Introduction and Summary of Findings
While radiation therapy has been standard of care for newly diagnosed glioblastoma for several decades, it only delays but does not prevent recurrence of these aggressive tumors. Therefore, the identification and targeting of factors allowing glioblastoma cells to escape the deleterious effects of radiation is essential to allow this therapeutic modality to fulfill its potential. In an elegant study published in 2010, the Brown lab reported that irradiation induces recruitment of bone marrow-derived cells (BMDCs) into glioblastoma to form blood vessels de novo, a process defined as vasculogenesis, in contrast to angiogenesis which involves the remodeling of existing vessels in the tumor bed.1 In that study, pharmacologic inhibition of HIF-1 or of the interaction between the chemokine stromal derived factor-1 (SDF-1, CXCL12) and its receptor CXCR4 prevented the influx of BMDCs, primarily CD11b+ myelomonocytes, and the development of functional tumor vasculature after radiation, resulting in abrogation of tumor regrowth.1
Now, in a follow-up study entitled “Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats” published in this issue of Neuro-Oncology2, Liu and colleagues from that same group sought to improve upon limitations previously reported when they1 and others3 targeted the SDF-1/CXCR4 interaction using the small molecule CXCR4 inhibitor plerixafor (AMD3100). Specifically, the authors noted that endothelial cells in tumors like glioblastoma express high levels of CXCR7, the more recently discovered second receptor for SDF-1.4 Of note, besides its blockade of CXCR4, plerixafor is actually an allosteric agonist of CXCR7,5 which can signal through β-arrestin and may activate the endothelium leading to increased invasiveness of cancer cells.6
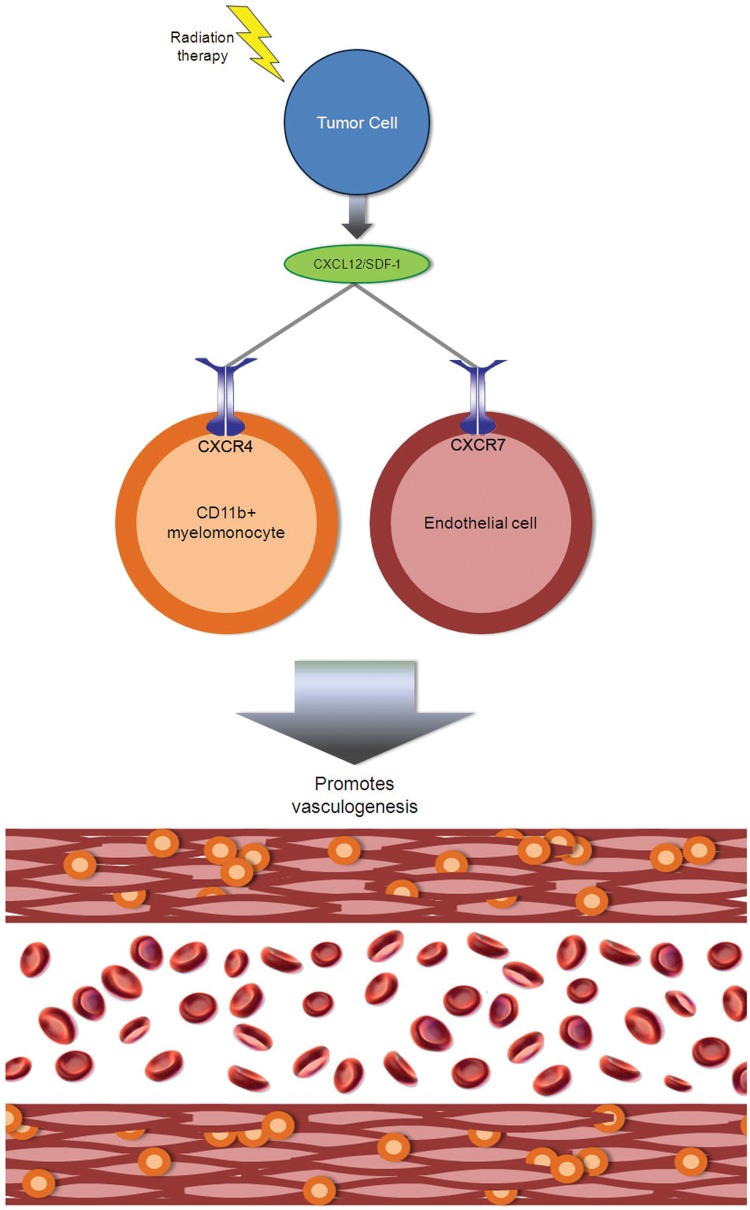
The authors therefore investigated combining radiation therapy with NOX-A12, 45 L-enantiomeric RNA nucleotides which form a structural scaffold that recognizes SDF-1 with high affinity and thus efficiently blocks the chemokine's interaction with CXCR4 and CXCR7 (Figure 1). They showed that NOX-A12 inhibits SDF-1 dependent chemotaxis of CXCR4 myelomonocytes and SDF-1 dependent CXCR7 internalization in endothelial cells.
Fig. 1.
Dual receptors lead to dual functions of SDF-1 (CXCL12) in glioblastoma vasculogenesis after radiation therapy. Irradiated tumor cells secrete SDF-1, which binds CXCR4 expressed by marrow-derived myelomonocytes and CXCR7 expressed by marrow-derived endothelial precursors, which together participate in vasculogenesis.
NOX-A12 also inhibits SDF-1 dependent migration of endothelial cells. Rats treated in utero with the carcinogen ethylnitrosourea (ENU) begin to die of brain tumors from approximately 120 days of age. The authors delivered a single dose of whole brain irradiation (20 Gy) on day 115 of age and began treatment with NOX-A12 immediately following irradiation and continued with either 5 or 20 mg/kg for 4 or 8 weeks, doses and times equivalent to well tolerated human exposures. The authors found a marked prolongation of rat lifespan that was dependent both on drug dose and duration of treatment. When treating tumors only when they were visible by MRI, the authors demonstrated complete regression of the tumors with combined treatment that was not achieved by radiation alone or with the addition of temozolomide to radiation.
Where do we go from here?
In terms of follow-up mechanistic questions, it will be important to determine the specific contribution of myelomonocytes to the vessels formed by vasculogenesis. Do these cells provide nutritional support for endothelial cells the way pericytes do, or do they produce extracellular matrix scaffolding that supports vessel stabilization? The individual roles of endothelial cells versus myelomonocytes in the vasculogenesis that this group previously showed to drive revascularization after radiation in glioblastoma could be investigated by specific inhibition of myelomonocyte receptor CXCR4 using specific inhibitor plerixafor and endothelial cell receptor CXCR7 using specific inhibitor CCX2066. Further studies will also be needed to determine the spatial distribution of these two cell types, which could vary because the spatial heterogeneity of glioblastoma will create varying degrees of factors stimulating vasculogenesis, most notably hypoxia and the subsequent HIF-1α expression that the authors show to play an important role in vasculogenesis.
Other follow-up studies could aim to render these findings more translational. Because glioblastoma patients are typically treated with fractionated external beam radiation therapy,7 it remains unclear if fractionated focal radiation will produce the same degree of SDF-1 upregulation noted after a single fraction of whole brain radiation used in this study. Furthermore, the contribution of vasculogenesis to tumor growth in humans remains unclear, with a study of cancers removed from patients who had undergone prior bone marrow transplantation suggesting minimal contribution to the tumor endothelium, although marrow-derived myelomonocytes in the vasculature were not assessed.8 Use of patient specimen-derived xenografts9 would also improve the translatability of these findings relative to cell line models. Furthermore, it will be worth investigating whether NOX-A12 disrupts SDF-1 production by other cellular sources beyond tumor cells given the recent finding that endothelial cell secretion of SDF-1 recruits glioblastoma tumor-initiating cells (TICs) to the perivascular niche.10
The applicability of the findings of Liu et al to the different molecular subtypes of glioblastoma will also warrant further investigation. Recent studies have suggested that resistance to radiation can be driven by microglia-secreted NF-κB promoting a transition to a mesenchymal subtype of tumors with more CD44+ TICs11 and with more expression of the receptor tyrosine kinase c-Met,12 features which have been shown to drive radiation resistance. The ability of radiation therapy to upregulate SDF-1 across molecular subtypes and whether SDF-1-mediated vasculogenesis interacts with c-Met and TIC-driven radiation resistance in mesenchymal tumors warrants further investigation.
Glioblastomas progress even after adjuvant therapy with VEGF inhibitors such as bevacizumab,13,14 as confirmed in two recently completed randomized phase III clinical trials in newly diagnosed glioblastoma in North America and Europe. In several studies, tumor progression after bevacizumab therapy has been associated with higher levels of SDF-1 and CXCR4.15,16 Per the report of Liu et al, the authors are currently investigating the relationship between anti-angiogenic therapy and changes in SDF-1 and CXCR4 using the C6 tumor implanted intracranially. If a role of SDF-1 in revascularization after anti-angiogenic therapy is confirmed, future studies could determine the effect of VEGF inhibitors, such as bevacizumab, in combination with NOX-A12 after radiation.
Conclusion
The current study by Liu et al continues the excellent series of studies from the Brown lab characterizing the role of vasculogenesis in revascularization after radiation of glioblastoma. Because NOX-A12 is currently in phase II studies with chronic lymphocytic leukemia (CLL) and multiple myeloma (MM), once the role of SDF-1 stimulation of CXCR4 and CXCR7 in driving revascularization after radiation has been verified, translating their findings into a clinical trial should prove feasible. Radiation and pharmacologic therapy remain the only two broad categories of treatments available to address the poor prognosis faced by glioblastoma patients. Efforts like the current study which seek to understand and overcome the mechanisms of resistance to these therapies will be essential.
References
- 1.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu SC, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, Merchant M, Zboralski D, Zöllner S, Kruschinski A, Klussmann S, Recht L, Brown JM. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro-Oncology. 2014;16:21–28. doi: 10.1093/neuonc/not149. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 4.Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 5.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75:1240–1247. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopal S, Kim J, Ahn S, et al. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Peters BA, Diaz LA, Polyak K, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 9.Giannini C, Sarkaria JN, Saito A, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat KP, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. Journal of the National Cancer Institute. 2011;103:645–661. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 13.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan- vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark AJ, Lamborn KR, Butowski NA, et al. Neurosurgical management and prognosis of patients with glioblastoma that progress during bevacizumab treatment. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e3182314f9d. [DOI] [PubMed] [Google Scholar]
- 15.DeLay M, Jahangiri A, Carbonell WS, et al. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 18:2930–2942. doi: 10.1158/1078-0432.CCR-11-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Duda DG, di Tomaso E, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]