Abstract
The serotonin 1A receptor (5-HT1A) has a major role in modulating the effects of serotonin on mood and behavior. Previous studies have shown that knockout of 5-HT1A selectively in the raphe leads to higher levels of anxiety during adulthood. However, it remains unclear whether this phenotype is due to variation in receptor levels specifically during development or throughout life. To test the hypothesis that developmental sensitivity may underlie the effects of 5-HT1A on anxiety, we used an inducible transgenic system to selectively suppress 5-HT1A levels in serotonergic raphe neurons from post-natal days (P) 14 to P30, with a maximal reduction of 40% at P21 and return to regular levels by P30. This developmental decrease in receptor levels has long-lasting consequences, increasing anxiety and decreasing social investigation in adulthood. In addition, post-natal knockdown of autoreceptors leads to long-term increases in the excitability of serotonergic neurons, which may represent a mechanism underlying the effects of post-natal receptor variation on behavior later in life. Finally, we also examined the interplay between receptor variation and juvenile exposure to stress (applied from P14 to P21). Similar to receptor knockdown, juvenile exposure to stress led to increased anxiety phenotypes but did not exacerbate 5-HT1A knockdown-mediated anxiety levels. This work indicates that the effects of 5-HT1A autoreceptors on anxiety and social behaviors are developmentally mediated and suggests that natural variations in the expression of 5-HT1A may act during development to influence individual anxiety levels and contribute to susceptibility to anxiety disorders.
Keywords: 5-HT1A, serotonin, development, gene environment interaction, anxiety, HTR1A
INTRODUCTION
Alterations in serotonergic neurotransmission have been implicated in the etiology of both mood and anxiety disorders (Ressler and Nemeroff, 2000). In particular, the inhibitory serotonergic receptor, serotonin receptor 1A (5-HT1A), is widely expressed in the mammalian brain and has a major role in modulating traits related to these disorders (Akimova et al, 2009; Albert and Lemonde, 2004; Savitz et al, 2009). Within the raphe, 5-HT1A acts as a presynaptic autoreceptor whose activation leads to reduced firing of serotonergic neurons and decreased serotonin levels in a variety of forebrain projection structures. Elsewhere in the brain, post-synaptic 5-HT1A heteroreceptors are located on both excitatory and inhibitory neurons and modulate the activity of a number of limbic structures involved in mood and behavior, including the hippocampus, prefrontal cortex, and amygdala (Barnes and Sharp, 1999; Beck et al, 1992; Hamon et al, 1990; Riad et al, 2000; Santana et al, 2004).
Multiple lines of evidence indicate that 5-HT1A receptors are important for mood and anxiety and that anxiety phenotypes may be developmentally mediated. In preclinical mouse models, complete knockout of 5-HT1A leads to increased anxiety and stress responsivity (Heisler et al, 1998; Parks et al, 1998; Ramboz et al, 1998). Clinically, the 5-HT1A agonist, buspirone, decreases anxiety levels and acts as a mild anti-depressant (Loane and Politis, 2012). Further, developmental blockade of 5-HT1A with WAY-100,635 from post-natal day (P)0 to P21 or from P13 to P34 recapitulates the increased anxiety phenotype, whereas similar antagonism during adulthood does not (Lo Iacono and Gross, 2008; Vinkers et al, 2010). However, it is unclear whether the increased anxiety phenotype is due to alteration of serotonin levels via the direct actions of the 5-HT1A autoreceptor on raphe neurons or through the altered response to released serotonin acting at 5-HT1A heteroreceptors in the limbic system. Thus, given the widespread distribution and potentially overlapping roles of 5-HT1A autoreceptors and heteroreceptors, conditional genetic strategies have been necessary to identify the specific behavioral roles of different 5-HT1A receptor populations.
To date, genetic models for investigating the behavioral consequences of specific 5-HT1A sub-populations have taken advantage of a tetracycline-sensitive genetic system in which transcription from the 5-HT1A gene can be suppressed in a spatially and temporally refined manner (Richardson-Jones et al, 2010; Richardson-Jones et al, 2011; Tanaka et al, 2010). Manipulation of 5-HT1A receptors at different time points has revealed a dissociable role for autoreceptor versus heteroreceptor populations during development and adulthood (Richardson-Jones et al, 2010; Richardson-Jones et al, 2011). In particular, whole life, but not adult-specific, suppression of autoreceptors leads to increased anxiety, a finding recently confirmed via RNAi knockdown of autoreceptors in adulthood (Bortolozzi et al, 2012; Richardson-Jones et al, 2010). In contrast, developmental suppression of forebrain heteroreceptors does not affect anxiety levels but appears to be important for modulating stress reactivity and depression-related behaviors (Richardson-Jones et al, 2011). This suggests that the developmental activation of autoreceptor and heteroreceptors leads to different behavioral outcomes. However, it is unclear whether autoreceptor knockdown induces increased anxiety levels by acting specifically during development or whether continued knockdown throughout life is necessary for this phenotype.
Although previous genetic experiments have focused on the role of variation in receptor levels as a potential biological factor underlying disease risk, environmental factors are also thought to contribute to differences in susceptibility to mood and anxiety disorders. In humans, exposure to stress or trauma, especially during childhood, is linked with increased risk for a variety of psychiatric disorders, including anxiety disorders and depression (Charney and Manji, 2004; Hammen, 2005; Heim et al, 2010). Similar findings have been observed in animal models of early-life stress (de Kloet et al, 2005; Holmes et al, 2005). Further, the effects of early-life stress may interact with heritable variation in serotonergic genes to shape individual differences in anxiety and depression risk. Within humans, variants that affect serotonergic function, such as the serotonin transporter polymorphism, interact with early-life stress to shape disease susceptibility (Caspi et al, 2003; Karg et al, 2011; Kendler et al, 2005). Similarly, phenotypes displayed by animal models of altered serotonergic function, such as 5-HTT and 5-HT1A knockout mice, are sensitive to environmental exposure (Carola and Gross, 2012; Zanettini et al, 2010). For instance, both 5-HTT heterozygous and knockout mice are more sensitive than their wild-type littermates to the effects of early-life and adult stress (Carola and Gross, 2012), and although less studied, environmental enrichment for the first 2 weeks of life alleviates the social anxiety phenotype characteristic of 5-HT1A knockout mice (Zanettini et al, 2010).
These findings suggest two hypotheses: (1) the effects of 5-HT1A auto-inhibition on anxiety levels are developmentally mediated and (2) the behavioral effects of variation in 5-HT1A autoreceptor levels may be modified by stress exposure during sensitive developmental periods. In order to address these hypotheses, we used a tetracycline-sensitive genetic system to reduce 5-HT1A autoreceptor levels by ∼40% from P14 to P30 (Figure 1). Specifically, autoreceptors were rescued to control levels by P30 (Figure 1) and were indistinguishable from controls through adulthood. A separate cohort of control and knockdown mice were also exposed to daily bouts of unpredictably timed restraint stress from P14 to P21. This represents the period of overlap in studies that found long-term increases in anxiety when administering the 5-HT1A antagonist, WAY100635, from P0 to P21 (Vinkers et al, 2010) and from P13 to P34 (Lo Iacono and Gross, 2008). Thus we sought to ask whether concurrent stress might interact with receptor knockdown to shape behavior later in life.
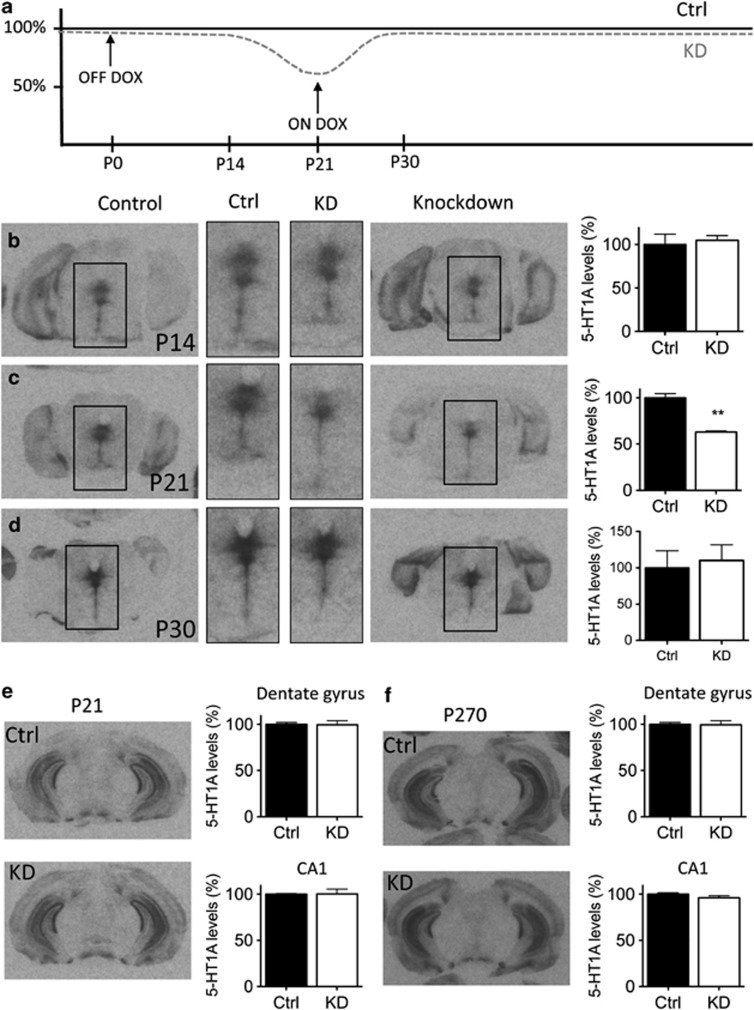
Figure 1.
Post-natal knockdown of 5-HT1A autoreceptors. (a) Doxycycline was removed from the chow from P0 to P21, resulting in a ∼40% reduction in 5-HT1A autoreceptor levels at P21 in animals carrying the tTs transgene (KD mice). (b–d) Receptor levels were the same in control and KD animals at P14 (n=2/grp), reach maximal knockdown at P21 (n=4–8/grp), and were rescued by P30 (n=3/grp). **p<0.001. (e and f) Consistent with previous reports, hippocampal 5-HT1A receptors are unaffected in control and knockdown animals at either P21, when raphe 5-HT1A receptor knockdown is maximal, or in adulthood (n=4–8/grp).
MATERIALS AND METHODS
Mice
Animal husbandry
Animals were housed in groups of 3–5, had ad libitum access to food and water, and were maintained on a 12:12 light:dark cycle. Animal protocols were approved by the Institutional Animal Care and Use Committee and in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
5-HT1A autoreceptor knockdown mice
Mice with suppressible 5-HT1A receptors (Htr1AtetO) were bred as previously described (Richardson-Jones et al, 2010; Richardson-Jones et al, 2011). Htr1AtetO/tetO mice are indistinguishable from WT mice in their patterns of 5-HT1A. Mice with inducible suppression of 5-HT1A autoreceptors were homozygous for the (Htr1AtetO/tetO) and carried one copy of the Pet1-tTs transgene. In the presence of doxycycline (DOX), these mice have normal 5-HT1A autoreptor levels, but in the absence of DOX, Htr1A (the gene that encodes 5-HT1A) transcription is repressed and 5-HT1A autoreceptor levels decrease. 5-HT1A receptors in other regions (hippocampus, entorhinal cortex, and amygdala) are not altered by removal of DOX (Richardson-Jones et al, 2010).
Animals were maintained on a mixed 129/Sv, C57Bl6/J, CBA background. For all the experiments, male Htr1AtetO/tetO/Pet1-tTs+ males were bred to Htr1AtetO/tetO females. Transmission of the tTs transgene through the male germline ensured that all offspring were raised by Htr1AtetO/tetO dams, which are not sensitive to the effects of DOX. For all experiments, Htr1AtetO/tetO/Pet1-tTs+ mice (referred to as knockdown; KD) were compared with Htr1AtetO/tetO littermates (control; Ctrl). As DOX is transmitted via the mother's milk, this design ensured that relevant comparisons occurred between littermates rather than between litters. All experimental mice were from litters from primiparous dams. Experiments were conducted exclusively with male offspring.
Autoreceptor suppression during post-natal development
In order to selectively repress 5-HT1A levels during post-natal development, mothers were maintained on DOX chow (Bio-serv F6118, Frenchtown, NJ) until the day of birth. From P0 to P21, DOX chow was replaced with normal laboratory diet (LabDiet 5001, Richmond, IN). On P21, males were weaned into cages with 4–5 animals and maintained on DOX chow for the remainder of the study.
Juvenile-restraint stress (JS)
Pups were removed from their home cage and placed in plastic restrainers in a brightly lit room (Fisher canisters 03-338-1F with air holes drilled in them) for an hour a day each day from P14 to P21. Restraint stress occurred during the dark portion of the light cycle and was applied at different times each day. Animals were weighed and corticosterone levels were measured following stress on P21 to ensure that animals had not habituated to the restraint paradigm. Corticosterone levels were measured using Assay Designs ELISA kit (Ann Arbor, MI), following the manufacturer's directions (n=2–4/grp).
Behavioral Assays
Tests for anxiety and stress coping were performed under normal light conditions beginning 1 h after lights were turned on. Social behavior was assessed under red light 2 h after lights were turned off. All animals were within 2 weeks of age at the time of testing, and behavioral testing began at P90. Tests were performed in the following order with at least 1 week between tests: open field, elevated plus, novelty-suppressed feeding (NSF), tail suspension, forced swim test, and social behavior. For all the experiments, genotype controls consisted of Htr1AtetO/tetO/Pet1-tTs- animals, which are indistinguishable from WT mice (Richardson-Jones et al, 2010). Animals raised using standard laboratory conditions (as outlined above, referred to as ‘no stress') served as controls for rearing condition.
Open field
A 10-min exploration in a novel open arena was performed as previously described (Richardson-Jones et al, 2010). Tracking was performed using AnyMaze ver4.82 Software (n=15–21/grp).
Elevated plus maze
Exploration of an elevated plus maze was assessed as previously described (Curley et al, 2009). Tracking was performed using AnyMaze ver4.82 Software (n=15–21/grp).
NSF
Testing was performed as previously described (Richardson-Jones et al, 2010). Animals were food restricted for 24 h and then placed in a 40 × 60 cm2 brightly lit arena (∼900 lux) with a food pellet placed in the center. Latency for the animal to begin eating the pellet was recorded. Immediately following testing, the animal was weighed and placed back in its home cage where food consumption over 5 min was recorded. Percentage of body weight loss and home cage food consumption were used as relative measures of hunger (n=11–21/grp).
Tail suspension test
Mice were suspended by their tail using tape to secure them to a horizontal bar. Immobility was assessed over a 5-min period using automated tracking with AnyMaze software (n=11–21/grp).
Forced swim test
Behavioral response to forced swimming was measured as described in David et al (2007). Mice were placed in clear glass buckets 20 cm deep, filled 2/3 of the way with 25 °C water, and videotaped from the side. The entire test was scored using Noldus Ethovision software as described in Juszczak et al (2008). All animals were exposed to swim stress on two consecutive days (n=11–21/grp).
Social behavior
Basic social interaction and novelty preference was assessed using the methods outlined by Yang et al (2011). Briefly, mice were habituated to a 40 × 60 cm2 arena lit by red lights for 10 min. At either end of the arena were two overturned pencil holder cups (Kitchen-Plus.com 31570). Following habituation to the test chamber, a novel adult male C57Bl/6J mouse was placed under one of the pencil holders (Phase 1). In Phase 2, this male became the ‘familiar animal' and a novel adult male C57Bl/6J was placed under the opposite pencil holder. In both the phases, total levels of social investigation were measured as the amount of time spent directly investigating the chamber(s) containing another mouse. In addition, preference for a social stimulus was measured in Phase 1 (social vs empty chamber) and social novelty preference (novel vs familial mouse) in Phase 2. For both the phases, a human observer blind to genotype and treatment group scored direct investigation of the chambers for the first 5 min of testing. Total test time, including habituation, was 30 min. Four animals were tested simultaneously, and the location of the novel and familiar animal was counter-balanced across testing chambers in both the test phases. In addition, overall distance travelled was measured using Anymaze ver4.82 software (n=11–21/grp).
Receptor Autoradiography
Mice were killed by cervical dislocation and decapitation. Brains were extracted, immediately frozen on dry ice, and maintained at −80 °C until sectioning. Brains were cryosectioned at a thickness of 20 μm and thaw mounted on Superfrost Plus slides (Fisher Scientific). Sections were maintained at −80 °C until processing. Sections were processed for 4-(2′-methopxyphenyl)-1-[2′- (n-2′′-pyridinyl)-p-[125I]iodobenzamido]ethylpiperazine (125I-MPPI) autoradiography as previously described (Richardson-Jones et al, 2010). All experimental and control brains within a group (n=2–8/grp, see Figure 1) were processed and exposed to film as a single batch. To the extent possible, Ctrl and KD animals used for receptor autoradiography at different time points were from the same litter with a maximum of four litters represented at any given time point. Receptor levels were quantified as follows. Films were scanned at 1600 dpi and analyzed using ImageJ software. For raphe, seven matching coronal sections containing dorsal raphe were identified for each brain (Supplementary Figure S1). The dorsal raphe was outlined by hand in a randomly selected control individual, and this outline was then applied to the corresponding section in all the other animals. Average gray value was calculated for the region. A background measurement was taken from each section by selecting a white matter tract or region known to not express 5-HT1A. Gray values for raphe and background regions were converted to pCi/region by comparing with an ARC146-F 14C standard, and the background value was subtracted from the raphe. All seven sections were graphed, and an area under the curve was calculated for each animal. To determine percentage of change in receptor level across the raphe, values were normalized to the average area under the curve for control animals (Supplementary Figure S1). Because the dentate gyrus and CA1 have more homogenous receptor levels than the raphe, these regions were analyzed in a similar fashion for nine matched sections/animal except that the gray value for these regions was taken from the average of three circles drawn within the region.
Electrophysiology
Adult animals (P270–P300) were used to assess the properties of raphe neurons in control compared with knockdown mice. We first verified that autoreceptor levels remained indistinguishable between control and knockdown animals at this later time point (t(11)=0.76, p=0.46; Figure 5a and b). For electrophysiological recordings, animals were euthanized by decapitation, and brain slices were prepared as previously described (Crawford et al, 2010, 2011). Midbrain coronal slices (200 μm) containing the dorsal raphe (DR), were placed in aCSF (in mM: NaCl 124, KCl 2.5, NaH2PO4 1.25, MgSO4 2.0, CaCl2 2.5, dextrose 10, and NaHCO3 26) bubbled with 95% O2/5% CO2 and maintained at 37 °C for 1 h after which slices were kept at room temperature until recording. Tryptophan (2.5 μM) was included in the holding chamber to maintain 5-HT synthesis but was not present in the aCSF during recording.
To obtain whole-cell recordings, slices were placed in a small recording chamber (Warner Instruments, Hamden, CT) and perfused with aCSF bubbled with 95% O2/5% CO2 at approximately 2 ml/min at 32 °C maintained by an in-line solution heater (TC-324, Warner Instruments). Neurons were visualized using a Nikon E600 upright microscope and targeted under DIC. Electrodes were filled with an intracellular solution of (in mM) K-gluconate, 130; NaCl, 5; Na phosphocreatine, 10; MgCl2, 1; EGTA, 0.02; HEPES, 10; MgATP, 2; and Na2GTP, 0.5; with biocytin, 0.1% pH 7.3. Whole-cell recordings were obtained using a Multiclamp 700B (Molecular Devices, Instruments,Sunnyvale, CA). Cell characteristics were recorded using current clamp techniques as previously described (Calizo et al, 2011; Crawford et al, 2010). Signals were collected and stored using a Digidata 1320 analog-to-digital converter and pClamp 9.0 software (Molecular Devices).
Membrane and cell excitability characteristics were recorded in current clamp by administering 500 ms current injections in 20 pA steps from −80 pA to 160 pA. Action potential characteristics were recorded in current clamp by administering enough current to elicit a single spike. The magnitude of the 5-HT1A receptor-mediated response was measured following bath administration of 100 nM 5-carboxyamidotryptamine (5-CT). Cells were voltage clamped at −60 mV, baseline current measurements obtained for 3–5 min followed by bath administration of 5-CT, and the current was measured for at least 3–5 min until a steady state response was obtained. The drug was removed from the aCSF to monitor the return to baselne. After electrophysiological recording, slices were placed in 4% paraformaldehyde and stored at 4 °C until processed for immunohistochemical analysis for the biocytin-filled neuron and tryptophan hydroxylase. Only those neurons identified as TPH containing were used for data analysis.
8-OH-DPAT-Induced Hypothermia
Animals were administered 0.5 mg/kg 8-OH-DPAT via intraperitoneal injection following three measurements of core body temperature to determine baseline. Change in core temperature was assessed every 10 min for 60 min as outlined in Richardson-Jones et al (2010) (n=6–9/grp).
Statistical Analysis
All statistical calculations are presented as mean±SEM and were performed in SPSS version 19. Initial behavioral analysis was performed using a 2-way ANOVA (genotype X rearing condition). If a significant interaction was observed, a simple main effects analysis with Sidak-adjusted α was used to investigate specific comparisons. In addition, in the absence of a significant interaction term in the open field test, we investigated a subset of planned comparisons using independent samples' t-tests. Planned comparisons were performed for each of the following: Ctrl vs KD mice in the no-stress condition, Ctrl vs KD mice in the stress condition, and Stress vs No Stress among Crtl mice. For the NSF paradigm, latency to eat was analyzed both via two-way ANOVA and via survival analysis using the Kaplan–Meier product-limit method. The latter accounts for animals that did not eat during the test; for the former, we applied a latency of 600 s (the test length) to animals that did not eat.
For electrophysiological recordings, two-way ANOVAs (genotype × sub-region) were used to test the significance of membrane characteristics, action potential characteristics, and 5-CT response. For membrane excitability, data were analyzed separately for each sub-region using a repeated-measure ANOVA, with a main factor of genotype and current injected as the repeated measure. 8-OH-DPAT-induced hypothermia was also assessed using a repeated-measures ANOVA. Follow-up Student's t-tests were used when indicated.
RESULTS
Selective Knockdown of 5-HT1A Autoreceptors from P14 to P30
In order to determine the feasibility of repressing 5-HT1A autoreceptors selectively during post-natal development, we removed DOX chow beginning on P0 and returned it on P21. We then compared the density of 5-HT1A receptors in the raphe at P14, P21, and P30 of tTS+ (knockdown; KD) and tTS− (control; Ctrl) littermates (Figure 1; Supplementary Figure S1). Somewhat surprisingly, differences in 5-HT1A levels were indistinguishable at P14 (t(2)=0.38; p=0.74) (Figure 1b). However, by P21, tTS+ animals showed a ∼40% reduction in 5-HT1A levels in the raphe raphe: (t(10)=−4.89, p<0.001) (Figure 1c). We also confirmed that receptor levels did not differ in the hippocampus (Figure 1e and f), consistent with previous reports that autoreceptors, but not heteroreceptors, are altered in this mouse model at P21 (CA1: t(8)=0.023, p=0.98; dentate gyrus: t(8)=0.239, p=0.82) or later in life at P270 (CA1: t(12)=−1.575, p=0.14; dentate gyrus: t(11)=−0.113, p=0.91). Animals were returned to DOX chow on P21, and by P30 receptor levels were indistinguishable between tTS+ and tTS− littermates (raphe: t (4)=0.318, p=0.77) (Figure 1d). The slow onset of the knockdown may be explained by a combination of time needed for DOX to clear the organism and half-life of the 5-HT1A protein (Stark et al, 2007).
JS has Physiological Impacts
In order to determine the validity of our restraint stress paradigm in juvenile mice, we examined corticosterone levels immediately following restraint on P21 and weighed mice at weaning. Restraint stress elicited a robust corticosterone response (JS=189.7 ng/ml, unhandled controls=13.9 ng/ml; t(4)=16.69; p<0.001; Supplementary Figure S2). In addition, restrained animals weighed approximately 10% less than non-restrained animals (standard reared=8.4±0.20 g; JS=7.5±0.13 g; main effect of rearing condition: F(1,61)=12.80, p=0.001; main effect of genotype: F(1,61)=0.11, p=0.74; interaction F(1, 61)=0.24, p=0.63). JS animals did achieve normal adult weight (main effect of rearing condition: F(1,61)=0.28, p=0.60; main effect of genotype: F(1,61)=0.27, p=0.60; interaction F(1,61)=0.60, p=0.44).
Post-Natal 5-HT1A Autoreceptor Knockdown and Juvenile Stress Alter Anxiety-Related Behaviors
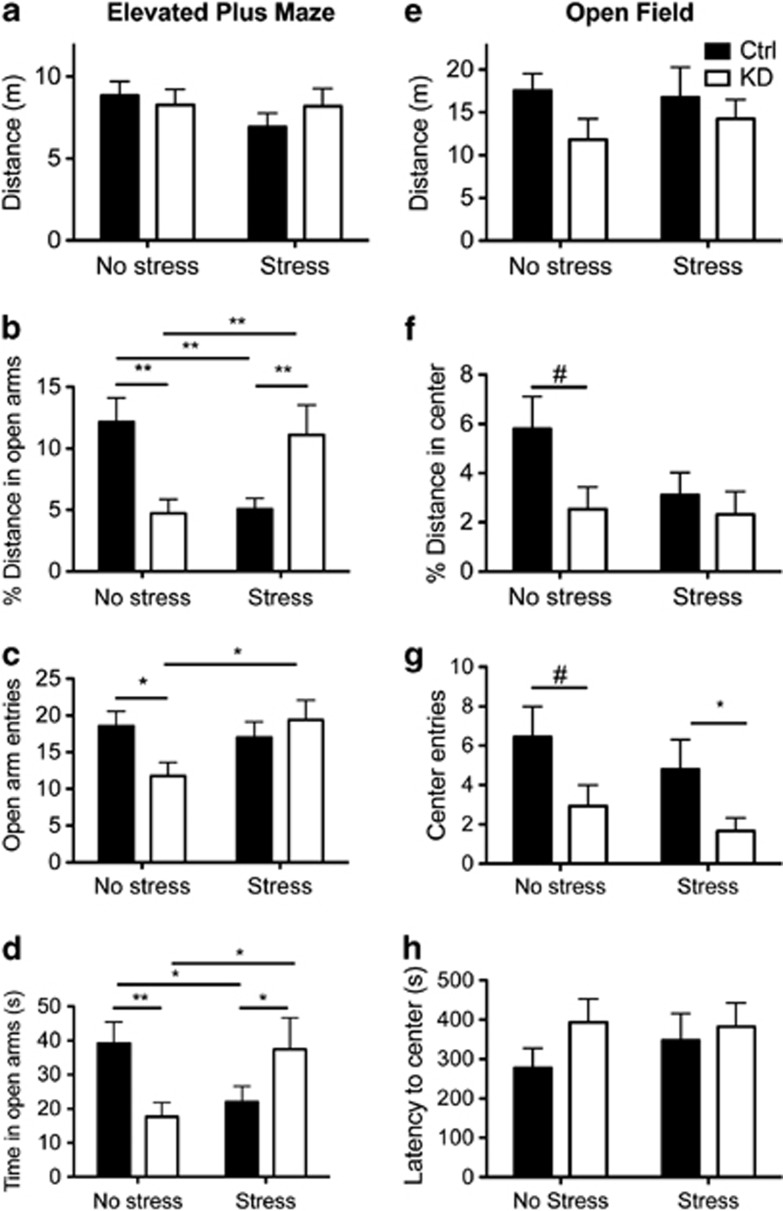
Mice lacking 5-HT1A autoreceptors throughout life have increased anxiety levels (Richardson-Jones et al, 2011). In order to assess whether developmental sensitivity underlies the effects of autoreceptor knockdown and stress on anxiety behaviors, we performed the elevated plus maze and open field tests in adulthood (>P90) (Figure 2). We further examined the interplay between receptor knockdown and JS by performing these tests on a separate cohort that underwent restraint stress from P14 to P21, concurrent with receptor knockdown. Results of two-way ANOVAs (rearing condition × genotype) are reported in the text while simple main effects with Sidak-adjusted α and planned comparisons are indicated in Figure 2. In animals reared under standard conditions (No stress), post-natal knockdown of autoreceptors resulted in increased anxiety levels. In the elevated plus maze, KD mice traveled less in the open arms (main effect of rearing condition: F (1, 66)=0.00, p=0.99; main effect of genotype: F(1, 66)=0.09, p=0.77; rearing × genotype: F(1, 66)=18.0, p<0.0001); Figure 2b), entered into the open arms less (main effect of rearing condition: F (1, 66)=1.96, p=0.17; main effect of genotype: F(1, 66)=1.02, p=0.32; rearing × genotype: F(1, 66)=4.45, p=0.04); Figure 2c), and spent less time in the open arms (main effect of rearing condition: F (1, 66)=0.19, p=0.67; main effect of genotype: F(1, 66)=0.127, p=0.72; rearing × genotype: F(1, 66)=11.3, p<0.0001); Figure 2d). We also observed a trend for increased levels of anxiety in the open field test (planned comparison t-tests are reported in Figure 2), as indicated by a decreased percentage of path length in the center of the arena (main effect of rearing condition: F (1, 68)=2.6, p=0.10; main effect of genotype: F(1, 68)=1.65, p=0.20; rearing × genotype: F(1, 66)=0.398, p=0.53); Figure 2f), decreased number of center entries (main effect of rearing condition: F (1, 68)=1.75, p=0.19; main effect of genotype: F(1, 68)=4.51, p=0.04; rearing × genotype: F(1, 66)=0.02, p=0.90; Figure 2g) but no strong difference in latency to enter the center (main effect of rearing condition: F (1, 68)=0.48, p=0.49; main effect of genotype: F(1, 68)=1.15, p=0.28; rearing × genotype: F(1, 66)=0.24, p=0.63; Figure 2h). Exposure to JS also resulted in increased anxiety levels in WT animals in the elevated plus maze in the percentage of distance and time in open arms (Figure 2b and d). Interestingly, a reversal of the anxiety phenotype determined by elevated plus maze was evident in early-life knockdown animals exposed to stress as indicated in in percentage of distance, number of entries, and time in open arms (Figure 2b–d), although this was not replicated in the open field test. No differences in locomotion were seen in either the elevated plus maze (main effect of rearing condition: F(1, 66)=1.12, p=0.29; main effect of genotype: F(1, 66)=0.15, p=0.71; rearing × genotype: F(1, 66)=0.96, p=0.33) or the open field (main effect of rearing condition: F(1, 68)=0.02, p=0.89; main effect of genotype: F(1, 68)=2.1, p=0.15; rearing × genotype: F(1, 68)=0.20, p=0.65). In addition, in a separate cohort of mice kept on DOX throughout life, we assessed the effect of the tTS transgene on anxiety. We found that expression of tTS neither altered ambulatory behavior nor led to increased anxiety measures (see Supplementary Methods and Supplementary Figure S3).
Figure 2.
Post-natal knockdown of 5-HT1A autoreceptors leads to increased anxiety. Anxiety levels were assessed via the elevated plus maze tests (a–d) and open field (e–h) during adulthood. Exposure to JS also increased anxiety in WT animals but yielded test-specific effects in mice with decreased autoreceptor levels. n=15–21/grp; #p<0.10, *p<0.05, **p<0.01.
Effects of JS and Autoreceptor Knockdown are Dissociable in the NSF Task
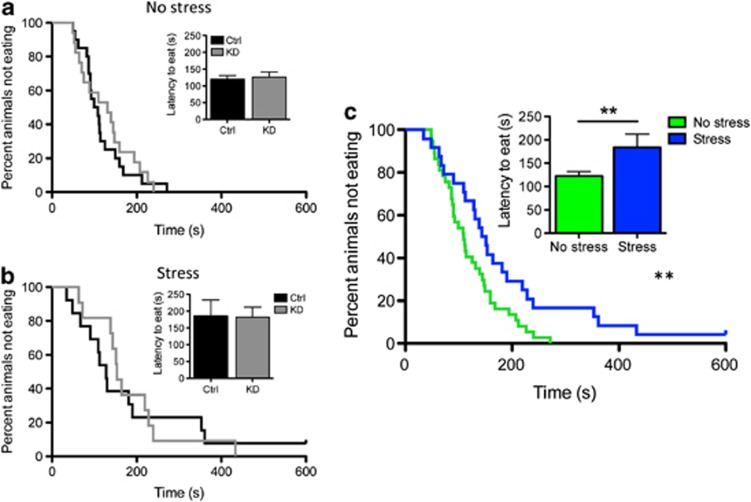
NSF measures a rodent's aversion to eating in a novel environment, specifically assessing stress-induced anxiety by measuring the latency of the mouse to eat a familiar food in a novel, aversive environment (Bodnoff et al, 1988). This trait has been shown in rats to be sensitive to early-life interventions. Specifically, environmental enrichment in the form of post-natal handling decreases the latency to eat (Bodnoff et al, 1987). In our study, we found that the latency to eat food in a brightly lit arena was greater for animals exposed to JS. This trait was not influenced by autoreceptor knockdown. Two-way ANOVA—main effect of rearing condition: F(1, 61)=5.6, p=0.02; main effect of genotype: F(1, 61)=0.007, p=0.93; rearing condition × genotype: F(1, 61)=0.04, p=0.84; Figure 3a and b). Because there was no main effect of genotype, we grouped animals by rearing condition and found that restrained animals showed an increased latency to eat, indicating that early-life stress increased anxiety levels (t(59)=2.43, p=0.02; Kruskal–Wallace p=0.03; Figure 3c). No effect of genotype or restraint stress was observed on weight loss (two-way ANOVA: main effect of rearing condition: F(1,61)=1.44, p=0.24; main effect of genotype: F(1,61)=0.01, p=0.91; interaction F(1, 61)=0.82, p=0.37) or home cage food consumption (two-way ANOVA: main effect of rearing condition: F(1,61)=0.62, p=0.44; main effect of genotype: F(1,61)=0.75, p=0.39; interaction F(1, 61)=0.03, p=0.87).
Figure 3.
Knockdown of 5-HT1A receptors did not affect the latency to feed in the novelty-suppressed feeding test for either standard reared (a) or stress-exposed (b) animals. In contrast, when the data are collapsed across genotypes, early-life stress increased the latency to feed in this test (c; **p< 0.05).
Neither Autoreceptor Knockdown nor JS Alters Stress Coping
Whole-life knockdown of 5-HT1A autoreceptors was shown to increase anxiety but not depression-related behaviors (Lo Iacono and Gross, 2008; Richardson-Jones et al, 2011). However, these studies were performed exclusively in standard reared mice with whole-life manipulation of 5-HT1A levels. In order to determine whether developmental autoreceptors alone or in the context of juvenile stress modulate differences in stress-coping behaviors, we assessed behavior in both the tail suspension and forced swim tests (n=11–21/group). As predicted, post-natal autoreceptor knockdown did not influence stress-coping behaviors. Similarly, exposure to juvenile stress did not alter stress-coping behaviors. Details and statistics are available in Supplementary Figure S4. These results suggest that both 5-HT1A knockdown and stress during post-natal development specifically affects anxiety but not depression-related traits.
Variation in Autoreceptor Levels and JS Influence Social Behavior
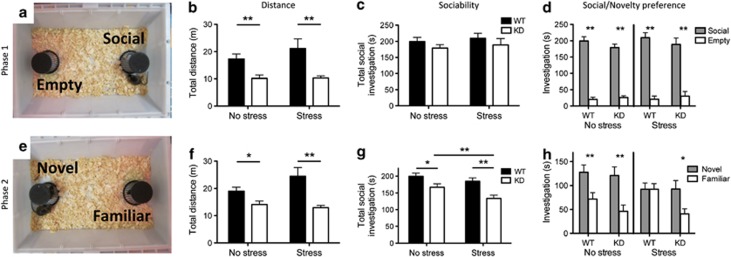
Differences in anxiety can affect many complex behaviors, including social behavior. Previously, it was shown that 5HT1A knockout mice display increased social anxiety, spending less time investigating an unfamiliar mouse, and display higher rates of anxiety-associated behaviors during social interactions, such as stretch-attend postures (Zanettini et al, 2010). In order to explore the role of 5-HT1A autoreceptors and juvenile stress in modulating social behavior, we used a two-phase test designed to investigate both levels of social investigation as well as the natural preference of mice for social novelty, as outlined by Yang et al (2011). KD mice showed decreased exploratory activity compared with their WT littermates (Figure 4). Introduction of a single novel mouse in Phase 1 did not reveal any group differences; all groups of mice showed normal preference for a social stimulus (compared with the empty chamber) and equivalent levels of overall social interest (Figure 4; main effect of rearing condition: F(1,60)=0.45, p=0.51; main effect of genotype: F(1,60)=1.87, p=0.18; interaction F(1, 60)=0.00, p=0.98). However, during the social choice phase (Phase 2), both receptor knockdown and exposure to juvenile stress decreased total levels of social investigation (Figure 4; main effect of rearing condition: F(1,60)=5.16, p=0.027; main effect of genotype: F(1,60)=15.74, p<0.001; interaction F(1, 60)=0.79, p=0.37), and notably, the effects of receptor knockdown were exacerbated by exposure to stress (t(21)=3.65; p=0.0015). In addition, WT animals exposed to JS failed to distinguish between novel and familiar individuals in this test, a phenotype previously reported in both mice and rats exposed to early-life stress (Franklin et al, 2011; Lukas et al, 2011). However, KD animals exposed to restraint stress showed normal social novelty preference.
Figure 4.
Effects of autoreceptor knockdown on social behavior. In Phase 1 (a), knockdown mice showed decreased locomotion (b), but there was no effect of genotype or rearing condition on time spent in social investigation (c) or preference for a social stimulus (d). In Phase 2 (e), 5-HT1A knockdown mice traveled a shorter distance (f) and displayed lower levels of overall sociability (total time spent investigating either mouse) (g), a phenotype that is exacerbated by exposure to early-life stress. Wild-type animals exposed to early-life stress failed to discriminate between novel and familiar individuals, whereas their knockdown littermates retained this ability (h). n=11–21; *p<0.05, **p<0.01.
5-HT1A Autoreceptor Knockdown has a Long-Lasting Impact on Serotonergic Raphe Neuron Cell Characteristics
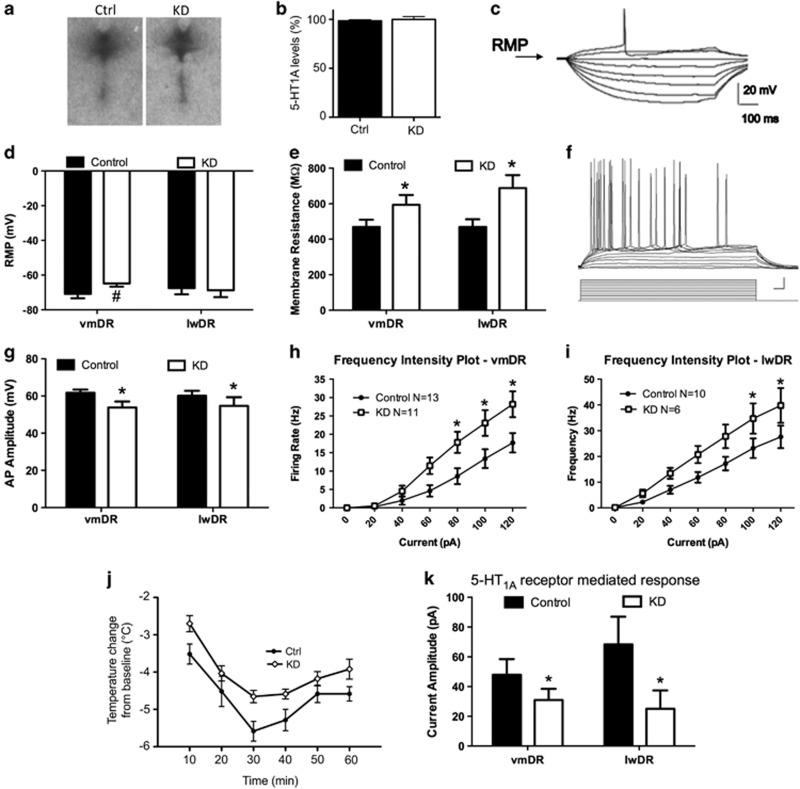
As the primary inhibitory autoreceptor present on serotonergic neurons, 5-HT1A has a critical role in negative feedback on serotonergic signaling (Barnes and Sharp, 1999). Thus we hypothesized that alteration of 5-HT1A autoreceptor levels could lead to long-term changes in the physiology of the serotonergic neurons of the raphe. We directly tested this by measuring the electrophysiological properties of the serotonergic raphe neurons in both the lateral wings (lwDR) and ventromedial (vmDR) aspect of the dorsal raphe in WT and KD animals as adults. We examined both of these subfields, because we previously found differences in their cell parameters (Crawford et al, 2010) and because they are known to have different projections, with the vmDR sending axons to forebrain structures such as the amygdala, medial prefrontal cortex, and caudate-putamen (Bang et al, 2012; Lowry et al, 2008; Michelsen et al, 2008), whereas the lwDR projects to sympathomimetic nuclei (Hale and Lowry, 2011; Johnson et al, 2004). We found that post-natal knockdown of receptors led to long-lasting changes in several of the membrane characteristics of the neurons, the sum of which resulted in increased cell excitability (Figure 5). We measured the resting membrane potential (RMP) at baseline, as well as the membrane resistance as indicated by the slope of the current–response curve obtained from the response to hyperpolarizing current pulses (Figure 5c). RMP was almost significantly depolarized in the vmDR serotonin neurons (t(22)=1.833, p=0.08; Figure 5d). The membrane resistance was significantly higher for the neurons recorded from both the vmDR and the lwDR (two-way ANOVA, F=8.150, p=0.006 for significant main effect of genotype, vmDR n=13 control, n=10 KD, lwDR, n=18 control, n=15 KD; Figure 5e). Also, the action potentials were shunted, as indicated by a significant decrease in amplitude in both the subfields (F=4.336, p=0.043 for the main effect of genotype, Figure 5f). In both the subfields, the excitability of the neurons was greatly enhanced, displaying increased firing frequency in response to depolarizing current pulses (Figure 5g–i; ANOVA significant interaction F=2.442, p=0.028 for vmDR and F=2.401, p=0.031 for lwDR). These data indicate that post-natal autoreceptor knockdown has long-lasting impacts on raphe neuron physiology and that these alterations may represent at least a partial mechanism underlying the long-lasting effects of post-natal 5-HT1A variation on behavior.
Figure 5.
Characteristics of adult 5-HT neurons in autoreceptor knockdown animals. Despite fully recovered 5-HT1A levels, developmental knockdown of 5HT1A receptor elicits long-lasting changes in neuron characteristics. (a and b) Autoreceptor levels are indistinguishable between control and knockdown mice at P270 (n=7-8/grp; p=0.46). (c) Raw data trace showing how the resting membrane potential and membrane resistance were measured, by taking the magnitude of the response elicited by hyperpolarizing current pulses, plotting a current–voltage response curve, and measuring the slope. (d) The RMP of vmDR neurons was slightly depolarized. (e) Membrane resistance was greater in the neurons recorded from both the subfields from the KD mice. (f) Depolarizing current pulses were injected, and the frequency of firing was measured as an indicator of membrane excitability. (g) Action potential amplitude was smaller or shunted in the neurons from KD mice in both the vmDR and lwDR. (h and i) Frequency–intensity plots demonstrate increased excitability of the vmDR and lwDR neurons. (j and k) KD mice also display a blunted 5-HT1A autoreceptor response in adulthood. (j) KD animals show an attenuated hypothermic response to 8-OH-DPAT injection (n=6–9/grp; p=0.026). (k) Application of a 5-HT1A receptor agonist resulted in a lower amplitude response in neurons from KD mice. #p<0.10, *p<0.05.
5-HT1A Autoreceptor Knockdown Results in Decreased Receptor Sensitivity Despite Normalization of Receptor Levels
In mice, 8-OH-DPAT-induced hypothermia has been shown to be mediated by 5-HT1A autoreceptors Therefore, to assess functionality of 5-HT1A autoreceptors in adulthood, we assessed body temperature in response to the 5-HT1A agonist 8-OH-DPAT. Baseline temperatures did not differ between the two groups before treatment: Ctrl=36.4 +/− 0.24 °C; KD=35.92+/−0.18 °C; t(13)=1.611; p=0.13. However, control mice had a significantly larger hypothermic response to the application of 8-OH-DPAT than KD mice (Richardson-Jones et al, 2010; effect of genoptype: F(1, 13)=6.289; p=0.026; Figure 5j). We also assessed the impact of the 5-HT1A agonist, 5-CT, in raphe neurons. Early-life knockdown resulted in a decreased magnitude of the outward current elicited by administration of a 5-HT1A agonist in both the vmDR and the lwDR (main effect of genotype, F=5.638, p=0.023, Figure 5k). These data indicate that both behavioral and electrophysiological responses to 5-HT1A agonist administration are blunted in animals with a history of post-natal receptor knockdown. This suggests that even though the receptor protein is expressed at normal levels, there may be some deficiency in the receptor–effector coupling or in the downstream signaling cascade.
DISCUSSION
5-HT1A-Mediated Anxiety Phenotypes are Developmental in Origin
This study directly demonstrates that a reduction in 5-HT1A autoreceptors between P14 and P30 leads to long-term increases in anxiety levels. Specifically, autoreceptor knockdown during post-natal development increases anxiety and decreases levels of social engagement but does not alter stress coping. These findings support and refine previous pharmacological and transgenic work suggesting that the effects of 5-HT1A autoreceptors on anxiety are a developmentally mediated phenotype (Bortolozzi et al, 2012; Lo Iacono and Gross, 2008; Richardson-Jones et al, 2011; Vinkers et al, 2010) and provide insights into how perturbations to the 5-HT1A autoreceptor system during post-natal life can impact more complex social phenotypes, an area that has not been extensively explored in previous literature.
Importantly, this work addresses a number of limitations of previous studies. Unlike broad-spectrum pharmacological and traditional knockout strategies, we selectively targeted 5-HT1A autoreceptors (Heisler et al, 1998; Lo Iacono and Gross, 2008; Parks et al, 1998; Ramboz et al, 1998). Furthermore, temporal control of autoreceptor levels via DOX administration limited the perturbation of 5-HT1A levels to a narrow window of post-natal development. By knocking down the endogenous receptor, we improved on gain-of-function strategies, which are plagued by ectopic expression (Gross et al, 2002). Finally, autoreceptor levels were reduced by only 40%, which is representative of the range of 5-HT1A expression variation observed in the human population (Drevets et al, 2007). As a result, we have been able to hone in on the behavioral importance of this specific 5-HT1A receptor sub-population during a relatively small window of time.
Implications for Understanding the Impact of Natural Variation on Receptor Levels
5-HT1A receptor levels are sensitive to both genetic and environmental variation. Exposure to stressors modulates 5-HT1A levels in a variety of primate and non-primate species (Franklin et al, 2011; Jovanovic et al, 2011; Leventopoulos et al, 2009; Lopez et al, 1998; Spinelli et al, 2010; Wang et al, 2009). Also, in humans, 5-HT1A levels are impacted by a functional C(-1019)G single-nucleotide promoter polymorphism (SNP; rs6295) (Albert, 2012). The G-allele of this SNP impairs the binding of the repressor, Nuclear Deaf-1 Related factor (NUDR), in a raphe cell line. Possibly as a result, G-allele carriers have higher levels of 5-HT1A autoreceptors, an increased risk for depression, and paradoxically, decreased amygdala reactivity (Albert and Lemonde, 2004; Fakra et al, 2009; Parsey et al, 2010). However, no study has assessed the brain region-specific impact of this polymorphism on 5-HT1A receptor expression during development. Our results show that decreases in 5-HT1A autoreceptors during development impact anxiety-related behaviors but not depression-related behaviors. In contrast, decreases in 5-HT1A autoreceptors in adulthood have no effect on anxiety-related behavior and result in lower levels of depression-related behaviors (Bortolozzi et al, 2012; Richardson-Jones et al, 2010). Thus, the temporal- and region-specific aspects of receptor variability, as impacted by either genetic or epigenetic factors, may have dissociable effects on psychiatric disease risk at different points of the life course, adding a layer of complexity to our understanding of psychiatric risk alleles. This is consistent with findings in multiple systems where a particular molecule has a different role during development compared with adulthood (Laurent et al, 2002; Schwarting et al, 2006; Young-Davies et al, 2000). For example, serotonin transporter blockers such as fluoxetine appear to have opposite effects during development, where they increase anxiety, than in adulthood, where they decrease anxiety (Ansorge et al, 2004).
Long-Lasting Effects of 5-HT1A Knockdown on Serotonin-Producing Neuron Physiology
Because 5-HT1A activation directly impacts the firing of raphe neurons, we hypothesized that decreased autoreceptor levels during post-natal development may have a long-term impact on neural physiology within the raphe (Vinkers et al, 2010). In order to test this hypothesis, we examined electrophysiological properties of serotonin-producing neurons in KD and WT littermates late in life (>P270). Despite full rescue of 5-HT1A levels, there were long-lasting alterations in the cell membrane characteristics of serotonin neurons in two different subfields of the raphe, the vmDR and the lwDR. Within both of these regions, serotonergic neurons of KD mice were more excitable due to alterations in multiple membrane properties. In particular, KD mice had greater membrane resistance. As membrane resistance is indirectly related to ionic conductance, this may suggest abnormal developmental expression of ion channels in these neurons.
In addition, even though adult 5-HT1A autoreceptor levels did not differ between KD and WT mice, the 5-HT1A autoreceptor-mediated response was dampened in the neurons recorded from KD mice. Other paradigms, such as antidepressant treatment (Le Poul et al, 2000), have also yielded decreases in 5-HT1A responsiveness in the raphe, even though receptor levels are unchanged. These data suggest that, among the myriad effects of serotonin on neural circuit development, tuning of raphe neuron excitability and differences in 5-HT1A responsiveness may represent mechanisms by which early-life variation in 5-HT1A autoreceptor levels leads to long-lasting effects on behavior.
Complex Interplay between 5-HT1A and Early-Life Stress
Genetic and environmental factors act in concert to determine individual differences in predisposition to psychiatric illness, including anxiety disorders and depression. In particular, it is thought that inherent genetic variation may contribute to a person's reactivity to environmental factors (Caspi et al, 2003; Manolio et al, 2009). In order to better understand the interplay between variation in 5-HT1A autoreceptor levels and stress during development, we established a novel juvenile stress paradigm designed to coincide with receptor knockdown. Previously, a single study using handling, a form of environmental enrichment, as an early-life intervention in 5-HT1A knockout mice had shown that this intervention alleviated social deficits but not anxiety (Zanettini et al, 2010). We found that the effects of JS in 5-HT1A autoreceptor knockdown animals similarly impacted aspects of social behavior. Specifically, autoreceptor knockdown mice displayed decreased levels of social engagement, similar to the whole-life 5-HT1A knockout (Zanettini et al, 2010). This phenotype was exacerbated by exposure to JS, suggesting that stress exposure may compound the effects of genetic predisposition for this phenotype. Interestingly, however, KD mice exposed to JS retained normal social novelty preference, whereas their WT littermates failed to display this trait. Social novelty preference, or social recognition, is often considered a form of learning (Kogan et al, 2000), and this may suggest that lower post-natal levels of 5-HT1A are protective against the effects of stress for certain memory-related phenotypes. Future research examining other learning and memory phenotypes will focus on this question. These findings touch on the complex interplay between these genetic and non-genetic developmental factors and support the idea that specific symptom subsets, rather than disease diagnoses per se, may show stronger correlations with genetic and environmental variation.
In sum, this study directly demonstrates that the long-lasting effects of 5-HT1A autoreceptor variation on anxiety behaviors are developmentally mediated. Further, post-natal knockdown also results in long-term changes in raphe physiology. From a clinical perspective, these data support the idea that anxiety disorders may have developmental origins. Additional studies are warranted to understand the factors that contribute to normal developmental variation in 5-HT1A levels in humans. Specifically, we are currently investigating whether the C(-1019)G polymorphism in the promoter of the 5-HT1A gene impacts adult anxiety levels via its actions on 5-HT1A autoreceptor levels during development.
FUNDING AND DISCLOSURE
This work was funded by a grant from the Robert Wood Johnson Foundation (to ZRD), T32 MH015144 (to ZRD); NIH Grant DP2OD001674 (to FAC); NIMH (R01 MH088542), NARSAD, and HDRF (to RH); NIMH RC1MH089800 (to SGB and RH); NIMH R01MH075047 (to SGB); NIMH R01MH091427 (to EDL). Transgenic mice were provided by EDL. RH is a consultant for Lundbeck, Roche, and Servier. The authors declare no conflict of interest.
Acknowledgments
We thank Laurie Tomashaw, Brian Choi, Dr Lyngine Calizo and David Piel and Alex Jonokuchi for technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci. 2012;367:2402–2415. doi: 10.1098/rstb.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beck SG, Choi KC, List TJ. Comparison of 5-hydroxytryptamine1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther. 1992;263:350–359. [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. Postnatal handling reduces novelty-induced fear and increases [3H]flunitrazepam binding in rat brain. Eur J Pharmacol. 1987;144:105–107. doi: 10.1016/0014-2999(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Castane A, Semakova J, Santana N, Alvarado G, Cortes R, et al. New antidepressant strategy based on acute siRNA silencing of 5-HT1A autoreceptors. Mol Psychiatry. 2012;17:567. doi: 10.1038/mp.2012.52. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, Pan YZ, Lemos JC, Craige C, et al. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, Gross C. Mouse models of the 5-HTTLPR x stress risk factor for depression. Curr Top Behav Neurosci. 2012;12:59–72. doi: 10.1007/7854_2011_190. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG. Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J Neurophysiol. 2010;103:2652–2663. doi: 10.1152/jn.01132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG. Glutamatergic input is selectively increased in dorsal raphe subfield 5-HT neurons: role of morphology, topography and selective innervation. Eur J Neurosci. 2011;34:1794–1806. doi: 10.1111/j.1460-9568.2011.07882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev Neurosci. 2009;31:318–331. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, et al. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-m ethylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Linder N, Russig H, Thony B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hamon M, Lanfumey L, el Mestikawy S, Boni C, Miquel MC, Bolanos F, et al. The main features of central 5-HT1 receptors. Neuropsychopharmacology. 1990;3:349–360. [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann NY Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Perski A, Berglund H, Savic I. Chronic stress is linked to 5-HT(1A) receptor changes and functional disintegration of the limbic networks. Neuroimage. 2011;55:1178–1188. doi: 10.1016/j.neuroimage.2010.12.060. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Lisowski P, Sliwa AT, Swiergiel AH. Computer assisted video analysis of swimming performance in a forced swim test: simultaneous assessment of duration of immobility and swimming style in mice selected for high and low swim-stress induced analgesia. Physiol Behav. 2008;95:400–407. doi: 10.1016/j.physbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, Laporte AM, Laaris N, Chauveau J, et al. Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39:110–122. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology. 2009;56:692–701. doi: 10.1016/j.neuropharm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane C, Politis M. Buspirone: what is it all about. Brain Res. 2012;1461:111–118. doi: 10.1016/j.brainres.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, et al. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann NY Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer's disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 (Suppl 1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, et al. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebral Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting GA, Henion TR, Nugent JD, Caplan B, Tobet S. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J Neurosci. 2006;26:6834–6840. doi: 10.1523/JNEUROSCI.1728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, et al. Effects of early-life stress on serotonin(1A) receptors in juvenile Rhesus monkeys measured by positron emission tomography. Biol Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, Gross C, Richardson-Jones J, Zhuang X, Hen R. A novel conditional knockout strategy applied to serotonin receptors. Handbook Exp Pharmacol. 2007;178:347–363. doi: 10.1007/978-3-540-35109-2_14. [DOI] [PubMed] [Google Scholar]
- Tanaka KF, Ahmari SE, Leonardo ED, Richardson-Jones JW, Budreck EC, Scheiffele P, et al. Flexible Accelerated STOP Tetracycline Operator-knockin (FAST): a versatile and efficient new gene modulating system. Biol Psychiatry. 2010;67:770–773. doi: 10.1016/j.biopsych.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers CH, Oosting RS, van Bogaert MJ, Olivier B, Groenink L. Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biol Psychiatry. 2010;67:309–316. doi: 10.1016/j.biopsych.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Res. 2009;170:245–251. doi: 10.1016/j.psychres.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011;Chapter 8:Unit 8 26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Davies CL, Bennett-Clarke CA, Lane RD, Rhoades RW. Selective facilitation of the serotonin(1B) receptor causes disorganization of thalamic afferents and barrels in somatosensory cortex of rat. J Comp Neurol. 2000;425:130–138. doi: 10.1002/1096-9861(20000911)425:1<130::aid-cne11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Carola V, Lo Iacono L, Moles A, Gross C, D'Amato FR. Postnatal handling reverses social anxiety in serotonin receptor 1A knockout mice. Genes Brain Behav. 2010;9:26–32. doi: 10.1111/j.1601-183X.2009.00531.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.