Most images and cartoons of cell-matrix or cell-cell adhesion show the plasma membrane of the cell connected to its binding partner by the nanomolar-scale extracellular domain of a transmembrane protein extending from a lipid bilayer to its equally small ligand in the adjacent cell or extracellular matrix. Cell adhesion, however, differs fundamentally from adhesion between liposomes or bubbles, even when mediated by the same adhesion proteins. Between the outermost edge of the cell membrane and that part of the extracellular matrix nearest to the cell lies a part of the cell that is largely overlooked: the pericellular matrix (PCM) or glycocalyx. The PCM was initially detected in electron microscopy studies of polysaccharide distribution as a fuzzy coat that covered, for example, the surface of cells lining the capillary lumen (Fig. 1), or the tips of microvilli in intestinal epithelia (1). This glycocalyx could extend several microns from the cell surface. Most light microscopy methods however, do not detect the PCM, and despite its robust appearance in electron micrographs, the PCM is generally too delicate to study by most rheology methods. The difficulties of studying the PCM in its native state have left the question of its potential mechanical role in cell function unresolved. In this issue of Biophysical Journal, McLane et al. (2) make an important advance in defining the physical properties of the PCM by exploiting the versatility and precision of optical traps to measure the resistance of the PCM to penetration by micron-sized probes.
Figure 1.
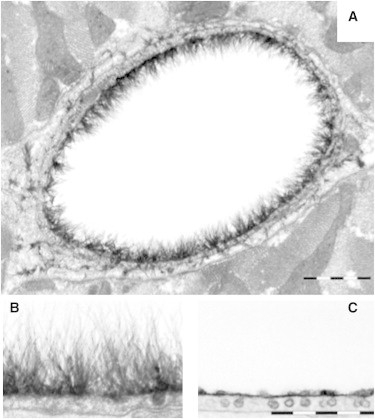
(A) Electron microscope cross-section image of an Alcian blue-stained rat myocardial capillary (bar = 1 μm). (B) Side-view image of the endothelial glycocalyx before and (C) after treatment with hyaluronidase (bar = 0.5 μm). Adapted from van den Berg et al. (19).
The PCM is composed primarily of hyaluronic acid (HA), a flexible highly anionic linear polymer of disaccharide repeats that also forms the gel within the vitreous body of the eyeball. Existing in detachment from the cell, it is a major constituent of many extracellular matrices. HA chains are produced by HA synthases on the cell membrane which project the long molecules into the extracellular space (3). HA can remain attached to HA synthase or to specific HA receptors on the cell membrane such as CD44 (4), RHAMM (5), and layilin (6). Other components of the glycocalyx are sulfated proteoglycans such as chondroitin sulfate, heparan sulfate, neurocan, and versican. These proteoglycans have been proposed to be either cross-linkers between protruding HA chains (suggesting that they form a true three-dimensional viscoelastic matrix), or stabilizers of the flexible HA chains that stiffen them and extend the length to which the PCM protrudes. The results of McLane and colleagues argue against the formation of a cross-linked gel, at least for the thick pericellular matrices around chondrocytes.
The abundance of extracellular polysaccharides in mammalian tissues was first shown with histological techniques using the periodic-acid Schiff stain (7). This technique revealed a thick polysaccharide layer in several tissues (8). That this layer was—at least partly—an inherent part of the cells was proposed in the 1960s, when several researchers noticed that a significant fraction of polysaccharides, especially HA, produced by fibroblast and lymphocytes in the synovial membrane of a joint could only be recovered in culture media after trypsin treatment (9), which suggested that the HA produced by the synovial cells remained “fairly firmly attached to the cells”. (10).
A possible function of the mammalian PCM or glycocalyx was suggested by studies of the interaction between synovial cells and lymphocytes that revealed “an optically clear zone beyond the cytoplasm of the synovial cells which appeared to prevent contact between the two types of cells” (11). This exclusion zone disappeared upon treatment with hyaluronidase, suggesting that the basal structure of the glycocalyx was formed by HA. A similar effect occurs in the vasculature and is especially evident in capillaries, where the luminal volume accessible to blood cells and large solutes is significantly smaller than the volume accessible to small solutes such as fluorescent dyes, because the cells and large particles cannot penetrate into the space filled by the endothelial PCM (12,13). These findings led to the now classic particle exclusion assay to visualize the PCM by using fixed erythrocytes added to an in vitro cell culture and observing that the erythrocytes did not make direct contact with the cultured cell membrane but left “a clear area which was sometimes more than 20 μm across…giving the impression that they were held back by a barrier of appreciable depth” (11). McLane and colleagues also use this method to visualize the dimensions of the PCM but extend it by using particles of different sizes to determine the mesh size of the PCM and the extent to which it can sieve particles of different size.
The presence of HA in extracellular matrix and other gel-like biomaterials suggested that the pericellular matrix could be a gel-like layer around the cell membrane, but direct rheological evidence for the elastic character of the PCM has been lacking. AFM and optical trap-based microrheology methods have been used to determine the mechanical properties of reconstituted gels with common glycocalyx constituents (14) as well as probing the glycocalyx directly (15–17). These studies revealed the PCM to be so soft as to test the limits of even these sensitive techniques. The technical advance of McLane et al. is to use a very sensitive optical trap in a dynamic mode in which a 3-μm passivated bead is pushed at a constant rate to various depths within the PCM. This study was performed using cultured chondrocytes, which are a cell type with an unusually large PCM that particle exclusion assays show to be 7 μm, and when aggrecan is added to extend the HA chain, as large as 17 μm. Because the trap moves at rates between 2 μm/s and 50 μm/s and the sampling rate is 500 Hz, changes in resistance as the probe moves into the PCM can be measured with high precision. These measurements reveal some surprising features of the PCM. The dynamic probe can detect the PCM even at distances beyond where the particle exclusion method reports it to be, suggesting that at least some chains of the PCM extend farther away from the cell than previously thought. If the probe movement is abruptly stopped before the bead reaches the cell membrane, some of the stress rapidly decays but a significant fraction remains, as an apparently elastic resistance to the bead that expels it from the PCM if the trap is turned off. The authors calculate that this elastic resistance, which increases exponentially as the bead comes nearer to the cell, arises not from the mechanical deformation of the HA chains, but from an osmotic pressure gradient caused by the increasing density of the tethered HA chains and their counterions near the cell surface. The increased chain density, or equivalently, the decreased mesh size as a function of distance from the surface, is confirmed by measurements of the extent to which particles of different sizes can approach the cell surface.
One of the most striking results illustrating the soft, highly dynamic, but not gel-like property of the PCM makes use of holography to produce two optical traps that manipulate two beads in sequence. The two optical traps are moved sequentially along the same path through the pericellular matrix. The resulting force measurements show that both beads have the same force distribution along their paths and that the first bead does not feel the approach of the second bead when they are both within the PCM. These results strongly suggest that the PCM is not a viscoelastic gel and the HA is not strongly cross-linked. Although McLane et al. do not directly calculate elastic and viscous moduli from their measurements, another recent study using a different form of optical microrheology reports that the shear storage modulus of the PCM is <1 Pa, strongly time-dependent, and similar in magnitude to its loss modulus (17), consistent with the results of McLane et al. To put this value in context, the mesh size of the PCM near the cell surface found by McLane et al. is <100 nm, even smaller than the mesh of the cortical actin network on the other side of the membrane. But the elastic modulus of the cell cortex is in the kPa range, consistent with expectations from theories for cross-linked polymer networks with this density. This elastic modulus is more than a thousand times greater than that of the PCM, further evidence that the HA chains in the PCM are not cross-linked into a three-dimensional gel.
McLane et al. conclude that the glycocalyx is not a true three-dimensional solid-like matrix that can be broken when deformed too much, but more like a bed of grass at the bottom of a lake. In the absence of hydrodynamic flow, the polymers of the glycocalyx extend from the surface with a roughly exponential density distribution, but they bend easily under hydrodynamic stress, a response that alters the polymer and charge density profile and might affect signaling. At the base of the PCM lie adhesion and other receptors that await stimulation by those ligands that manage to penetrate the mesh above. Whether this model applies generally to the PCM of other cell types remains to be seen, and some previous studies show significant differences in the mechanical stability of PCMs from different cell types (18).
One aspect of the PCM that McLane et al. leaves to future studies is the electrostatic effect of the PCM. HA, the major constituent of the PCM, has a similar negative charge density as DNA, and its structure will be altered by multivalent counterions or coions that penetrate its surface. The probe particles that McLane et al. use are uncharged polyethylene glycol-coated beads, but the native solutes and particles designed for drug delivery or transfection are generally charged and often cationic. The combination of viscoelastic properties, size-exclusion, and electrostatic repulsion or attraction generated by the PCM that are now beginning to be revealed will likely motivate more study and better understanding of this ubiquitous and often ignored structure.
Acknowledgments
This work was supported by grants from the National Institutes of Health (grant No. DK083592, to P.A.J.), the IIE Fulbright Program (to A.S.v.O.), and the Kuitse Fund-Prins Bernhard Culture Fund (to A.S.v.O.).
References
- 1.Ito S. Structure and function of the glycocalyx. Fed. Proc. 1969;28:12–25. [PubMed] [Google Scholar]
- 2.McLane L.T. Ultrastructure of the pericellular matrix on living cells. Biophys. J. 2013;104:986–996. doi: 10.1016/j.bpj.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weigel P.H., Hascall V.C., Tammi M. Hyaluronan synthases. J. Biol. Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 4.Aruffo A., Stamenkovic I., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 5.Turley E.A., Torrance J. Localization of hyaluronate and hyaluronate-binding protein on motile and non-motile fibroblasts. Exp. Cell Res. 1985;161:17–28. doi: 10.1016/0014-4827(85)90486-0. [DOI] [PubMed] [Google Scholar]
- 6.Bono P., Rubin K., Hynes R.O. Layilin, a novel integral membrane protein, is a hyaluronan receptor. Mol. Biol. Cell. 2001;12:891–900. doi: 10.1091/mbc.12.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McManus J.F.A. Histological and histochemical uses of periodic acid. Stain Technol. 1948;23:99–108. doi: 10.3109/10520294809106232. [DOI] [PubMed] [Google Scholar]
- 8.Leblond C.P. Distribution of periodic acid-reactive carbohydrates in the adult rat. Am. J. Anat. 1950;86:1–49. doi: 10.1002/aja.1000860102. [DOI] [PubMed] [Google Scholar]
- 9.Morris C.C., Godman G.C. Production of acid mucopolysaccharides by fibroblasts in cell cultures. Nature. 1960;188:407–409. doi: 10.1038/188407a0. [DOI] [PubMed] [Google Scholar]
- 10.Daniel M.R., Dingle J.T., Lucy J.A. Cobalt-tolerance and mucopolysaccharide production in rat dermal fibroblasts in culture. Exp. Cell Res. 1961;24:88–105. doi: 10.1016/0014-4827(61)90250-6. [DOI] [PubMed] [Google Scholar]
- 11.Clarris B.J., Fraser J.R.E. On the pericellular zone of some mammalian cells in vitro. Exp. Cell Res. 1968;49:181–193. doi: 10.1016/0014-4827(68)90530-2. [DOI] [PubMed] [Google Scholar]
- 12.Henry C.B.S., Duling B.R. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am. J. Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 13.van Haaren P.M.A., van Bavel E., Spaan J.A. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2848–H2856. doi: 10.1152/ajpheart.00117.2003. [DOI] [PubMed] [Google Scholar]
- 14.Nijenhuis N., Mizuno D., Spaan J.A. Microrheology of hyaluronan solutions: implications for the endothelial glycocalyx. Biomacromolecules. 2008;9:2390–2398. doi: 10.1021/bm800381z. [DOI] [PubMed] [Google Scholar]
- 15.Boehm H., Mundinger T.A., Curtis J.E. Mapping the mechanics and macromolecular organization of hyaluronan-rich cell coats. Soft Matter. 2009;5:4331–4337. [Google Scholar]
- 16.Iyer S., Gaikwad R.M., Sokolov I. Atomic force microscopy detects differences in the surface brush of normal and cancerous cells. Nat. Nanotechnol. 2009;4:389–393. doi: 10.1038/nnano.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijenhuis N., Mizuno D., Schmidt C.F. High-resolution microrheology in the pericellular matrix of prostate cancer cells. J. R. Soc. Interface. 2012;9:1733–1744. doi: 10.1098/rsif.2011.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen M., Klein E., Addadi L. Organization and adhesive properties of the hyaluronan pericellular coat of chondrocytes and epithelial cells. Biophys. J. 2003;85:1996–2005. doi: 10.1016/S0006-3495(03)74627-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg B.M., Vink H., Spaan J.A.E. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]


