Abstract
Background
IDH1 gene mutations identify gliomas with a distinct molecular evolutionary origin. We sought to determine the impact of surgical resection on survival after controlling for IDH1 status in malignant astrocytomas—World Health Organization grade III anaplastic astrocytomas and grade IV glioblastoma.
Methods
Clinical parameters including volumetric assessment of preoperative and postoperative MRI were recorded prospectively on 335 malignant astrocytoma patients: n = 128 anaplastic astrocytomas and n = 207 glioblastoma. IDH1 status was assessed by sequencing and immunohistochemistry.
Results
IDH1 mutation was independently associated with complete resection of enhancing disease (93% complete resections among mutants vs 67% among wild-type, P < .001), indicating IDH1 mutant gliomas were more amenable to resection. The impact of residual tumor on survival differed between IDH1 wild-type and mutant tumors. Complete resection of enhancing disease among IDH1 wild-type tumors was associated with a median survival of 19.6 months versus 10.7 months for incomplete resection; however, no survival benefit was observed in association with further resection of nonenhancing disease (minimization of total tumor volume). In contrast, IDH1 mutants displayed an additional survival benefit associated with maximal resection of total tumor volume (median survival 9.75 y for >5 cc residual vs not reached for <5 cc, P = .025).
Conclusions
The survival benefit associated with surgical resection differs based on IDH1 genotype in malignant astrocytic gliomas. Therapeutic benefit from maximal surgical resection, including both enhancing and nonenhancing tumor, may contribute to the better prognosis observed in the IDH1 mutant subgroup. Thus, individualized surgical strategies for malignant astrocytoma may be considered based on IDH1 status.
Keywords: anaplastic astrocytoma, IDH1, glioblastoma, malignant glioma, surgical resection
Malignant astrocytomas—World Health Organization (WHO) grade III anaplastic astrocytomas (AAs) and WHO grade IV glioblastoma (GBM)—are aggressive primary brain tumors.1 They show substantial variability in clinical course, with some patients succumbing to progressive disease within weeks, while others can survive for a decade or more. AAs are histologically distinct from glioblastomas, lacking the characteristic endothelial microvascular proliferation or pallisading necrosis found in glioblastomas.1 Precise grading of malignant astrocytomas is difficult and can be highly dependent upon the volume of tissue provided for pathology review, with stereotactic biopsy associated with a substantial frequency of undergrading,2,3 and interobserver agreement can remain elusive even when sufficient tissue is available.4
Malignant astrocytomas are further distinct from gliomas within the oligodendroglial lineage, which are characterized by loss of chromosomes 1p and 19q, CIC (capicua transcriptional repressor) mutations,5–7 and more indolent growth and chemosensitivity.8–11 The mainstay of treatment for patients with malignant astrocytoma is based on 3 modalities: surgical resection, radiation therapy, and chemotherapy (most typically with the oral alkylating agent temozolomide). Patients with glioblastoma derive a survival benefit when treated with concomitant chemoradiation therapy12; however, this benefit has not been demonstrated for AAs.13 The optimal adjuvant treatment of AAs is currently the focus of the international “CATNON” EORTC 26053-22054/RTOG-0834 study.
Greater surgical resection of enhancing disease is associated with improved survival in both GBM and AA patients in retrospective analyses.14–18 Gliomas typically have a penumbra of nonenhancing disease (detectable on T1 noncontrast and T2/fluid attenuated inversion recovery [FLAIR] MRI sequences), and up to 30% of grade III and 10% of grade IV tumors have no contrast enhancement evident on presentation. However, minimization of postoperative nonenhancing disease volume has not been reported to be associated with improved survival in glioblastomas or AAs.17 This lack of evident surgical benefit stands in stark contrast to the clear evidence of improved survival in association with greater resection of nonenhancing disease found in low-grade (WHO grade II) gliomas.19 The evaluation of the “completeness” of surgical resection may therefore be dependent upon the initial histologic and radiographic appearance of a given lesion and raises the question of whether aggressive resection of the nonenhancing disease “infiltrated margin” may improve survival for a subset of malignant astrocytomas. Due to the highly individualized nature of surgery, the association of various measures of surgical resection (residual enhancing or nonenhancing volume) with survival may be further impacted by other prognostic factors, such as age, KPS score, and tumor location in relation to critical functional regions of the brain.15,20
Recently, isocitrate dehydrogenase (IDH) gene mutations were discovered in a subset of GBM (∼5%–10%) and AAs (∼50%–70%).21–26 The vast majority (∼90%) of IDH mutations in malignant astrocytomas are recurrent arginine to histidine heterozygous mutations in codon 132 of the IDH1 gene (R132H), with only a small fraction of noncanonical mutations found in IDH1 and the related family member IDH2, which are more typically associated with oligodendroglial histology.27 Immunohistochemical scoring of R132H mutations in glioma has been reported to have 100% interobserver agreement,28 unlike histologic grading, representing an opportunity to reduce diagnostic variability in the study of anaplastic gliomas.
IDH gene mutations identify tumors with markedly different clinical presentations, concurrent molecular genetic alterations, and overall natural history. It therefore has been proposed that IDH status can be used as a classifier of different molecular etiologic subtypes of glioma.29–31 Patients with IDH1 mutant astrocytomas have a better overall prognosis compared to wild-type IDH astrocytomas, even after controlling for histologic grade. However, whether this better prognosis is due primarily to an improved intrinsic natural history or response to therapy (or both) is not known. Examinations of response to radiation or chemotherapy in randomized trials of malignant astrocytomas have not found a therapeutic interaction between adjuvant treatment and IDH genotype.32–34 Studies of IDH and adjuvant chemotherapy and radiation treatment in lower-grade tumors have yielded conflicting results.35,36 In the assessment of surgical resection, studies may be biased due to their retrospective design; should IDH1 mutant tumors prove to be more “resectable,” they will be enriched in the “complete resection group” assignment of a post-hoc binning schema, and confound the isolated assessment of the relationship between surgical resection and outcome. The role of surgery in relation to IDH genotype has not been well studied.
Given their distinct molecular origins, we hypothesized that the optimal surgical strategies for IDH1 mutant versus wild-type malignant astrocytomas may be different. We therefore sought to understand the contribution of surgical resection to survival in patients stratified by IDH1 mutation status. By performing a detailed study of these clinical and molecular factors in well-defined patient populations, we aimed to determine the independent contribution of surgical resection to overall survival in each of these 2 groups of malignant astrocytomas.
Materials and Methods
Institutional Review Board Statement and Clinical Database
This study was conducted under an M.D. Anderson Cancer Center (MDACC) institutional review board–approved protocol, LAB09-0987. The MDACC Brain and Spine Center Database prospectively records treatment and survival data for all glioma patients. The database was queried for patients with a centrally reviewed diagnosis of supratentorial AA or GBM, whose first therapeutic intervention was an open surgical resection at our institution from June 1993 to April 2009, with paraffin tissue scored for IDH. To minimize histopathologic sampling issues, biopsy-only patients were excluded, unless they proceeded to debulking surgery and confirmed AA or GBM diagnosis within the subsequent 2 months, without intervening treatment. At our institution, surgical resection typically employs stereotactic navigation and other technical adjuncts, such as intraoperative MRI and motor mapping to maximize resection. Patients with a KPS score <50, documented 1p/19q allelic loss characteristic of oligodendroglial histology, or concomitant secondary malignancy were also excluded. These criteria identified 130 AAs and 213 cases of GBM, respectively.
Tumor Blocks, Immunohistochemistry, and DNA Sequencing
Formalin-fixed, paraffin-embedded sections were scored using immunohistochemistry with an R132H IDH1 mutation–specific antibody (clone H09, Dianova).37 Primers for PCR amplification of the IDH1 R132 mutation hotspot were: forward: 5′-CTCCTGATGAGAAGAGGGTTG-3′ and reverse: 5′-M13Forward-CACATTATTGCCAACATGAC-3′, and products were sequenced (Beckman Coulter Genomics). Tumors were categorized as IDH wild-type and mutant by multiple (>2) scoring runs of at least one method. Based on the low reported frequency of noncanonical IDH1 mutations and IDH2 mutations in malignant astrocytomas, these were not characterized due to tissue limitations.
Tumor Volume Measurements
MRI volume calculations were performed using Vitrea 2 three-dimensional volumetric software (Vital Images). Personnel scoring the tumor volumes were blinded to molecular stratification and patient survival. Imaging-based tumor volumes were calculable on 335 of 343 cases; these 335 cases constituted the final study population. Total tumor volume was calculated as equivalent to the T2/FLAIR volume, or the sum of enhancing and nonenhancing T1 volume in 41 cases where T2/FLAIR sequences were not available. Complete resection of enhancing disease was defined as no residual postoperative enhancement. In the classical volumetric surgical study of GBM,14 extent of resection has been calculated as a percentage of preoperative enhancing volume that is removed on volumetric calculations on the postoperative scan; our treatment of the presence of preoperative and postoperative contrast enhancement as factors separate from total tumor volume attempts to capture the core component of this variable while allowing for inclusion of nonenhancing lesions in the overall volumetric analysis. This consideration is particularly important given the inclusion of grade III tumors in the analysis, a significant fraction of which are nonenhancing lesions. Tumor functional grade was defined by relationship of the tumor to cortical eloquence and scored as eloquent, near-eloquent, and noneloquent according to prior published methods.38 Tumor location was scored as frontal, temporal, parietal, or other, according to the primary localization of the tumor. Multifocal tumors were assigned location based on the largest component of the tumor mass.
Statistical Analysis
Analyses were performed using Stata software v10.0 and the PASW 17–SPSS statistical package. The chi-square test and Fisher's exact test, as appropriate, were used to establish associations between categorical factors; the independent samples t-test was used for continuous factors, and the Mann–Whitney test for variables not normally distributed. Binary logistic regression was performed to determine factors associated with complete resection. Survival analyses were performed using the Kaplan–Meier method and the Cox proportional hazards regression method. Patients who were alive at the last known follow-up were considered censored in those analyses. Bivariate interaction terms were included in the Cox model where appropriate to assess whether the effects of a given variable varied with the levels of other variables. Smoothed martingale residual plots were used to assess the need for modeling continuous variables as categorical. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were obtained. All tests were 2-tailed. P ≤ .05 was considered significant.
Results
Descriptive Characteristics
Our study cohort consisted of 128 AA patients (52 females and 76 males) and 207 GBM patients (86 females and 121 males). The descriptive characteristics of our patient dataset are detailed by genotype in Table 1 (and by classical histology in Supplementary material, Table S1). IDH1 mutations were identified in 86 of 128 AAs (67%) and 27 of 207 GBM (13%). The baseline differences between mutant and wild-type tumors were similar to those previously reported.21,23,30 IDH1 mutant tumors were more commonly found in younger patients and were more commonly located in the frontal lobe (55% vs 40% for wild-type; P = .008). The prevalence and volume of preoperative contrast enhancement was significantly higher in wild-type IDH1 tumors. Where 87% of the wild-type tumors were enhancing, only 40% of the mutant lesions (P < .001) displayed enhancement. Wild-type enhancing tumors had a significantly greater median preoperative volume of enhancing disease (31.0 cm3 vs 11.1 cm3 in mutant enhancing tumors, P < .001). Wild-type tumors also had a greater volume of total (enhancing and nonenhancing) disease preoperatively, with a median of 75.3 cm3 versus 58.6 cm3, but this difference was not significant (P = .23).
Table 1.
Clinical characteristics of the study population by IDH1 status
| Factor |
IDH1 Status |
|||
|---|---|---|---|---|
| All Patients (N = 335) | Mutant (n = 113) | Wild-type (n = 222) | P | |
| Histology, n (%) | <.001 | |||
| Glioblastoma | 207 (62) | 27 (24) | 180 (81) | |
| Anaplastic astrocytoma | 128 (38) | 86 (76) | 42 (19) | |
| Age, y, median (range) | 50.3 (18.3–87.8) | 37.0 (18.3–79.0) | 57.0 (18.9–87.8) | <.001 |
| Preoperative KPS score, median (range) | 90 (60–100) | 90 (60–100) | 90 (60–100) | .022 |
| ≥80 | 299 (89) | 107 (95) | 192 (86) | |
| <80 | 36 (11) | 6 (5) | 30 (14) | |
| Location, n (%) | .004 | |||
| Frontal | 150 (45) | 62 (55) | 88 (40) | |
| Parietal | 41 (12) | 7 (6) | 34 (15) | |
| Temporal | 124 (37) | 34 (30) | 90 (40) | |
| Other supratentorial locations | 20 (6) | 10 (9) | 10 (5) | |
| Tumor functional grade, n (%)a | .012 | |||
| Eloquent | 142 (42) | 53 (47) | 89 (40) | |
| Near-eloquent | 160 (48) | 43 (38) | 117 (53) | |
| Noneloquent | 33 (10) | 17 (15) | 16 (7) | |
| Presence of preoperative enhancement, n (%) | 239 (71) | 45 (40) | 194 (87) | <.001 |
| Volume of preoperative enhancement, cc; median (range)b | 28.5 (0.1–164.8) | 11.1 (0.1–158.0) | 31.0 (0.4–164.8) | <.001 |
| Presence of postoperative enhancement, n (%) | 67 (20) | 3 (3) | 64 (29) | <.001 |
| Volume of postop enhancement, cc; median (range)c | 2.3 (0.4–44.8) | 28.3 (1.5–42.6)* | 2.3 (0.4–44.81)* | <.001 |
| Preoperative enhancing and nonenhancing volume, cc; median (range) | 70.8 (3.8–340.0) | 58.6 (8.3–340.0) | 75.3 (3.8–294.4) | .23 |
| Postoperative enhancing and nonenhancing volume, cc; median (range) | 24.0 (0.0–190.9) | 16.5 (0.0–190.9) | 29.4 (0.0–170.5) | .008 |
| Long-term survivor (>5 y; remaining cases; % surviving) | 59 (35%) | 40 (72%) | 19 (17%) | |
| Overall survival, mo; median (95% CI) | 26.8 (20.1–33.5) | 163.4 (93.3–233.5) | 16.2 (14.5–17.9) | <.001 |
| Follow-up in survivors, mo; median (range)d | 47.8 (0.2–207.7) | 47.9 (0.2–207.7) | 45.3 (0.5–186.9) | >.5 |
Bold indicates statistical significance at level of <.05.
*Note: comparison includes non-enhancing postop (ie, complete resections)
aBased on initial tumor location.
bAmong those with preop enhancement, 6 missing.
cAmong those with postop enhancement, 4 missing.
dAmong 123 patients.
Importantly, we noted a uniform consistency of objective clinical measures across the period of case accrual. The divided cohort of 2 groups based on cases accrued early versus late (168 cases on or prior to November 12, 2004 and 167 thereafter), the median age (49.3 vs 49.8 y), and KPS (90 vs 90) were indistinguishable between early-half versus late-half cases. Fifty-six of the first 168 cases collected scored mutant for IDH1, compared with 57 of 167 subsequent cases scoring mutant. Similarly, examination of radiographic measures revealed no significant differences in the frequency of preoperative or postoperative enhancement or volume of preoperative or postoperative enhancing disease between early-half versus later-half accrued cases. Tumors within the later-half of the series did have a larger measured median total preoperative volume (85.7 cm3 later-half versus 57.4 early-half cm3) and a larger measured median postoperative total tumor volume (28.4 cm3 later-half versus 16.9 cm3 early-half), but the median extent of resection of total volume was no different (66.9% later-half versus 70.6% early-half), but the median extent of resection of total volume was no different (66.9% vs 70.6%, respectively), and the median extent of resection of enhancing volume was 100% for both groups, indicating that the maximization of surgical resection was maintained throughout the study period.
Interestingly, there was a difference in histopathologic diagnoses between the 2 eras, with the histologic assignment of glioblastoma applied to 55.4% of the cases in the early cohort but in 68.3% of later-cohort cases. We speculate this discrepancy could arise from the known interaction between histopathologic diagnosis and volume of tissue provided to the neuropathologist for centrally reviewed diagnosis,2 since there was a paucity of objective demographic, radiographic, or tumor molecular alterations in tumors between the 2 eras, but there was a significant difference in resected tumor volume, as already noted. Notwithstanding this difference in histopathologic assignment, there was no significant difference in survival between the early (median 105 wk; 95% CI = 82.2–123.0 wk) and later (median 132 wk, 75.3–188.5 wk) cohorts, suggesting that the underlying disease process of malignant astrocytic glioma was uniform throughout the cohort.
Survival Analysis by Histology
Consistent with prior reports,14–18 we observed improved survival associated with complete resection of enhancing disease in both AAs (Supplementary material, Fig. S2) and GBM (Supplementary material, Fig. S3), although our dataset had only 3 AAs with postoperative enhancement, limiting our conclusions regarding this comparison. We found that minimization of total tumor volume (enhancing disease and nonenhancing disease) was not associated with improved survival in AA (Supplementary material, Fig. S4) or in GBM (Supplementary material, Fig. S5). There was, however, a notable difference in survival when comparing the AAs identified by Keles et al,17 a cohort of 102 patients with a median age of 49, where 67 patients had preoperative enhancement and the median survival was 163.8 weeks, with our AA cohort of 128 patients, median age 38, 38% enhancing, with a median survival of greater than 10 years. Since there can be an association between surgical resection and histologic diagnosis (“undersampling”) that can inappropriately undergrade GBM as AA,2 we sought to examine the underlying molecular features of these tumors as a potential explanation for this difference.
IDH1 Mutation and Complete Resection of Enhancing Disease
Cursory inspection of the baseline tumor characteristics demonstrated that of 45 IDH1 mutant malignant astrocytomas with preoperative enhancement, 42 (93%) underwent complete resection of enhancing disease (as evidenced by the absence of postoperative enhancement), a remarkably high frequency for this surgical result—indeed, all 24 mutant IDH1 glioblastomas underwent complete resection of enhancing disease, as did 18 of 21 AAs (86%; P = .1). The corresponding figure in the wild-type tumors was 130 (67%; P = .002). All 17 AAs (100%) and 113 (64%) of glioblastomas achieved complete resection.
The clinical factors associated with IDH1 mutant tumors (younger age, frontal location, nonenhancing disease component) may impact the ability to achieve a complete resection of enhancing disease. We therefore sought to analyze the molecular factors that were associated with the achievement of complete resection of enhancing disease in our cohort in univariate and multivariate models. Since not all tumors display enhancement preoperatively, we restricted this analysis to the subset of 239 lesions (mutant and wild-type) that displayed preoperative enhancement. In a univariate binary logistic regression analysis (Table 2), we found that age, KPS, histology (AA vs GBM), IDH1 mutation, and frontal tumor location were factors associated with the achievement of complete resection of enhancing disease. In the multivariate analysis including these factors, IDH1 mutation, tumor location, and KPS were found to be independent factors significantly associated with complete resection of enhancing disease.
Table 2.
Complete resection of enhancing disease—univariate and multivariate modeling of factors associated with survival among 239 patients with preoperative enhancement
| Factor | Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|---|
| Number With CR (%) | ORa | 95% CI | P | OR | 95% CI | P | |
| Age, yb | – | 1.03 | 1.01–1.06 | .002 | 1.01 | 0.99–1.04 | .39 |
| Preoperative KPSb | – | 0.96 | 0.93–0.99 | .006 | 0.97 | 0.94–1.00 | .053 |
| Tumor functional grade | |||||||
| Eloquent | 75 (73) | 1.00 | – | – | 1.00 | – | – |
| Near-eloquent | 81 (68) | 1.26 | 0.70–2.24 | .44 | 1.01 | 0.53–1.90 | .99 |
| Noneloquent | 16 (94) | 0.17 | 0.02–1.32 | .09 | 0.28 | 0.03–2.31 | .24 |
| Location | |||||||
| Frontal | 77 (84) | 1.00 | – | – | |||
| Parietal | 25 (66) | 2.67 | 1.12–6.36 | .03 | – | ||
| Temporal | 62 (63) | 3.06 | 1.54–6.09 | .001 | – | ||
| Other supratentorial locations | 8 (80) | 1.28 | 0.25–6.65 | .77 | – | ||
| Location | |||||||
| Frontal | 77 (84) | 1.00 | – | 0.41 | 0.20–0.82 | .01 | |
| Other supratentorial locations | 95 (65) | 0.36 | 0.19–0.68 | .002 | 1.00 | – | – |
| Histology | |||||||
| GBM | 137 (68) | 5.45 | 1.62–18.38 | .006 | 1.98 | 0.52–7.54 | .32 |
| Anaplastic astrocytoma | 35 (92) | 1.00 | – | – | 1.00 | – | – |
| IDH1 status | |||||||
| Mutant | 42 (93) | 0.14 | 0.04–0.49 | .002 | 0.24 | 0.07–0.90 | .03 |
| Wild-type | 130 (67) | 1.00 | – | – | 1.00 | – | – |
Bold indicates statistical significance at level of <.05.
Abbreviations: OR, odds ratio; CR, complete resection.
aAn OR <1 means higher chance of achieving complete resection. An OR >1 means lower chance of achieving complete resection; an OR = 1 means equal chance of achieving complete resection (bOR expressed per unit increase for these 2 factors).
Similar linear regression analysis was performed to identify factors associated with total residual tumor volume (inclusive of enhancing and nonenhancing disease). Not unexpectedly, the factor most predictive of postoperative tumor volume was preoperative tumor volume.
Surgical Resection Measures in Wild-type IDH Malignant Astrocytomas
We analyzed the parameters associated with prolonged survival in IDH wild-type malignant astrocytomas (42 AAs and 180 GBM). This cohort had a median overall survival after surgical resection of 16.2 months (95% CI = 14.5–17.9 mo). Patients remaining alive at the end of the follow-up period were followed for a median of 45.3 months (range, 0.5–186.9 mo).
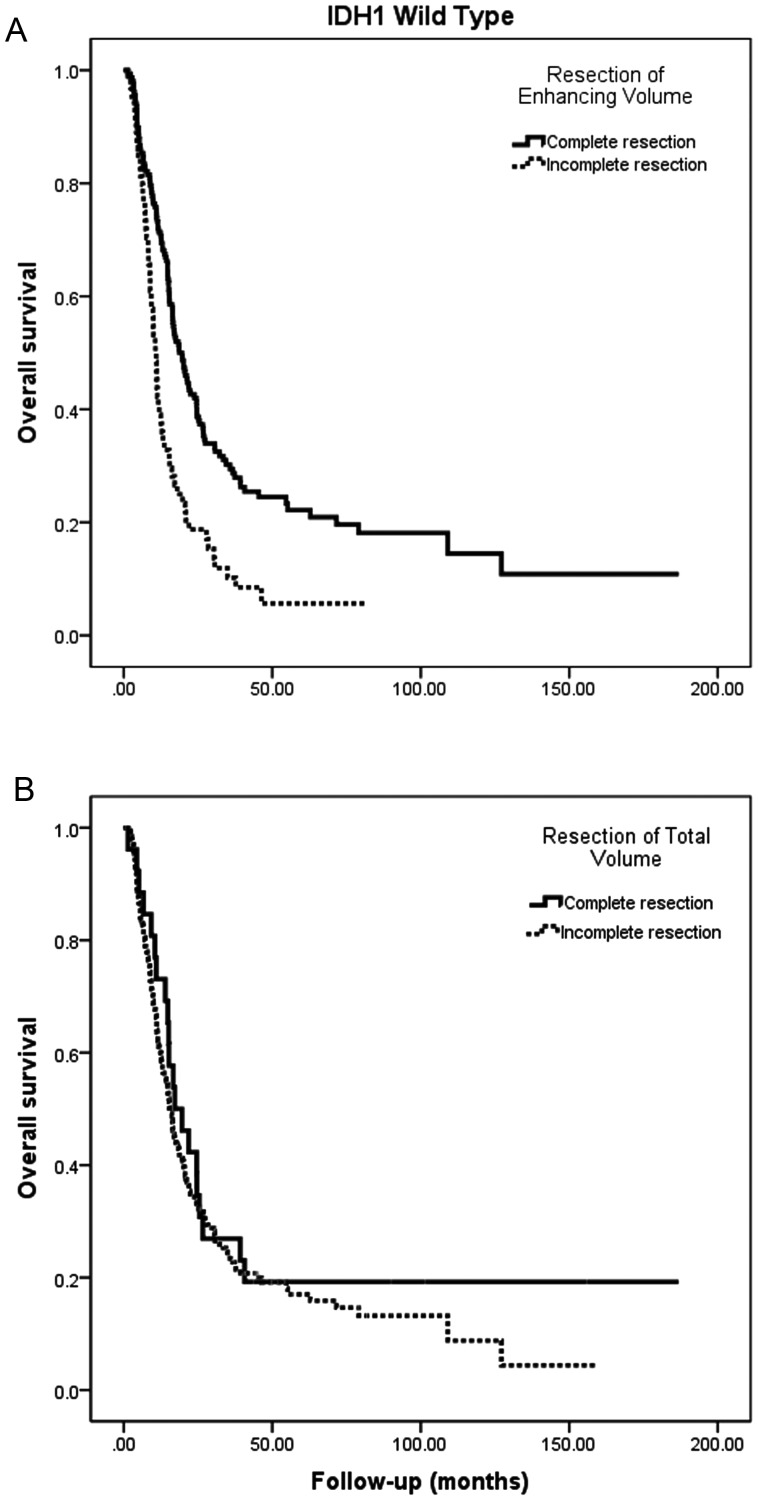
In univariate modeling of survival in this subgroup with wild-type IDH status, age, KPS, histologic grade (AA vs GBM), and tumor location, the presence of preoperative and postoperative enhancement and the overall postoperative tumor volume (enhancing and nonenhancing) were each associated with survival (Table 3). Complete resection of enhancing disease was associated with a median survival of 19.6 months versus 10.7 months for incomplete resection (HR = 1.96 for incomplete resection; 95% CI = 1.42–2.68; P < .001; Fig. 1A).
Table 3.
Wild-type IDH1 malignant astrocytoma—univariate and multivariate modeling of overall survival
| Factor | Univariate |
Multivariate |
Cox Stepwise |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | N Event | Median | 95% CI | HR | 95% CI | P | HR | 95% CI | P | |
| Age, y | 1.04 | 1.03–1.05 | <.001 | 1.03 | 1.02–1.04 | <.001 | 1.03 | 1.02–1.04 | <.001 | |||
| Preoperative KPS | 0.97 | 0.96–0.98 | <.001 | 0.98 | 0.97–1.00 | .06 | 0.98 | 0.97–0.99 | .04 | |||
| Tumor functional grade | ||||||||||||
| Noneloquent | 1.00 | – | – | 16/10 | 20.6 | 13.3–27.9 | 1.00 | – | – | |||
| Near-eloquent | 2.01 | 1.05–3.86 | .04 | 117/100 | 15.1 | 12.3–17.9 | 1.25 | 0.62–2.53 | .53 | |||
| Eloquent | 1.55 | 0.80–3.02 | .19 | 89/70 | 16.3 | 10.7–21.8 | 1.19 | 0.59–2.40 | .62 | |||
| Tumor location | ||||||||||||
| Frontal | 1.00 | – | – | 88/62 | 20.5 | 15.4–25.6 | 1.00 | – | – | |||
| Parietal | 1.52 | 0.98–2.36 | .06 | 34/30 | 15.1 | 12.7–17.5 | 0.91 | 0.57–1.45 | .69 | |||
| Temporal | 1.55 | 1.11–2.16 | .01 | 90/79 | 13.2 | 9.3–17.1 | 1.15 | 0.79–1.67 | .46 | |||
| Other supratentorial | 1.68 | 0.83–3.38 | .15 | 10/9 | 15.1 | 4.8–25.5 | 1.50 | 0.71–3.16 | .28 | |||
| Preop enhancement | ||||||||||||
| No | 1.00 | – | – | 28/12 | 127.2 | 7.4–246.9 | 1.00 | – | – | 1.00 | – | - |
| Yes | 3.16 | 1.76–5.70 | <.001 | 194/168 | 14.9 | 12.6–17.1 | 1.77 | 0.83–3.80 | .14 | 2.10 | 1.14–3.86 | .02 |
| Postop enhancement | ||||||||||||
| No | 1.00 | – | – | 158/121 | 19.6 | 16.0–23.1 | 1.00 | – | – | 1.00 | – | - |
| Yes | 1.96 | 1.42–2.68 | <.001 | 64/59 | 10.7 | 9.3–12.1 | 1.24 | 0.87–1.76 | .23 | 1.33 | 0.96–1.85 | .09 |
| Preoperative volume–enhancing and nonenhancing, cc | 1.01 | 1.00–1.00 | .16 | 1.00 | 0.99–1.01 | .46 | ||||||
| Postoperative volume–enhancing and nonenhancing, cc | 1.01 | 1.01–1.01 | .021 | 1.01 | 0.99–1.02 | .39 | ||||||
| Extent of resection based on total postoperative volume | 1.00 | 0.99–1.01 | .10 | 1.01 | 0.99–1.01 | .50 | ||||||
| Histology | ||||||||||||
| Anaplastic astrocytoma | 1.00 | – | – | 42/24 | 1.00 | – | – | |||||
| Glioblastoma | 2.68 | 1.72–4.19 | <.001 | 180/156 | 1.32 | 0.72–2.41 | .37 | |||||
Bold indicates statistical significance at level of <.05.
Abbreviation: NR, not reached.
Multivariate cohorts are based on cases with data on all variables (n = 222).
Fig. 1.
Kaplan–Meier estimates of IDH1 wild-type malignant astrocytoma overall survival according to (A) presence or absence of postoperative enhancing disease and (B) presence or absence of postoperative total disease volume (enhancing and nonenhancing). IDH1 wild-type malignant astrocytomas (AA and GBM) display an improved survival in association with resection of enhancing disease, but not minimization of overall tumor volume (enhancing and nonenhancing).
In the multivariate model, age (HR = 1.03, P < .001) and KPS (HR = 0.98, P = .06) remained prognostic at P < .1. A stepwise backward conditional Cox proportional hazards model identified 4 factors associated with overall outcome at P < .1: age, KPS, presence of preoperative enhancement, and resection of enhancing tumor (Table 3).
The association of postoperative residual contrast enhancement with survival remained significant in the subset of patients (n = 90; 27 with missing data) who received combined chemoradiation, in both univariate and multivariate analyses, with complete resection associated with a near-doubling of the median survival estimate to 22.4 months (95% CI = 15.7–29.1 mo) compared with that seen with incomplete resection (13.2 mo; 95% CI = 8.5–18.0 mo). Notably, in this multivariate analysis, postoperative residual tumor volume (scored as continuous volumetric cubic centimeters) was not significantly associated with survival for wild-type IDH1 tumors; for comparison with the previously noted univariate association of contrast enhancing disease, Fig. 1B demonstrates that complete resection of enhancing and nonenhancing volume was not associated with prolonged survival (median, 17.2 mo; 95% CI = 8.8–25.6 vs 15.5 mo [95% CI = 13.2–17.8 among patients with incomplete resection; P = .38]).
Surgical Resection Measures in Mutant IDH Malignant Astrocytomas
Next, we analyzed the parameters associated with improved overall survival in IDH mutant tumors (27 GBM and 86 AAs). Patients remaining alive at the end of the follow-up period were followed for a median of 47.9 months (range, 0.2–207.7 mo). This cohort had an estimated median overall survival after surgical resection exceeding the median follow-up period, at 163.4 months (95% CI = 93.3–233.5 mo). Since only 3 IDH1 mutant cases (all AAs) had postoperative enhancement, and therefore conclusions about the association of postoperative enhancement and survival would be limited, we did not include this variable in our analyses. These patients experienced poor survival indistinguishable from their wild-type counterparts, as might be expected. Importantly, exclusion of these patients did not meaningfully impact the findings regarding the association between overall postoperative tumor volume and survival in the remainder of the cohort.
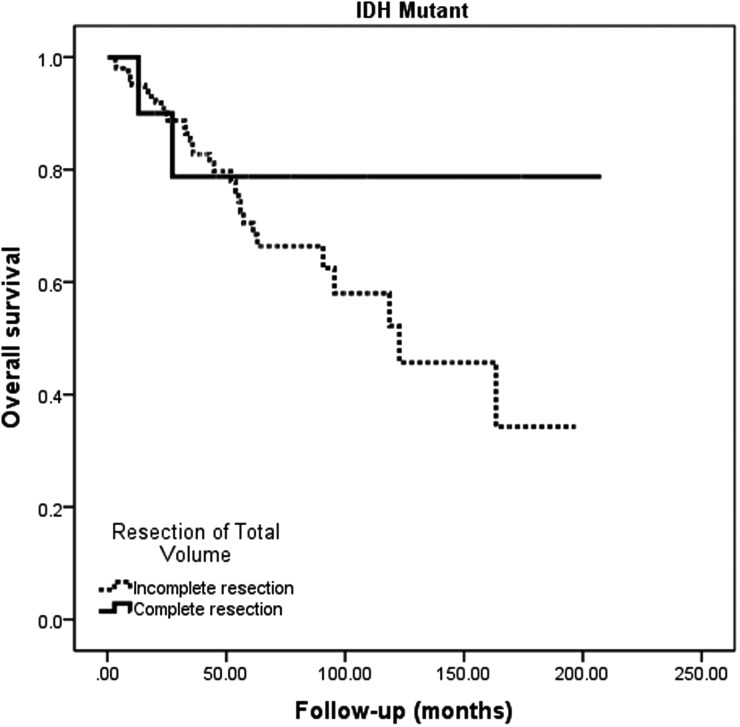
In univariate modeling, we found that age, KPS, histology (GBM vs AA), tumor location, the presence of preoperative enhancement, the volume of preoperative and postoperative disease, and the extent of resection of enhancing and nonenhancing disease were significantly associated with survival. After controlling for these variables in multivariate models, age, postoperative volumes (expressed as continuous variables in cubic centimeters), and histology (GBM vs AA) were significantly associated with overall survival (Table 4). The strong association between postoperative residual tumor volume and survival (Fig. 2) is in notable contrast to the findings in wild-type malignant astrocytomas.
Table 4.
Univariate and multivariate modeling of overall survival—mutant IDH1 malignant astrocytoma
| Factor | Univariate |
Multivariate |
Cox Stepwise |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | N Event | Median | 95% CI | HR | 95% CI | P | HR | 95% CI | P | |
| Age, y | 1.04 | 1.01–1.08 | .04 | 1.05 | 1.01–1.09 | .03 | 1.04 | 1.01–1.08 | .02 | |||
| Preoperative KPS | 0.95 | 0.91–0.99 | .03 | 0.96 | 0.92–1.02 | .17 | ||||||
| Tumor functional grade | ||||||||||||
| Noneloquent | 1.00 | 0.99–12.42 | – | 17/6 | 61.2 | ND | 1.00 | – | – | |||
| Near-eloquent | 0.63 | 0.23–1.74 | .37 | 41/10 | NR | – | 0.44 | 0.13–1.47 | .18 | |||
| Eloquent | 0.77 | 0.29–2.06 | .60 | 52/13 | 118.7 | 70.4–166.9 | 0.55 | 0.18–1.66 | .29 | |||
| Tumor location | ||||||||||||
| Frontal | 1.00 | – | – | 60/14 | NR | – | 1.00 | – | – | |||
| Parietal | 3.51 | 0.99–12.42 | .05 | 6/3 | 34.9 | ND | 4.09 | 0.93–17.92 | .06 | |||
| Temporal | 2.07 | 0.92–4.63 | .08 | 34/11 | 95.5 | 53.0–138.0 | 1.46 | 0.55–3.91 | .45 | |||
| Other supratentorial locations | 1.25 | 0.16–9.82 | .83 | 10/1 | NR | – | 1.40 | 0.15–13.37 | .77 | |||
| Preop enhancement | ||||||||||||
| No | 1.00 | – | – | 68/14 | NR | – | 1.00 | – | – | |||
| Yes | 2.23 | 1.06–4.70 | .035 | 42/15 | 118.7 | 72.6–164.7 | 0.69 | 0.23–2.04 | .50 | |||
| Preoperative volume–enhancing and nonenhancing, cc | 1.01 | 1.00–1.01 | .054 | 0.98 | 0.97–1.00 | .04 | 0.98 | 0.97–0.99 | .01 | |||
| Postoperative volume–enhancing and nonenhancing, cc | 1.01 | 1.01–1.02 | .001 | 1.04 | 1.01–1.08 | .006 | 1.04 | 1.02–1.06 | <.001 | |||
| Extent of resection based on total postoperative volume | 0.99 | 0.98–0.99 | .03 | 1.00 | 0.98–1.03 | .78 | ||||||
| Histology | ||||||||||||
| Anaplastic astrocytoma | 1.00 | – | – | 83/15 | NR | – | 1.00 | – | – | 1.00 | – | – |
| Glioblastoma | 3.67 | 1.74–7.74 | .001 | 27/14 | 63.1 | 18.6–107.6 | 6.36 | 1.95–20.77 | .002 | 5.30 | 2.15–13.06 | <.001 |
Bold indicates statistical significance at level of <.05.
Abbreviation: NR, not reached.
Multivariate cohorts are based on cases with data on all variables.
Fig. 2.
Kaplan–Meier estimates of IDH1 mutant malignant astrocytoma overall survival according to presence or absence of postoperative total disease volume (enhancing and nonenhancing). IDH1 mutant malignant astrocytomas (AA and GBM) display an improved survival in association with minimization of overall tumor volume (enhancing and nonenhancing). Importantly, no “cutoff” value was apparent within the continuous volumetric data.
Discussion
Here we report the relationship between IDH1 mutation status and the benefit of surgical resection in malignant astrocytic gliomas. We report for the first time that IDH1 mutation is an independent predictor of complete resection of enhancing disease in malignant astrocytomas. This relationship between IDH1 mutation and resection serves as a potential explanation for the yet unexplained association of younger age and tumor eloquent location with complete resection of enhancement seen in the recent randomized trial of 5-aminolevulinic acid.15 IDH1 mutation frequency decreases significantly after age 40 (a cutpoint significant by martingale residuals analysis). Our findings raise the possibility that ongoing use of the clinical heuristics of age and contrast enhancement may reflect, in part, their incomplete surrogacy for IDH1 status. Since one trivial explanation of our finding that IDH1 mutant malignant astrocytomas are more resectable is that IDH1 status could represent merely a proxy for these other well-established prognostic factors, it is important to note that we find no combination of clinical factors that can a priori identify IDH1 mutant cases with 100% certainty and that IDH1 status remains an independent predictor of complete resection after controlling for these potentially confounding factors, including histology. In our anecdotal experience, we find that IDH mutant tumors have a less fibrous vascular quality, which may afford a greater extent of resection; however, the reason that IDH mutant tumors are more resectable will need further study to definitely determine.
As already noted, the criteria for diagnosis of AA versus GBM have evolved over time and, even in the current era, display institutional variability on central review.4 The stability over time of clinical measures, including survival, and underlying molecular genotype within our cohort provides evidence that the intrinsic tumor composition of the study population was not impacted by variable diagnostic criteria (by either the inclusion or, more importantly, the selective exclusion of cases affecting the underlying composition of the patient population with malignant astrocytic gliomas over time). Thus, our findings are likely to be generalizable to the population of malignant astrocytomas (AA and GBM) considered as a whole.
The association between IDH1 mutation and more complete resection of enhancement in malignant astrocytomas raises an important cautionary note for prior surgical studies; a critical molecular reappraisal is warranted, as IDH1 mutant tumors are likely to disproportionately populate the “complete resection” category in any retrospectively designed histology-based analyses, carrying a significant survival bias that will confound the assessment of surgical efficacy. For example, in our series, all 24 IDH1 mutant GBMs (a subgroup with a median survival >5 y) achieved complete resections of enhancing disease, contributing a substantial survival advantage to a retrospective “complete resection” cohort.
With this in mind, our analyses of resection stratified by IDH1 mutation reassesses the evidence base for complete resection of enhancing disease in the surgical treatment of malignant astrocytomas. It has recently been noted that wild-type IDH1 AA and wild-type IDH1 GBM have a nearly identical time to progression,39 suggesting that the underlying natural history of these lesions is similar.31 The recent discovery of hotspot somatic mutations in the promoter of the telomerase reverse transcriptase gene in nearly all IDH1 wild-type AA and GBM40 further provides objective molecular evidence that IDH wild-type malignant astrocytomas likely arise from a common precursor cell that is distinct from IDH mutant malignant astrocytomas. We also observe a lack of association between grading and outcome in multivariate analyses of our wild-type IDH1 tumor cohort; an observation that largely mirrors prior age-based stratification (as older age skews cohorts toward wild-type tumors), where minimal difference in overall survival has been observed for AA and GBM patients initially diagnosed at advanced age (>50 y).41,42 Thus, we propose that surgical treatment for IDH wild-type malignant astrocytomas can be guided by the existing clinical framework that has been developed for histologically classic GBM14,15 and AA17 (ie, complete resection of enhancing disease). Notably, however, neither our study nor these prior studies uncover a survival association in favor of (or against) the resection of the tumor-infiltrated nonenhancing margin. Malignant astrocytomas defined by the absence of IDH mutations display marked heterogeneity43–45 and likely require further subclassification to determine optimal therapies.
Our cohort consists of a retrospective review of a single institution practice, highlighting the potential limitation in the comparison of histology-based patient cohorts from different institutions. For example, our GBM cohort had 13% IDH1 mutant tumors, a higher frequency compared with other, more locoregional, cohorts. The molecular profile of gliomas from our referral center has been demonstrated to differ from other histology-matched cohorts,44 likely as a result of the well-established selection bias associated with travel and tertiary care coordination. With this in mind, IDH1 status can provide an objective stratification factor for comparison with other patient cohorts, thus improving methodologically on prior studies in this field and preventing diagnostic heterogeneity from obscuring the impact of otherwise effective therapeutic interventions.46 These factors may explain the discrepancy between our positive association of nonenhancing disease resection with survival in our IDH mutant cohort compared with the lack of association reported in a prior study of AA17 and serves as a justification for IDH1 molecular testing routinely for all cases of malignant astrocytoma.
Indeed, our most striking observation is that IDH1 mutant AAs and GBM have substantially improved survival in association with more aggressive resection of nonenhancing disease, compared with IDH1 wild-type tumors. Though we cannot formally infer a causal relationship in our retrospective analysis, our study attempts to minimize surgical selection bias through the examination of prognostic factors collected prospectively and thus supports the proposal that maximal surgical resection positively impacts survival in this patient subgroup. This finding is comparable to the benefit of nonenhancing disease resection in low-grade gliomas,19 the majority of which are IDH1 mutant, indicating that as IDH1 mutant lesions progress from lower to higher grades, the optimal surgical strategy may remain aggressive resection of both enhancing and nonenhancing disease (ie, total tumor volume).
Our data are particularly intriguing compared with other malignant astrocytoma cohorts, considering the absolute blinding of surgeons and clinicians to molecular bias prior to the discovery of IDH1 mutations. As an internal control, the survival of our wild-type cohort comparably overlapped existing modern-era reports of survival for GBM, as histologically defined, a primarily wild-type IDH1 disease. In contrast, given the current stage of follow-up on surviving IDH1 mutant patients (estimated median survival, 163.4 mo) in our cohort, the finalized median survival of this subgroup can be expected to exceed previous reports of survival for IDH1 mutant AAs (65 mo23) or GBM (31–46 mo21,23). Thus, our findings support the potential utility of surgical resection to improve survival for IDH1 mutant malignant astrocytoma patients. Focusing on the specific goals of resection, aggressive minimization of the total volume of MRI-detectable disease burden (enhancing and nonenhancing) is associated with improved survival in our IDH1 mutant cohort. We conclude that IDH1 mutation in malignant astrocytoma may serve as a predictive molecular biomarker to guide aggressive surgical resection, allowing for individualized therapy based on tumor genotype.
Supplementary Material
Funding
This work was supported by funding from the Burroughs Wellcome Trust, the James S. McDonnell Foundation, and the Texas Neurofibromatosis Foundation.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
We thank the patients who participated in this research through donation of blood and tumor samples. We acknowledge the technical contributions of Rebecca Maywald and Lindsey Heathcock, and thank Drs Andrew Chi and Fred Barker for critical comments on the manuscript. A portion of this work was presented at the 2012 American Association of Neurological Surgeons annual meeting and the 2012 American Society of Clinical Oncology annual meeting.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glantz MJ, Burger PC, Herndon JE, 2nd, et al. Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. 1991;41(11):1741–1744. doi: 10.1212/wnl.41.11.1741. [DOI] [PubMed] [Google Scholar]
- 3.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 4.Prayson RA, Agamanolis DP, Cohen ML, et al. Interobserver reproducibility among neuropathologists and surgical pathologists in fibrillary astrocytoma grading. J Neurol Sci. 2000;175(1):33–39. doi: 10.1016/s0022-510x(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 5.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333(6048):1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226(1):7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahm F, Koelsche C, Meyer J, et al. CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol. 2012;123(6):853–860. doi: 10.1007/s00401-012-0993-5. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 9.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 11.Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms—an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11(2):165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 13.Shonka NA, Theeler B, Cahill D, et al. Outcomes for patients with anaplastic astrocytoma treated with chemoradiation, radiation therapy alone or radiation therapy followed by chemotherapy: a retrospective review within the era of temozolomide. J Neurooncol. 2013;113(2):305–311. doi: 10.1007/s11060-013-1116-4. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 15.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576. doi: 10.1227/01.neu.0000317304.31579.17. discussion 564–576. [DOI] [PubMed] [Google Scholar]
- 16.Nitta T, Sato K. Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer. 1995;75(11):2727–2731. doi: 10.1002/1097-0142(19950601)75:11<2727::aid-cncr2820751115>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Keles GE, Chang EF, Lamborn KR, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105(1):34–40. doi: 10.3171/jns.2006.105.1.34. [DOI] [PubMed] [Google Scholar]
- 18.Chaichana KL, Kosztowski T, Niranjan A, et al. Prognostic significance of contrast-enhancing anaplastic astrocytomas in adults. J Neurosurg. 2010;113(2):286–292. doi: 10.3171/2010.2.JNS091010. [DOI] [PubMed] [Google Scholar]
- 19.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 20.Curran WJ, Jr, Scott CB, Horton J, et al. Does extent of surgery influence outcome for astrocytoma with atypical or anaplastic foci (AAF)? A report from three Radiation Therapy Oncology Group (RTOG) trials. J Neurooncol. 1992;12(3):219–227. doi: 10.1007/BF00172709. [DOI] [PubMed] [Google Scholar]
- 21.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 23.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174(4):1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but are rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 28.van den Bent MJ, Hartmann C, Preusser M, et al. Interlaboratory comparison of IDH mutation detection. J Neurooncol. 2013;112(2):173–178. doi: 10.1007/s11060-013-1056-z. [DOI] [PubMed] [Google Scholar]
- 29.Weller M, Wick W, von Deimling A. Isocitrate dehydrogenase mutations: a challenge to traditional views on the genesis and malignant progression of gliomas. Glia. 2011;59(8):1200–1204. doi: 10.1002/glia.21130. [DOI] [PubMed] [Google Scholar]
- 30.Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 32.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 33.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27(34):5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 34.Hegi ME, Janzer RC, Lambiv WL, et al. Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol. 2012;123(6):841–852. doi: 10.1007/s00401-011-0938-4. [DOI] [PubMed] [Google Scholar]
- 35.Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73(21):1792–1795. doi: 10.1212/WNL.0b013e3181c34ace. [DOI] [PubMed] [Google Scholar]
- 36.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 37.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 38.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044–1055. doi: 10.1097/00006123-199805000-00054. discussion 1055–1046. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 40.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim TS, Halliday AL, Hedley-Whyte ET, Convery K. Correlates of survival and the Daumas-Duport grading system for astrocytomas. J Neurosurg. 1991;74(1):27–37. doi: 10.3171/jns.1991.74.1.0027. [DOI] [PubMed] [Google Scholar]
- 42.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 43.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brennan C, Momota H, Hambardzumyan D, et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4(11):e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betensky RA, Louis DN, Cairncross JG. Influence of unrecognized molecular heterogeneity on randomized clinical trials. J Clin Oncol. 2002;20(10):2495–2499. doi: 10.1200/JCO.2002.06.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.