Abstract
Background
Pseudoprogression (PsP) occurs at a higher rate in glioblastoma multiforme with a methylated MGMT promoter—a subset with increased sensitivity to chemoradiotherapy and better overall prognosis. In oligodendroglioma (OG) and oligoastrocytoma (OA), presence of 1p/19q codeletions is highly predictive of response to treatment and is often associated with the methylated MGMT promoter; hence, this study queries whether the presence of 1p/19q codeletions in OG/OA correlates with a higher rate of PsP following therapy.
Methods
A retrospective analysis was performed on all OG/OA in a database of patients with brain tumors who underwent resection of their tumor since 1998. Eighty-eight cases (37 with and 51 without 1p/19q codeletions) met inclusion criteria, and their patient data were analyzed to determine whether the presence of 1p/19q codeletions was associated with PsP and survival.
Results
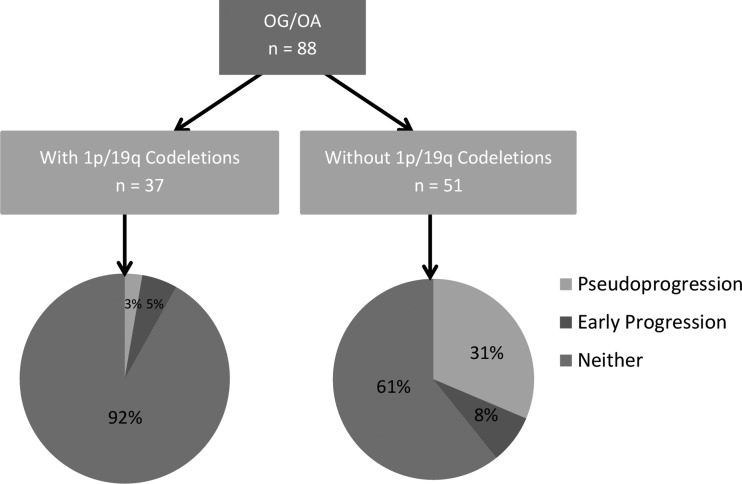
OG/OA (World Health Organization grades II and III) with 1p/19q codeletions had a significantly improved survival (P = .041). Multivariate analysis found that PsP occurred less frequently in OG/OA with 1p/19q codeletions compared with tumors without codeletions (odds ratio, 0.047; 95% confidence interval, 0.005–0.426; P = .0066). The rate of PsP was 19% for the entire cohort, 31% for tumors without codeletions, and 3% for tumors with codeletions. When early posttreatment contrast enhancement developed in tumors with 1p/19q codeletions, it occurred exclusively in tumors that were histologically OA and not OG.
Conclusion
Codeletions of 1p/19q are a marker of good prognosis but are unexpectedly associated with a lower likelihood of PsP. PsP does not correlate with sensitivity to treatment and improved survival in OG/OA.
Keywords: oligodendroglioma, oligoastrocytoma, pseudoprogression, 1p/19q codeletions, p53.
Pseudoprogression (PsP) is now appreciated to be a common radiologic phenomenon that occurs shortly after gliomas are treated with chemotherapy and radiation. Chemoradiotherapy has been suggested to alter capillary permeability and disrupt the blood–brain barrier, resulting in the development of areas of contrast enhancement that are largely indistinguishable from true tumor progression. PET and new MRI techniques such as perfusion and MR spectroscopy have shown promise in differentiating between these 2 findings. However, these techniques have not been validated in a prospective study and are currently considered investigational.1
At present, the only definitive method of distinguishing PsP from true tumor progression, besides histological confirmation, is close follow-up imaging to monitor for stability of the contrast enhancement over time.2,3 This diagnostic dilemma is an outstanding challenge in neuro-oncology, as it often results in either overtreatment—unnecessary neurosurgical interventions and/or escalation of chemotherapy—or treatment delay, since early progression can be misdiagnosed as treatment effect. For this reason, molecular markers are needed to risk stratify contrast-enhancing lesions that occur shortly after chemoradiotherapy.
PsP has primarily been studied in the context of glioblastoma multiforme (GBM), though it has long been known to occur in lower-grade gliomas as well.4 In GBM, PsP has been documented in 21% to 47% of tumors following chemoradiotherapy and has been shown to be associated with MGMT promoter methylation and improved overall survival.5 It is hypothesized that the enhanced response to chemoradiotherapy conferred by the methylated MGMT promoter predisposes these tumors and the peritumoral tissue to the effects of chemoradiation and the ensuing robust inflammatory reaction that is believed to occur in PsP.
In lower-grade gliomas, the incidence of PsP and the molecular markers associated with its development have not been well defined. In one series, the rate of PsP was found to be 18% among a cohort of World Health Organization (WHO) grade III oligodendrogliomas (OGs), oligoastrocytomas (OAs), and astrocytomas.6 A recent study reported a correlation between p53 overexpression and higher rates of PsP; this study examined a patient cohort comprising primarily GBM and only a small number of WHO grade III OGs and astrocytomas.7 Whether p53 overexpression is predictive of PsP in OG/OA and whether it is an independent factor or functions in association with other molecular markers (eg, IDH1/2 mutations, 1p/19q codeletions, MGMT promoter methylation) in predicting PsP in OG/OA remains unexplored.
Codeletions at 1p and 19q are a common recurrent cytogenetic abnormality in 50%–70% of OG/OA.8 Testing for codeletions is now routinely performed because its presence is strongly associated with prolonged survival and increased sensitivity to treatment.9 This correlation with better prognosis and treatment responsiveness suggests that 1p/19q codeletions may be associated with the development of PsP, especially given that 1p/19q codeletions have been found by many groups to be associated with the methylated MGMT promoter.10–15 To that end, we investigated whether the presence of 1p/19q codeletions in OG/OA is a molecular marker of increased risk for the development of PsP.
Patients and Methods
Case Ascertainment and Data Collection
An institutional review board–approved database was queried for patients whose pathology was reviewed by a neuropathologist at Washington University, and the glioma was determined to be either an OG or OA based on WHO diagnostic and grading criteria. Resected since 1998 were 67 low-grade OGs (grade II), 46 low-grade OAs (grade II), 54 anaplastic OGs (grade III), and 65 anaplastic OAs (grade III). All patient cases were included in this retrospective analysis if (i) their medical records were available for review, (ii) serial imaging was performed for at least 6 months after radiotherapy, (iii) treatment included radiation with or without chemotherapy, and (iv) cytogenetic testing on 1p and 19q was performed.
Prior to 2003, cytogenetic testing for 1p and 19q codeletions was performed by fluorescence in situ hybridization (FISH) on paraffin-embedded tissue with a bacterial artificial chromosome (BAC) probe from chromosomes 1p32 (260I23, a gift from Dr Paul Goodfellow) and 19q (telomere probe, Vysis). After 2003, FISH was performed on paraffin-embedded tissue with either human RPCI-11 BAC library–derived probes (Research Genetics) localizing to 1p32, 1q42, 19p13, and 19q13 or commercial probes (Abbott Molecular) localizing to 1p36, 1q25, 19p13, and 19q13.
PsP is defined as previously reported.2,3,16,17 Specifically, PsP was diagnosed when the official radiology report identified new areas of enhancement on MRI of concern for possible recurrence within 6 months after the completion of radiotherapy, and these new areas of contrast enhancement (i) spontaneously resolved, (ii) significantly improved, or (iii) remained stable on subsequent imaging without salvage therapy such as bevacizumab. PsP could also be histologically proven by biopsy or resection. True early progression of the glioma was defined as the development of areas of contrast enhancement that continued to increase on serial imaging without spontaneous improvement; alternatively, true tumor progression could be histologically proven by biopsy or resection.16,17
In this analysis, areas of enhancement that first occurred more than 6 months after radiotherapy were not evaluated. Late PsP, defined as PsP first occurring between 6 months and 2 years after the completion of radiotherapy, can occur; however, its etiology is thought to be related to radiation-induced ischemic injury rather than the increased inflammatory reaction observed in early PsP. The high frequency of bevacizumab use in this setting to treat presumed disease precludes any meaningful analysis of late PsP versus true tumor progression.
The surgical pathology reports for the included cases were also reviewed for p53 overexpression and for the R132H-mutated version of the isocitrate dehydrogenase 1 (IDH1) protein as identified by immunohistochemistry. However, immunohistochemistry on IDH1 was reported on only 25 of 88 tumors; the low availability of data precluded further analysis.
Statistical Methods
Statistical analysis was performed using the statistical package SAS 9.3. All tests were 2-sided and the significance level was set at P = .05.
Patient and tumor characteristics were compared between the 2 groups (OG/OA with 1p/19q codeletions and OG/OA without 1p/19q codeletions) by the Kruskal–Wallis test for continuous variables and by Fisher's exact test for categorical variables.
Patient cases were analyzed as a binary outcome; either they did or did not develop PsP (progression thus fell into the latter category). A univariate logistic regression model was used to examine the association of age, gender, volume of the tumor at diagnosis, degree of resection, location, p53 expression, WHO grade, adjuvant treatments, and volume of T2 abnormality at the time of adjuvant treatment against this binary outcome. Multivariate analysis through stepwise selection was performed to examine the relationship between PsP and those covariates, when the binary group indicator (presence or absence of 1p/19q codeletions) was forced into the model. A significance level of 0.2 was required to allow a predictor into the model, and a significance level of 0.2 was required for a predictor to stay in the model. The significance of the predictors in the final model was examined by the likelihood ratio test, and the performance of the model was tested by C-statistics. The Hosmer–Lemeshow test was used to test goodness of fit.
Overall survival (OS) was a secondary endpoint. OS was defined as the months from pathologic diagnosis to death from any cause or last patient contact. Kaplan–Meier curves were generated that provided unadjusted survival estimates for all patients. Differences between the 2 groups were determined by the log-rank test.
Results
Patient Characteristics
Based on the inclusion criteria we have detailed, 88 cases—37 cases of OG/OA with 1p/19q codeletions and 51 cases without 1p/19q codeletions—that underwent resection since 1998 were evaluable. Patient and treatment characteristics were analyzed between the 2 groups (Table 1). The groups were well balanced with the exception of age, p53 expression, and tumor location—patients with 1p/19q codeletions were significantly older than patients without 1p/19q codeletions at the time of radiotherapy, and their tumors were less likely to overexpress p53 and were more frequently in the frontal lobe.
Table 1.
Patient and treatment characteristics
| Variable | Without 1p/19q Codeletions (n = 51) | With 1p/19q Codeletions (n = 37) | P |
|---|---|---|---|
| Age, y, at the time of radiotherapy | |||
| 20–39 | 30 (58%) | 11 (30%) | .020 |
| 40–49 | 13 (25%) | 13 (35%) | |
| 50+ | 8 (16%) | 13 (35%) | |
| Gender | |||
| Male | 27 (53%) | 25 (68%) | .19 |
| Female | 24 (47%) | 12 (32%) | |
| WHO grade | |||
| II | 18 (35%) | 10 (27%) | .49 |
| III | 33 (65%) | 27 (73%) | |
| Tumor volume at diagnosis | |||
| Range (cm3)a | 5–212 | 5–163 | .46 |
| Median (cm3)a | 48 | 28 | |
| Resection | |||
| Gross total resection | 26 (51%) | 20 (54%) | .77 |
| Partial resection | 19 (37%) | 12 (32%) | |
| Biopsy | 6 (12%) | 4 (11%) | |
| AutoLITTb | 0 | 1 (3%) | |
| T2 abnormality at time of adjuvant therapy | |||
| Range (cm3)c | 0–146 | 0–40 | .48 |
| Median (cm3)c | 16 | 9 | |
| Location | |||
| Frontal lobe | 35 (69%) | 33 (89%) | .038 |
| Not frontal lobe | 16 (31%) | 4 (11%) | |
| p53 | |||
| p53 overexpression | 28/34 (82%) | 4/16 (25%) | .00027 |
| Normal p53 expression | 6/34 (18%) | 12/16 (75%) | |
| Radiation | |||
| Range (cGy)d | 5400–6134 | 5000–6300 | .48 |
| Median (cGy)d | 5940 | 5940 | |
| Chemotherapy | |||
| Concurrent chemotherapy | 42 (82%): 40 with temozolomide, 2 with hydroxyurea | 32 (86%): 31 with temozolomide, 1 with hydroxyurea | .29 |
| Radiation followed by chemotherapy | 6e (12%): 5 with temozolomide, 1 with PCV | 1 (3%): 1 with temozolomide | |
| Radiation alone | 3 (6%) | 4 (11%) | |
aData on tumor volume prior to resection were available for 41/51 tumors without 1p/19q codeletions and 33/37 tumors with 1p/19q codeletions.
bAutoLITT is a trademark of Monteris Medical, Plymouth, MN, and is an FDA-approved system that is used to thermally ablate brain tumors via a fiber-optic laser probe.
cData on T2 abnormality at the time of adjuvant therapy were available for 42/51 tumors without 1p/19q codeletions and 30/37 tumors with 1p/19q codeletions.
dData on dose administered were available for 39/51 tumors without 1p/19q codeletions and 33/37 tumors with 1p/19q codeletions.
eIn 1 of these 6 cases, it is unclear whether temozolomide was started before or after completion of radiotherapy. cGy, centigray; PCV, procarbazine, lomustine, and vincristine.
Likelihood of PsP in OG/OA With and Without 1p/19q Codeletions
Among WHO grades II and III OG/OA without 1p/19q codeletions, PsP occurred in 16 patients (31%) by radiographic criteria (Fig. 1, Table 2). There was no correlation between the rate of PsP and WHO grade of these tumors without 1p/19q codeletions; specifically, the rate was 39% for grade II tumors and 27% for grade III tumors. In contrast, PsP rarely occurred in OG/OA with 1p/19q codeletions regardless of the WHO grade; the rate was 3% (1 in 37 cases). In that one case of PsP, the amount of early contrast enhancement was minimal, with 2 small areas of nodular enhancement within the operative bed measuring 2 mm in size that appeared on the 6-month MRI and resolved by the 9-month MRI. Histologically, that tumor was a grade II OA.
Fig. 1.
Rates of PsP and early progression depending on the status of 1p/19q codeletions.
Table 2.
Rates of PsP and early progression (ePg)
| Frequency (WHO grade) | Without 1p 19q Codeletions (n = 51) |
With 1p19q Codeletions (n = 37) |
||
|---|---|---|---|---|
| Rate of PsP among OG (II) | 0% (0/1) | 39% (7/18) | 0% (0/8) | 10% (1/10) |
| Rate of PsP among OA (II) | 41% (7/17) | 50% (1/2) | ||
| Rate of PsP among OG (III) | 20% (1/5) | 27% (9/33) | 0% (0/21) | 0% (0/27) |
| Rate of PsP among OA (III) | 29% (8/28) | 0% (0/6) | ||
| Rate of ePg among OG (II) | 0% (0/1) | 0% (0/18) | 0% (0/8) | 0% (0/10) |
| Rate of ePg among OA (II) | 0% (0/17) | 0% (0/2) | ||
| Rate of ePg among OG (III) | 0% (0/5) | 12% (4/33) | 0% (0/21) | 7% (2/27) |
| Rate of ePg among OA (III) | 14% (4/28) | 33% (2/6) | ||
The only patient and tumor characteristic that was significantly associated with PsP in the univariate logistic regression model was the absence of 1p/19q codeletions (P = .0081; Table 3). A multivariate analysis was performed to address potentially confounding variables (Table 4), and again PsP was found to occur less frequently in OG/OA with 1p/19q codeletions than in OG/OA without codeletions (odds ratio [OR], 0.047; 95% confidence interval [CI], 0.005–0.426; P = .0066). This multivariate model was statistically significant (χ2(5) = 23.0341; P = .0003) with an associated C-statistic of 0.843 and Hosmer–Lemeshow test result of 4.0853 (degrees of freedom = 6; P = .6651), indicating excellent explanatory power and fit to the data.
Table 3.
Results of univariate analysis examining the relationship between PsP and covariates
| Covariate | P |
|---|---|
| Presence/absence of 1p/19q codeletions | .0081 |
| Gender | .1023 |
| Resection | .2333 |
| Location | .0720 |
| Age | .3916 |
| Grade | .2092 |
| Volume of tumor at diagnosis | .4639 |
| Volume of T2 abnormality during adjuvant therapy | .4846 |
| p53 | .7832 |
Table 4.
Results of multivariate analysis through stepwise selection examining the relationship between PsP and covariates with the status of 1p/19q codeletions forced into the model
| Covariate | P value, OR (95% CI) |
|---|---|
| + Gender | .0654, 3.699 (0.920, 14.873) |
| + Resection | .1655, |
| GTR vs biopsy/Other 0.226 (0.036, 1.432) | |
| PR vs biopsy/Other 0.144 (0.019, 1.090) | |
| + Location | .1498, 2.574 (0.711, 9.321) |
| + Age | - |
| + Grade | - |
| + Volume of tumor at diagnosis | - |
| + Volume of T2 abnormality during adjuvant therapy | - |
| + p53 | - |
| Presence/absence of 1p/19q codeletions | .0066, 0.047 (0.005, 0.426) |
Gender, resection, and location (but not age, grade, volume of the tumor at diagnosis, volume of the T2 abnormality during adjuvant therapy, nor p53 status) met the significance level of 0.2 required for the predictor to remain in the model.
Abbreviations: GTR, gross total resection; PR, partial resection.
Even though radiotherapy is typically not complete for at least 2 months after surgery (the period when postoperative periresection cavity infarcts are most likely to enhance), to evaluate the possibility that these early enhancing abnormalities could be due to postoperative infarcts, we compared the rate of postoperative infarcts on brain MRIs that were performed within 2 weeks following surgery in the subset that underwent resection over the past 5 years—the time period during which diffusion weighted imaging was routinely reported. Fifty-nine percent (10/17) in the cohort with 1p/19q codeletions had a postresection infarct compared with 78% (14/18) in the cohort without codeletions. There was no association between PsP and postoperative infarction by Fisher's exact test (P = .44).
The patients who developed PsP were rarely symptomatic. Detailed clinical data were available for 88% of patients who developed PsP. Of those patients, only 4 were symptomatic: the first had mild cognitive complaints, the second had cognitive complaints and simple partial seizures, the third had a partial seizure with secondary generalization, and the fourth had headaches. At the time PsP developed, all the patients were either off or in the process of being weaned off of dexamethasone except for the patient with the persistent headache.
Early Progression and Overall Survival
To determine whether the inverse relationship between loss of heterozygosity at 1p/19q and the development of PsP affected survival, we measured the rates of true early progression and OS in these 2 groups. Early progression in the first 6 months after radiotherapy occurred at a similar frequency for the grade III OG/OA group regardless of the 1p/19q status of the tumor; in contrast, early progression did not occur among the grade II tumors. In the subset of grade III gliomas without 1p/19q codeletions, early progression occurred in 4 cases at a rate of 12% (Table 2), 2 of which were diagnosed by radiologic criteria and the other 2 by resection or biopsy of the enhancing area, demonstrating evidence of recurrent or residual disease and marked radiation effect. Among the grade III tumors with 1p/19q codeletions, early progression was diagnosed by radiologic criteria in 2 patients or 7% of the cohort (Table 2). Histologically, both of these tumors were grade III OAs.
In these patients who developed early progression, radiologic progression preceded clinical progression, as they were largely asymptomatic, with the exception of 1 patient who complained of headache and 1 patient with cognitive impairment. At least 5 out of 6 patients were tapered off or in the process of being tapered off of dexamethasone at the time early progression was diagnosed.
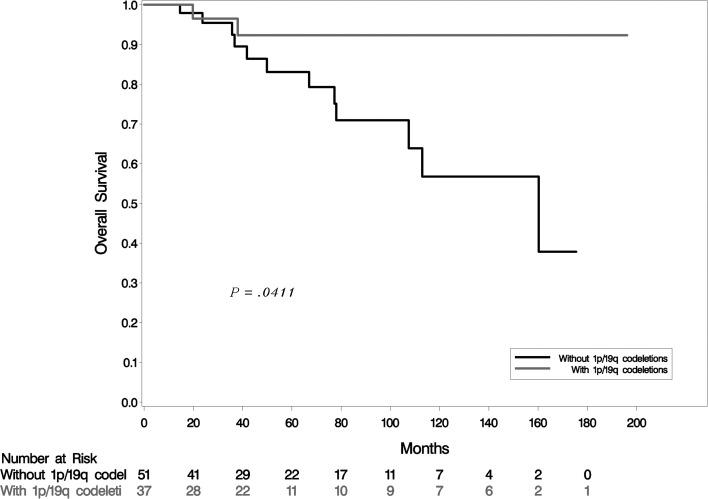
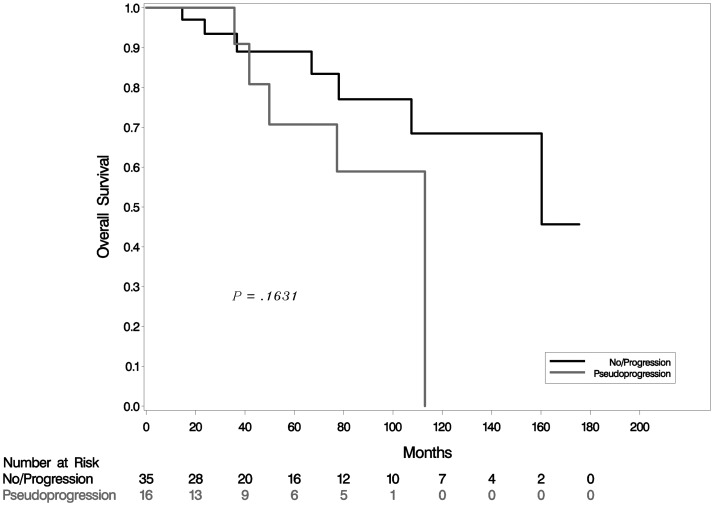
In accordance with previous data that show that 1p/19q codeletions have a strong prognostic value,9 OS was significantly better in the cohort with 1p/19q codeletions compared with the cohort without codeletions (P = .041; Fig. 2). Survival analysis did not identify a significant difference in OS among patients without codeletions who developed PsP compared with patients who did not develop it (P = .16;Fig. 3).
Fig. 2.
Kaplan–Meier estimates of OS by status of 1p/19q codeletions.
Fig. 3.
Kaplan–Meier estimates of OS in tumors without 1p/19q codeletions with and without PsP.
P53 Overexpression
Immunohistochemistry for p53 was performed on 50 of the 88 tumors. Overexpression of p53 was present in 82% of OG/OA without 1p/19q codeletions and present in only 25% of OG/OA with 1p/19q codeletions (Table 1), a statistically significant difference (P = .00027). Of the 50 tumors for which p53 status was known, PsP occurred in 12 tumors, among which p53 was overexpressed in 9 cases or 75%, all of which were without 1p/19q codeletions. Using a logistic regression model, there was a suggestion that PsP occurs less frequently in OG/OA with normal p53 expression compared with OG/OA with p53 overexpression (OR, 0.51; 95% CI, 0.12–2.20; P = .37).
Discussion
Methylation of the MGMT promoter in GBM both carries favorable prognostic and predictive values18 and has been identified as a molecular risk factor for PsP.5 With few exceptions,19–21 1p/19q codeletions have been shown to be associated with MGMT promoter methylation10–15 and are a well-defined marker of favorable prognosis and treatment response.9 For this reason, 1p/19q codeletions in OG/OA would be predicted to be a risk factor for the development of PsP. However, we found a strong correlation between the presence of 1p/19q codeletions and a decreased risk for the development of PsP.
The 2 cohorts were significantly different in terms of age and location of the tumor. The propensity of OG/OA with 1p/19q codeletions to develop more frequently in the frontal lobe as seen in our data has been previously reported.22,23 The etiology of the difference in age is unclear but may be due to the more indolent nature of tumors with 1p/19q codeletions. The prognostic value of 1p/19q codeletions, which has been previously reported, was re-demonstrated in this study confirming the general integrity of the dataset. Notably, 1p/19q codeletions remained a significant predictor of OS despite the significantly greater age of this cohort at baseline.
The difference in rates of PsP between the 2 groups is striking: PsP is 10 times more common in OG/OA without 1p/19q codeletions than in tumors with 1p/19q codeletions. Because of the high rates of PsP in the cohort without 1p/19q codeletions, PsP is 4 times more common than early progression. This high propensity for developing PsP should be taken into consideration in the management of patients with these tumors when contrast enhancement develops shortly after chemoradiotherapy, and it should be recognized in both clinical practice and clinical trials involving this patient population. On the other hand, tumors with 1p/19q codeletions rarely develop PsP, especially if they are histologically OG. It is possible that some cases of early tumor progression in our data analysis were actually unrecognized cases of PsP because these areas of contrast enhancement frequently occurred in the setting of steroid treatment being tapered, and they may have occurred within the 80% isodose line. However, since early progression occurred more frequently in the cohort without 1p/19q codeletions and occurred in only a small number of patients, this potential bias was unlikely to confound the results of this study.
When PsP occurs in OG/OA without 1p/19q codeletions, it is not due to increased sensitivity to chemoradiation, as these tumors have been shown to respond less favorably9 and no survival advantage was identified among patients without codeletions who developed PsP in this analysis. When PsP occurs, it may be due in part to the higher rate of TP53 mutations seen in tumors without 1p/19q codeletions when compared with those with codeletions.21,22,24–27 Consistent with this hypothesis, we identified a significant correlation between the absence of 1p/19q codeletions and p53 overexpression (a surrogate marker for TP53 mutations because mutations frequently result in the stabilization and accumulation of the protein).28 The positive correlation between p53 overexpression and PsP was suggestive, although it did not reach statistical significance, possibly because p53 overexpression is an imperfect marker of TP53 mutations and the sample size was small.
In GBM and lower-grade astrocytic tumors, the presence of MGMT promoter methylation, except in rare instances, has been shown to correlate with TP53 mutations,20,29–32 thus TP53 mutations would be predicted to be associated with a higher risk for developing PsP in GBM, as suggested by Kang et al.7 Therefore, the presence of TP53 mutations could be the common risk factor for the development of PsP in both high- and lower-grade gliomas, independent of both predictive biomarkers: MGMT promoter methylation and 1p/19q codeletions. In addition, TP53 mutations are a known genetic feature of astrocytomas,28 which may explain in part the observation that PsP preferentially occurred in gliomas with 1p/19q codeletions that have an astrocytic component.
It is possible that some of the OA in our cohort were actually GBM with oligodendroglial features—OA with areas of necrosis and endothelial proliferation.33 However, it is unlikely that this accounts for the higher frequency of PsP in the noncodeleted cohort for 2 reasons: (i) low-grade noncodeleted tumors develop PsP at the same, if not a higher, rate as anaplastic OA and (ii) on univariate analysis, there was no relationship between PsP and biopsy-only cases, in which undersampling and misclassification of histologic grading might have occurred.
While the results of this study are highly suggestive, this is a retrospective analysis of a patient database and as such has clear limitations: (i) it is based on the retrospective analysis of OGs/OAs, which were treated by multiple practitioners with individual practice patterns, (ii) there is a paucity of data on IDH1/2 status and no data on MGMT promoter methylation, (iii) TP53 mutations are identified indirectly by p53 overexpression rather than sequencing techniques, and (iv) there is potential bias in our evaluation of PsP and true tumor progression, in spite of our attempts to limit that bias by using the official neuroradiology reports, which are less likely to be influenced by knowledge of 1p/19q status, instead of the source images. Because of these limitations, the results of this study will be best validated in a prospective trial subjecting all patients to the same chemoradiotherapy with the status of 1p/19q codeletions blinded.
In summary, our results suggest that the status of 1p/19q codeletions in OG/OA may be used to stratify the risk for developing PsP. If confirmed, the unexpected finding that 1p/19q codeletions predict a lower risk for developing PsP may force the reassessment of the assertion that PsP occurs more frequently in tumors that are sensitive to chemoradiotherapy. Further investigation is needed to determine whether the difference observed in this study was truly a function of TP53 mutations or was due to another molecular marker.
Funding
The brain tumor database is supported in part by an unrestricted educational grant from IMRIS, Inc., Chanhassen, MN.
Conflict of interest statement. J.E., R.G.D., and M.R.C. received an unrestricted educational grant from IMRIS that supports the brain tumor database. G.P.L. served on the NCCN CNS panel from 2006 to 2011 without payment or remuneration.
References
- 1.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 2.Hygino da Cruz LC, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 4.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 5.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 6.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 7.Kang HC, Kim CY, Han JH, Choe GY, Kim JH, Kim IA. Pseudoprogression in patients with malignant gliomas treated with concurrent temozolomide and radiotherapy: potential role of p53. J Neurooncol. 2011;102(1):157–162. doi: 10.1007/s11060-010-0305-7. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 9.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Bent MJ, Gravendeel LA, Gorlia T, et al. A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. Clin Cancer Res. 2011;17(22):7148–7155. doi: 10.1158/1078-0432.CCR-11-1274. [DOI] [PubMed] [Google Scholar]
- 11.Brandes AA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J Clin Oncol. 2006;24(29):4746–4753. doi: 10.1200/JCO.2006.06.3891. [DOI] [PubMed] [Google Scholar]
- 12.Möllemann M, Wolter M, Felsberg J, Collins VP, Reifenberger G. Frequent promoter hypermethylation and low expression of the MGMT gene in oligodendroglial tumors. Int J Cancer. 2005;113(3):379–385. doi: 10.1002/ijc.20575. [DOI] [PubMed] [Google Scholar]
- 13.van den Bent MJ, Dubbink HJ, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2009;27(35):5881–5886. doi: 10.1200/JCO.2009.24.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Yan C, Huang L, Qiu X, Wang Z, Jiang T. Molecular prognostic factors of anaplastic oligodendroglial tumors and its relationship: a single institutional review of 77 patients from China. Neuro Oncol. 2012;14(1):109–116. doi: 10.1093/neuonc/nor185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 17.van den Bent MJ, Wefel JS, Schiff D, et al. Response Assessment in Neuro-Oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 18.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 19.Everhard S, Kaloshi G, Crinière E, et al. MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol. 2006;60(6):740–743. doi: 10.1002/ana.21044. [DOI] [PubMed] [Google Scholar]
- 20.Jha P, Suri V, Jain A, et al. O6-methylguanine DNA methyltransferase gene promoter methylation status in gliomas and its correlation with other molecular alterations: first Indian report with review of challenges for use in customized treatment. Neurosurgery. 2010;67(6):1681–1691. doi: 10.1227/NEU.0b013e3181f743f5. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T, Nakamura M, Kros JM, et al. Phenotype versus genotype correlation in oligodendrogliomas and low-grade diffuse astrocytomas. Acta Neuropathol. 2002;103(3):267–275. doi: 10.1007/s004010100464. [DOI] [PubMed] [Google Scholar]
- 22.Mueller W, Hartmann C, Hoffmann A, et al. Genetic signature of oligoastrocytomas correlates with tumor location and denotes distinct molecular subsets. Am J Pathol. 2002;161(1):313–319. doi: 10.1016/S0002-9440(10)64183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren X, Cui X, Lin S, et al. Co-deletion of chromosome 1p/19q and IDH1/2 mutation in glioma subsets of brain tumors in Chinese patients. PLoS One. 2012;7(3):e32764. doi: 10.1371/journal.pone.0032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177(6):2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto Y, Di Patre PL, Burkhard C, et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108(1):49–56. doi: 10.1007/s00401-004-0861-z. [DOI] [PubMed] [Google Scholar]
- 26.Maintz D, Fiedler K, Koopmann J, et al. Molecular genetic evidence for subtypes of oligoastrocytomas. J Neuropathol Exp Neurol. 1997;56(10):1098–1104. doi: 10.1097/00005072-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Huang L, Jiang T, Yuan F, et al. Correlation of chromosomes 1p and 19q status and expressions of O6-methylguanine DNA methyltransferase (MGMT), p53 and Ki-67 in diffuse gliomas of World Health Organization (WHO) grades II and III: a clinicopathological study. Neuropathol Appl Neurobiol. 2009;35(4):367–379. doi: 10.1111/j.1365-2990.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- 28.Qu M, Olofsson T, Sigurdardottir S, et al. Genetically distinct astrocytic and oligodendroglial components in oligoastrocytomas. Acta Neuropathol. 2007;113(2):129–136. doi: 10.1007/s00401-006-0142-0. [DOI] [PubMed] [Google Scholar]
- 29.Shamsara J, Sharif S, Afsharnezhad S, et al. Association between MGMT promoter hypermethylation and p53 mutation in glioblastoma. Cancer Invest. 2009;27(8):825–829. doi: 10.1080/07357900902783211. [DOI] [PubMed] [Google Scholar]
- 30.Komine C, Watanabe T, Katayama Y, Yoshino A, Yokoyama T, Fukushima T. Promoter hypermethylation of the DNA repair gene O6-methylguanine-DNA methyltransferase is an independent predictor of shortened progression free survival in patients with low-grade diffuse astrocytomas. Brain Pathol. 2003;13(2):176–184. doi: 10.1111/j.1750-3639.2003.tb00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe T, Katayama Y, Yoshino A, et al. Aberrant hypermethylation of p14ARF and O6-methylguanine-DNA methyltransferase genes in astrocytoma progression. Brain Pathol. 2007;17(1):5–10. doi: 10.1111/j.1750-3639.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groenendijk FH, Taal W, Dubbink HJ, et al. MGMT promoter hypermethylation is a frequent, early, and consistent event in astrocytoma progression, and not correlated with TP53 mutation. J Neurooncol. 2011;101(3):405–417. doi: 10.1007/s11060-010-0274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Li S, Chen L, et al. Glioblastoma with an oligodendroglioma component: distinct clinical behavior, genetic alterations, and outcome. Neuro Oncol. 2012;14(4):518–525. doi: 10.1093/neuonc/nor232. [DOI] [PMC free article] [PubMed] [Google Scholar]