilsson, 2005; Sung et al, 2003; Sweitzer et al, 2001; Tawfik et al, 2008).
Exposure to drugs of abuse leads to activation of both astrocytes and microglia. For example, noncontingent administration of cocaine upregulates glial fibrillary acidic protein and vimentin 3 weeks after drug cessation (Bowers and Kalivas, 2003). Likewise, noncontingent administration of methamphetamine and opiates also increase measures of glial activation, including cell morphology and expression of inflammatory markers (Beitner-Johnson et al, 1993; Reichel et al, 2012; Schwarz et al, 2011; Watkins et al, 2009). Moreover, GLT-1 and GLAST are chronically downregulated following cocaine self-administration and withdrawal (Fischer-Smith et al, 2012; Knackstedt et al, 2010; Reissner et al, 2011), and chronic administration of ceftriaxone or N-acetylcysteine, compounds which upregulate GLT-1, reduce reinstated drug seeking in animals trained to self-administer cocaine (Sari et al, 2009; Knackstedt et al, 2010; Moussawi et al, 2011).
Propentofylline (PPF) is an atypical methylxanthine derivative characterized as an adenosine uptake and phosphodiesterase inhibitor (Sweitzer and De Leo, 2011), that also reverses markers for reactive gliosis following nerve injury, including reduced expression of GLT-1 (Tawfik et al, 2008). Methylxanthines are a broad class of drugs that include caffeine, theophylline, and theobromine. FDA-approved methylxanthines include pentoxifylline for improved circulation in intermittent claudication (Muir, 2009) and theophylline and aminophylline as bronchodilators for chronic obstructive pulmonary disease and asthma (Dzierba and Jelic, 2009; Tilley, 2011). Further, systemic treatment with glial modulator PPF can block conditioned place preference to both methamphetamine and morphine (Narita et al, 2006). We hypothesized that the capacity of PPF to restore GLT-1 might confer capacity for this class of drugs to be medications for cocaine relapse. To evaluate PPF as a potential anti-relapse treatment for cocaine addiction, we trained rats to self-administer cocaine and determined if reinstated cocaine seeking initiated by conditioned cues was reduced by PPF pretreatment. Daily, but not acute, PPF reduced reinstatement to cocaine, and using a vivo-morpholinos oligomer antisense (AS) strategy to inhibit translation of GLT-1, we determined that the ameliorative effect of PPF relies on its ability to raise levels of GLT-1 in the nucleus accumbens (NAc).
MATERIALS AND METHODS
Reagents
Vivo-morpholinos were custom synthesized and purchased from Gene Tools, LLC. PPF was purchased from Sigma (P9689) and prepared in saline. Sequences for vivo-morpholinos were as described (Reissner et al, 2012). Animals were purchased from Charles River, and cocaine was provided by NIDA.
Animal Care and Surgical Techniques
All animal treatment was in accordance with National Institutes of Health guidelines for laboratory animal care, and all protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (∼350 g) were individually housed on a 12 h reverse light cycle and were food restricted (20 g chow per day). Catheters were surgically implanted into the right jugular vein (0.02 ID, 0.047 OD, Bio-sil). Prophylactic antibiotic (Timentin 10 mg/0.1 ml, i.v.) was administered during surgery and 3 days postoperatively. Catheters were flushed daily with heparin until the end of self-administration. Cannulae (26 gauge) were implanted into the NAc using the following coordinates: +1.5 A/P, +1.8 M/L, −5.5 D/V (Paxinos and Watson, 2005).
Behavioral Analysis
Prior to the onset of self-administration, animals received one 15 h food training session, to facilitate acquisition of the operant task. Self-administration was performed on an FR1 reinforcement schedule (2 h per day) during which an active lever press resulted in a 0.2 mg infusion (0.05 ml) of cocaine paired with light and tone (5 s each). All sessions were performed at the same time each day. Following a minimum of 10 days of ten infusions or more, animals entered extinction training, during which time lever presses no longer resulted in infusion or cue presentation. Extinction active lever presses are shown for the average of the last 2 days of extinction. In all cases, PPF or saline was administered i.p. 30 min prior to extinction training or reinstatement test. Cue-primed reinstatement tests were for 2 h and measured lever pressing in response to availability of contingent cues; in the case of cocaine-primed reinstatement, animals were given a 10 mg/kg i.p. injection of cocaine immediately before the onset of reinstatement testing in the absence of conditioned cues. Locomotor testing was performed in a photocell apparatus (Omnitech Electronics, Columbus, OH) 30 min after the last treatment (saline or PPF), and activity was recorded in 10 min increments for a total of 120 min. Activity was quantified as total distance traveled. PPF was prepared in saline, and doses were selected based on systemic doses used in studies on allodynia (Sweitzer et al, 2001; Tawfik et al, 2008).
Sucrose self-administration was performed identically to cocaine administration on an FR1 schedule of reinforcement, except instead of a drug infusion animals received a 45 mg sucrose pellet (Test Diet, Richmond, IN).
Microinjections and Histology
Control and AS vivo-morpholinos against GLT-1 (30 pmol per injection) were microinjected 2 mm below the cannula base using 33-gauge microinjectors at 0.5 μl/min for a total volume of 1 μl per hemisphere. Microinjectors were then left in place for 1 min before removal, to allow diffusion. Microinjections were made once per day for 3 days prior to treatment with PPF or saline, such that reinstatement testing would be performed 7 days after the last microinjection, based on a protocol previously optimized for suppression of GLT-1 (Reissner et al, 2012). The AS sequence was designed around the translation start site; vivo-morpholino AS sequence: 5′-TGTTGGCACCCTCGGTTGATGCCAT-3′. For control, a reverse sequence of the same bases was used. A BLAST search of the Rattus norvegicus genome revealed that neither sequence should recognize any nonspecific gene. Upon completion of behavioral analysis, animals were sedated with pentobarbital (200 mg/kg, i.p.) and perfused with saline. Brains were postfixed in 10% formalin, sliced 100 μm thick, and stained using Cresyl Violet.
For validation of GLT-1 knockdown, control and AS sequence microinjections were performed in contralateral hemispheres of the same animal. Seven days after the last injection, sham microinjections were performed approximately 30 min prior to tissue harvest, for visual identification of the microinjection site. Tissue surrounding the microinjection site was taken no more than 1 mm from the site of injection in any dimension.
Western Blotting
For analysis of GLT-1 protein expression, a crude membrane subfraction was prepared from fresh tissue (Knackstedt et al, 2010). The final pellet was resuspended in RIPA buffer supplemented with 1.0% SDS and Halt protease/phosphatase inhibitors and EDTA (Thermo Scientific, 1 : 100). Protein content was determined by the BCA method (Thermo Scientific) and 10 μg were separated on Bio-Rad BisTris gels and transferred to PVDF membranes, then probed using anti-GLT-1 (abcam, ab41621, 1 : 1000) and anti-Calnexin (Enzo ADI-SPA-860, 1 : 1000). Prior to electrophoresis, samples were heated for 30 min at 50°C.
Statistical Analyses
All data presented are mean and standard error about the mean. For all experiments, reinstatement criteria for control animals receiving saline i.p. was set at a minimum of 12 active lever presses (n=5 did not reach criteria). A Student's t-test was used to compare reinstated lever presses in the saline and PPF groups. For acute PPF, a one-way ANOVA was used since there were three treatment groups. A three-way ANOVA was used in the case of animals receiving GLT-1 vivo-morpholinos. The three factors for the ANOVA were drug treatment (PPF vs saline), morpholino treatment (AS vs control) and day (extinction vs reinstatement). Where appropriate, this was followed by two-way ANOVA split by corresponding factor with a Bonferroni correction. For analysis of GLT-1 knockdown in naïve animals, a paired t-test was used for within animal comparison. In the case of western blotting, significant difference was determined using a one-way ANOVA followed by Neuman–Keuls direct comparisons between groups (Figure 3) or a paired Student's t-test (Figure 4b). All tests were considered significant at p<0.05.
RESULTS
Daily PPF Pretreatment Inhibits Reinstatement to Cocaine
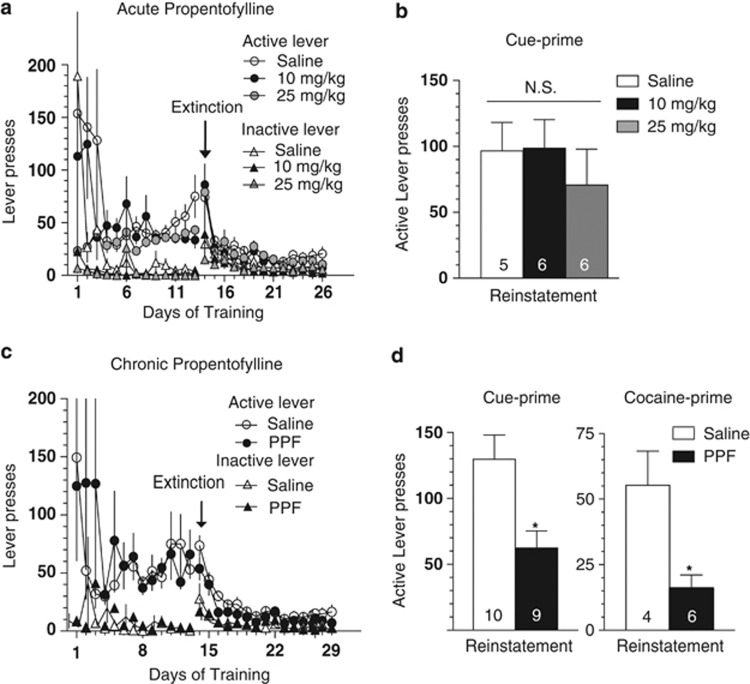
Animals were administered either a single injection of PPF prior to reinstatement (Figure 1b), or daily just prior to the last six extinction sessions, as well as prior to the reinstatement session (Figure 1d). There was no effect by either dose of acute PPF pretreatment on the number of cue-induced active lever presses during the reinstatement session (F(1,15)=0.4372). In contrast, for animals receiving chronic saline vs 10 mg/kg PPF, there was a significant reduction in the PPF compared with the saline group after either cue- or cocaine-induced reinstatement (cue t(17)=2.494, p= 0.028; cocaine t(8)=3.238, p=0.012), (Figure 1d).
Figure 1.
Chronic, but not acute, treatment with PPF impairs cocaine reinstatement. Top, lever presses during self-administration and extinction (a) and reinstatement (b) for acute treatment groups. (b) A repeated measure two-way ANOVA revealed significant reinstatement, but no difference in reinstatement between treatment groups receiving acute saline, or PPF 10 or 25 mg/kg. Bottom, lever presses during self-administration and extinction (c) and reinstatement (d) for chronic treatment groups. In contrast to acute PPF, chronic administration of 10 mg/kg PPF significantly impaired cue- and cocaine-primed reinstatement compared with saline treatment. Self-administration and extinction profiles were not significantly different for animals receiving chronic saline or PPF prior to cocaine-primed reinstatement (data not shown). *p<0.05.
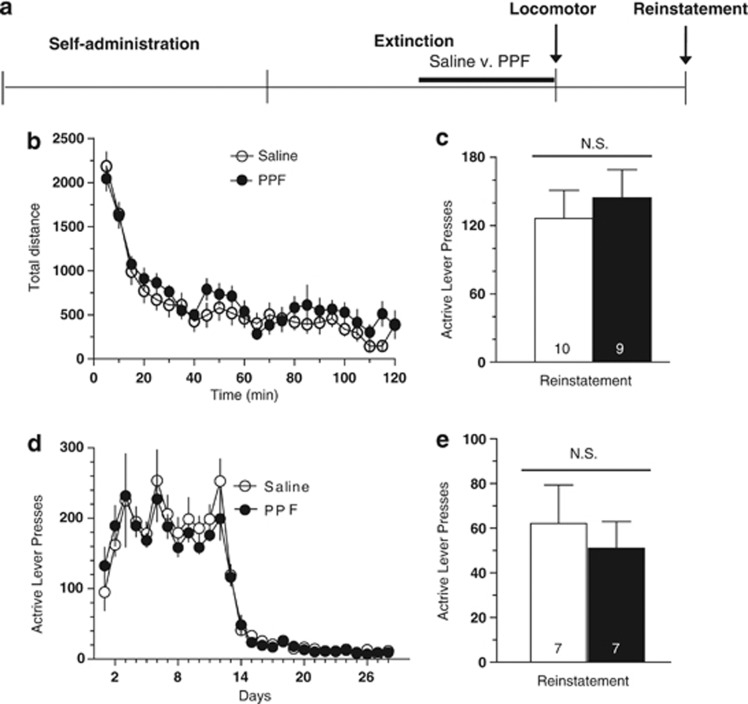
In order to determine whether the effect of chronic PPF administration on reinstatement was due to nonselective motor inhibition or memory for the drug-paired cues, locomotor and sucrose reinstatement experiments were performed (Figure 2a). For this purpose, animals were trained in self-administration and extinction exactly as described for Figure 1. However, following chronic PPF treatment, instead of reinstatement, animals were placed into a novel open field for 2 h. No difference was observed in the locomotor activity for animals receiving either saline or PPF (Figure 2b). In order to determine whether PPF-induced inhibition of reinstatement was enduring, these same animals were then allowed to re-enter extinction training without further PPF administration. In this case, there was no difference in reinstatement between the groups, indicating that the effect was reversible and required ongoing daily administration (Figure 2c).
Figure 2.
Chronic treatment with PPF does not have an enduring effect on drug seeking, nor does it affect locomotor activity or reinstatement for sucrose. (a) Experimental design for panels b and c. Animals were trained in self-administration and extinction, and treated with chronic PPF (10 mg/kg, ip) or saline, as described in Figure 1. (b) Instead of reinstatement, locomotor testing was performed on day 14 of extinction in an open field. (c) These animals were then allowed to continue an additional 6 days of extinction training without treatment, followed by a final cue-primed reinstatement test. (d,e) A separate cohort of animals were trained to self-administer sucrose pellets in a protocol identical to cocaine self-administration, followed by extinction and daily PPF or saline treatment. Cue-primed sucrose reinstatement was performed identically as for cocaine reinstatement. No significant difference was observed between sucrose-administering animals that received saline or PPF (10 mg/kg).
A separate group of animals was trained to self-administer sucrose using a protocol of self-administration and extinction similar to the cocaine experiment. Rats receiving chronic PPF or saline showed equivalent levels of cue-induced reinstatement of sucrose seeking, indicating the effect on reinstatement to cocaine seeking was not a generalized effect on motivation nor extended to pursuit of non-drug reward (Figure 2).
Modulation of GLT-1 by PPF is required for the Behavioral Therapeutic Effect
PPF has been shown to manifest a variety of cellular effects, including restoration of GLT-1 suppression (Sweitzer et al, 2001). Because expression of GLT-1 is reduced following cocaine self-administration and extinction, or incubation of cocaine craving (Fischer-Smith et al, 2012; Knackstedt et al, 2010), and because reduced GLT-1 expression has been postulated to be an important mediator of drug seeking (Kalivas, 2009; Knackstedt et al, 2010), we sought to determine whether PPF might restore the cocaine-dependent decrease in GLT-1 in the NAc. Animals were trained to self-administer cocaine, or received yoked infusions of saline, and were then injected with either daily saline or PPF (10 mg/kg, i.p.). Following self-administration and extinction, animals were killed for preparation of a membrane subfraction from NAc core or the dorsomedial prefrontal cortex, including prelimbic and anterior cingulate regions. We observed a significant cocaine-dependent decrease in GLT-1 expression in the NAc, and a non-significant trend toward a decrease in the PFC (core: (F(2,24)=3.833, p=0.037); PFC: (F(2,24)=0.872, p=0.432), Figure 3). Moreover, a chronic regimen of PPF fully restored expression of GLT-1 in the NAc. These results suggest that restored expression of GLT-1 in the NAc might contribute to PPF-induced antagonism of reinstated cocaine seeking.
Figure 3.
PPF restores the cocaine-dependent decrease in GLT-1. Animals were trained on cocaine (Coc) self-administration and extinction as in other experiments. Saline (Sal) animals received noncontingent infusions of saline dependent on behavior from cocaine-administering animals. Chronic treatment with PPF (10 mg/kg, ip, for 7 days) or saline was performed exactly as in Figure 1, except accumbens tissue was obtained instead of a reinstatement test, 24 h after the last extinction test. GLT-1 levels in a membrane-enriched subfraction were assessed by western blot and normalized to a Calnexin loading control. GLT-1 in cocaine-administering animals was then normalized to saline controls. *p<0.05.
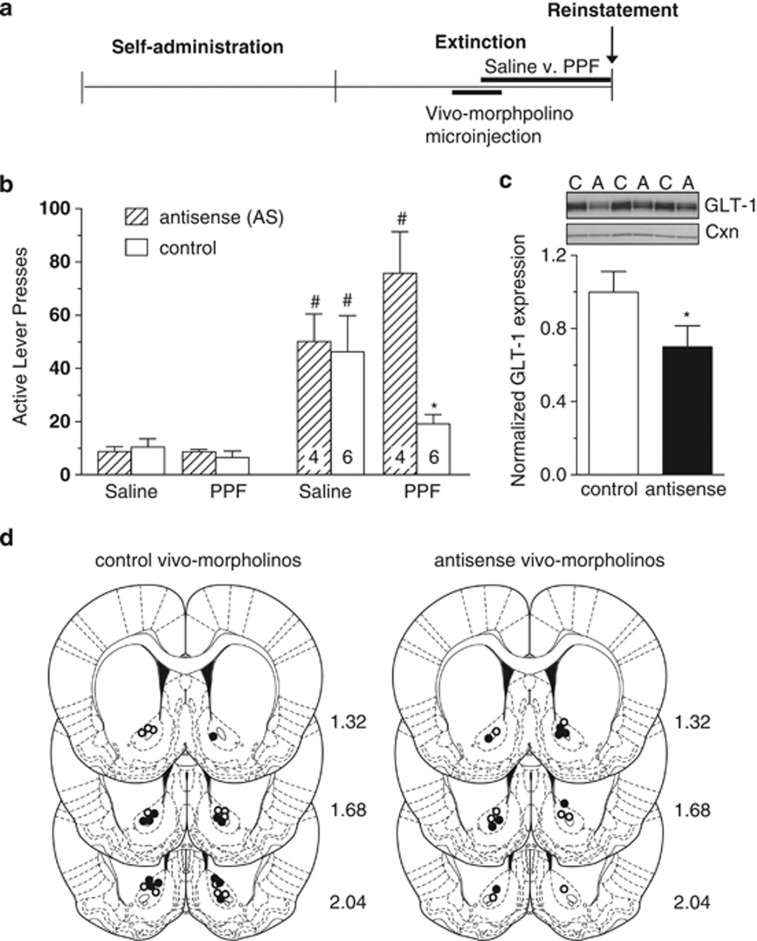
To test this hypothesis, control or AS vivo-morpholinos were microinjected into the NAc core, using a protocol previously demonstrated to reduce GLT-1 expression by ∼40% for 7–14 days after the last AS microinjection (Reissner et al, 2012), combined with chronic saline or PPF administration. Analysis by three-way ANOVA revealed a significant interaction between vivo-morpholino treatment (control vs AS), drug treatment (PPF vs saline) and day (extinction vs reinstatement) (F(1,32)=4.498, p=0.042). Thus, comparison between groups was performed by a two-way ANOVA split by corresponding factor with a Bonferroni correction. Analyses revealed a main effect of reinstatement compared to extinction in both AS and control morpholino groups that had been treated with saline (F(1,16)=15.126, p=0.001); in contrast, an interaction was observed in PPF-treated groups (F(1,8)=18.007, p=0.003). Although the group treated with GLT-1 AS plus PPF reinstated (p=0.003), the PPF-treated control vivo-morpholino group did not reinstate (p⩾0.05) and a significant difference was found between this group and the PPF-treated AS group (Figure 4b, far right). Moreover, reinstatement in the AS/PPF group was no different from reinstatement in animals that received saline i.p. and were microinjected with either control or AS vivo-morpholinos. Thus, restored expression of GLT-1 is required for PPF to suppress cue-primed reinstatement.
Figure 4.
Restored expression of GLT-1 is required for the PPF-dependent decrease in reinstatement. (a) Experimental design. Vivo-morpholino microinjections were performed on 3 sequential days, and saline or PPF administration began on the third day of vivo-morpholino microinjection. Animals then received chronic treatment with saline or PPF (10 mg/kg, i.p.). (b) Active lever presses during cue-primed reinstatement to cocaine. (#p<0.001). (c), In a separate group of animals, efficacy of antisense to GLT-1 was determined using western blot (n=6 per group). C, control sequence; A, antisense. (d) Placement of microinjections for use in behavioral analysis (b) was determined by Cresyl violet staining. Open circles indicate saline treatment, closed circles indicate PPF treatment. *p<0.05.
In order to validate control of GLT-1 protein expression, AS or control vivo-morpholinos against GLT-1 were microinjected in an independent, naive group of animals. In this case, control vs AS vivo-morpholinos were microinjected into contralateral hemispheres in an identical manner as performed for behavioral analysis. Seven days after the last microinjection, tissue surrounding the microinjected site was harvested for subcellular fractionation and western blotting (Figure 4c). Compared with control vivo-morpholino microinjection, AS against GLT-1 results in ∼30% suppression, similarly as reported previously (t(5)=3.45, p=0.018), (Reissner et al, 2012).
DISCUSSION
Restoration of GLT-1 is a Critical Mechanism for Control of Reinstatement for Cocaine
These data show that daily, but not acute, systemic administration of the glial modulator PPF can impair cue-primed reinstatement to cocaine. The impairment of cocaine reinstatement was not due to a sedative or generalized effect by PPF on motivation, as the same protocol that blocked reinstatement did not affect locomotor activity or reinstated sucrose seeking. However, the effect of PPF depended on its ability to partially restore expression of the glutamate transporter GLT-1 within the NAc. Several studies have demonstrated that administration of compounds N-acetylcysteine and ceftriaxone inhibit cocaine sensitization, self-administration, or reinstatement, and also increase GLT-1 expression (Knackstedt et al, 2010; Sari et al, 2009; Sondheimer and Knackstedt, 2011; Ward et al, 2011). However, no studies have directly shown that this increase is requisite for these behavioral effects. Results presented here indicate that when PPF is administered systemically, AS-mediated suppression of GLT-1 specifically within the NAc core, a nucleus known to be critically important in cue-primed reinstatement to multiple drugs (Feltenstein and See, 2008; Kalivas and McFarland, 2003), is sufficient to prevent PPF reductions in cue-reinstated cocaine seeking.
GLT-1 is predominantly localized near synapses to rapidly eliminate synaptically released glutamate and minimize access to the extrasynaptic space (Cholet et al, 2002; Minelli et al, 2001; Shigeri et al, 2004). Reinstatement to cocaine, heroin, alcohol, and nicotine is associated with potentiated release of glutamate within the NAc (Gass et al, 2011; Gipson et al, 2013; LaLumiere and Kalivas, 2008; Lutgen et al, 2012; McFarland et al, 2003). Given that all of these drugs reduce GLT-1 in the NAc, the reduced uptake of synaptically released glutamate is a likely mediator of the increase in extracellular glutamate associated with reinstatement. Accordingly, restoring astrocyte-mediated clearance of synaptic glutamate in the NAc is a hypothesized mechanism whereby PPF inhibits cocaine seeking in the present study.
Although our data indicate a necessity to restore GLT-1 expression for the therapeutic effect of PPF, contributions by other known effects of PPF cannot be conclusively ruled out. For example, PPF is a phosphodiesterase inhibitor, and phosphodiesterase inhibition reduces cocaine sensitization and reinforcement (Liddie et al, 2012; Meskini et al, 1994; Schroeder et al, 2012; Zhong et al, 2012). In addition, PPF can act as an agonist of A1 or antagonist of A2 adenosine receptors in a concentration-dependent manner (Borgland et al, 1998), and adenosine receptors have been implicated in cocaine-mediated behaviors (Brown et al, 2012; O'Neill et al, 2012; Soria et al, 2006; Tozzi et al, 2012).
Finally, the fact that the therapeutic effect of PPF requires daily administration and depends on GLT-1 expression suggests that it may be working through a transcriptionally dependent mechanism. However, the effect of daily PPF did not endure after discontinuing daily administration. PPF has a relatively short half-life (∼1 h) (Kwon et al, 1998; Kwon and Ryu, 2000) and is taken three times daily in most human studies. Thus, it is possible that a more frequent or longer dosing regimen would be required to observe an enduring effect.
PPF as a Medication in Cocaine Addiction and other Neuropsychiatric Disorders
Numerous human studies testing effects of PPF on cognition have demonstrated efficacy in age-related dementia, Alzheimer's disease, and vascular dementia (Frampton et al, 2003; Kittner et al, 1997; Marcusson et al, 1997; Mielke et al, 1998; Saletu et al, 1990). However, phase II and III clinical trials for use in Alzheimer's Disease and vascular dementia were discontinued due to variable results (for review, see Sweitzer and De Leo, (2011)). Use of the related compound pentoxifylline resulted in a non-significant trend toward a decrease in cocaine abuse in addicted individuals; however, in the case of this outpatient study, no measures of medication compliance were obtained (Ciraulo et al, 2005; Cooper et al, 2012). Although these studies collectively indicate a complex profile of clinical utility, they also demonstrate safety and tolerability of this class of molecules. Data presented here have important implications regarding the role of glial activation in the behavioral and cellular adaptations induced by chronic drug abuse, particularly with respect to GLT-1. Future studies will be important to more fully elucidate the mechanism(s) by which responsiveness of glial cells to drug exposure contributes to the behavioral pathologies, and to clarify the relationship between GLT-1 expression, astroglial function, and drug reward. For example, how do adaptations of glial cells in response to drug abuse affect neuronal signaling and synaptic strength? Also, can targeting glial cells directly restore these measures? A more thorough understanding of these fundamental mechanisms of cocaine-induced glial pathology will help identify new therapeutic mechanisms and inform on developing compounds to target these mechanisms.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
The authors thank members of the Kalivas lab for helpful comments on a previous version of this manuscript. This work was supported by DA026254 (KJR), DA015369/DA003906 (PWK), and American Australian Association and NHMRC (RMB).
References
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else. Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61:1766–1773. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- Binns BC, Huang Y, Goettl VM, Hackshaw KV, Stephens RL., Jr. Glutamate uptake is attenuated in spinal deep dorsal and ventral horn in the rat spinal nerve ligation model. Brain Res. 2005;1041:38–47. doi: 10.1016/j.brainres.2005.01.088. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Castanon M, Spevak W, Parkinson FE. Effects of propentofylline on adenosine receptor activity in Chinese hamster ovary cell lines transfected with human A1, A2A, or A2B receptors and a luciferase reporter gene. Can J Physiol Pharmacol. 1998;76:1132–1138. doi: 10.1139/cjpp-76-12-1132. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Kalivas PW. Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neurosci. 2003;17:1273–1278. doi: 10.1046/j.1460-9568.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- Brown RM, Duncan JR, Stagnitti MR, Ledent C, Lawrence AJ. mGlu5 and adenosine A2A receptor interactions regulate the conditioned effects of cocaine. Int J Neuropsychopharmacol. 2012;15:995–1001. doi: 10.1017/S146114571100126X. [DOI] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Chen JH, Dougherty PM. Altered discharges of spinal wide dynamic range neurons and down-regulation of glutamate transporter expression in rats with paclitaxel-induced hyperalgesia. Neuroscience. 2006;138:329–338. doi: 10.1016/j.neuroscience.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12:515–525. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Sarid-Segal O, Knapp CM, Ciraulo AM, LoCastro J, Bloch DA, et al. Efficacy screening trials of paroxetine, pentoxifylline, riluzole, pramipexole and venlafaxine in cocaine dependence. Addiction. 2005;100 (Suppl 1:12–22. doi: 10.1111/j.1360-0443.2005.00985.x. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Jones JD, Comer SD. Glial modulators: a novel pharmacological approach to altering the behavioral effects of abused substances. Expert Opin Investig Drugs. 2012;21:169–178. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierba AL, Jelic S. Chronic obstructive pulmonary disease in the elderly: an update on pharmacological management. Drugs Aging. 2009;26:447–456. doi: 10.2165/00002512-200926060-00001. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston AC, Rebec GV. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–339. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton M, Harvey RJ, Kirchner V.2003Propentofylline for dementia Cochrane Database Syst RevCD002853. [DOI] [PubMed]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16:215–228. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Staneviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kittner B, Rossner M, Rother M. Clinical trials in dementia with propentofylline. Ann N Y Acad Sci. 1997;826:307–316. doi: 10.1111/j.1749-6632.1997.tb48481.x. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OS, Chung YB, Kim MH, Hahn HG, Rhee HK, Ryu JC. Pharmacokinetics of propentofylline and the quantitation of its metabolite hydroxypropentofylline in human volunteers. Arch Pharm Res. 1998;21:698–702. doi: 10.1007/BF02976760. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Ryu JC. Identification of propentofylline metabolites in rats by gas chromatography/mass spectrometry. Arch Pharm Res. 2000;23:374–380. doi: 10.1007/BF02975450. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddie S, Anderson KL, Paz A, Itzhak Y. The effect of phosphodiesterase inhibitors on the extinction of cocaine-induced conditioned place preference in mice. J Psychopharmacol. 2012;26:1375–1382. doi: 10.1177/0269881112447991. [DOI] [PubMed] [Google Scholar]
- Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89:141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Lutgen V, Kong L, Kau KS, Madayag A, Mantsch JR, Baker DA.2012Time course of cocaine-induced behavioral and neurochemical plasticity Addict Biol(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Marcusson J, Rother M, Kittner B, Rossner M, Smith RJ, Babic T, et al. A 12-month, randomized, placebo-controlled trial of propentofylline (HWA 285) in patients with dementia according to DSM III-R. The European Propentofylline Study Group. Dement Geriatr Cogn Disord. 1997;8:320–328. doi: 10.1159/000106650. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskini N, Nemoz G, Okyayuz-Baklouti I, Lagarde M, Prigent AF. Phosphodiesterase inhibitory profile of some related xanthine derivatives pharmacologically active on the peripheral microcirculation. Biochem Pharmacol. 1994;47:781–788. doi: 10.1016/0006-2952(94)90477-4. [DOI] [PubMed] [Google Scholar]
- Mielke R, Moller HJ, Erkinjuntti T, Rosenkranz B, Rother M, Kittner B. Propentofylline in the treatment of vascular dementia and Alzheimer-type dementia: overview of phase I and phase II clinical trials. Alzheimer Dis Assoc Disord. 1998;12 (Suppl 2:S29–S35. [PubMed] [Google Scholar]
- Minelli A, Barbaresi P, Reimer RJ, Edwards RH, Conti F. The glial glutamate transporter GLT-1 is localized both in the vicinity of and at distance from axon terminals in the rat cerebral cortex. Neuroscience. 2001;108:51–59. doi: 10.1016/s0306-4522(01)00375-x. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, Kalivas PW. Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci. 2011;5:1–9. doi: 10.3389/fnsys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir RL. Peripheral arterial disease: pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs. 2009;27:26–30. doi: 10.1016/j.jvn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, et al. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- O'Neill CE, LeTendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology. 2012;37:1245–1256. doi: 10.1038/npp.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2005The rat brain in stereotaxic coordinates5 edn.Elsevier Academic: Amsterdam [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Sartor GC, Vazey EM, Dunn TE, Aston-Jones G, Kalivas PW. Use of vivo-morpholinos for control of protein expression in the adult rat brain. J Neurosci Methods. 2012;203:354–360. doi: 10.1016/j.jneumeth.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Uys JD, Schwacke JH, Comte-Walters S, Rutherford-Bethard JL, Dunn TE, et al. AKAP signaling in reinstated cocaine seeking revealed by iTRAQ proteomic analysis. J Neurosci. 2011;31:5648–5658. doi: 10.1523/JNEUROSCI.3452-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu B, Moller HJ, Grunberger J, Deutsch H, Rossner M. Propentofylline in adult-onset cognitive disorders: double-blind, placebo-controlled, clinical, psychometric and brain mapping studies. Neuropsychobiology. 1990;24:173–184. doi: 10.1159/000119482. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JA, Ruta JD, Gordon JS, Rodrigues AS, Foote CC. The phosphodiesterase inhibitor isobutylmethylxanthine attenuates behavioral sensitization to cocaine. Behav Pharmacol. 2012;23:310–314. doi: 10.1097/FBP.0b013e3283536d04. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2006;31:978–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer S, De Leo J. Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol. 2011;200:235–250. doi: 10.1007/978-3-642-13443-2_8. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Tawfik VL, Regan MR, Haenggeli C, Lacroix-Fralish ML, Nutile-McMenemy N, Perez N, et al. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience. 2008;152:1086–1092. doi: 10.1016/j.neuroscience.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley SL. Methylxanthines in asthma. Handb Exp Pharmacol. 2011;200:439–456. doi: 10.1007/978-3-642-13443-2_17. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Marsili V, Romano R, Tantucci M, Di Filippo M, et al. A2A adenosine receptor antagonism enhances synaptic and motor effects of cocaine via CB1 cannabinoid receptor activation. PLoS One. 2012;7:e38312. doi: 10.1371/journal.pone.0038312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, et al. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The ‘toll' of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wang W, Yu F, Nazari M, Liu X, Liu QS. Phosphodiesterase 4 inhibition impairs cocaine-induced inhibitory synaptic plasticity and conditioned place preference. Neuropsychopharmacology. 2012;37:2377–2387. doi: 10.1038/npp.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]