Abstract
Medulloblastoma (MB) is the most frequent malignant brain tumor in children. Patients with MB who are classified as having high-risk disease or those with recurrent disease respond poorly to current therapies and have an increased risk of MB-related mortality. Preclinical studies and molecular profiling of MB tumors have revealed upregulation or activation of several key signaling pathways such as the sonic hedgehog and WNT pathways. Although the exact mechanisms underlying MB tumorigenesis remain poorly understood, inhibiting these key pathways with molecularly targeted therapies represents an important approach to improving MB outcomes. Several molecularly targeted therapies are already under clinical investigation in MB patients. We discuss current preclinical and clinical data, as well as data from clinical trials of targeted therapies that are either ongoing or in development for MB.
Keywords: hedgehog, medulloblastoma, oncogenic signaling, smoothened, targeted therapy
urrent treatments can cure a majority of patients diagnosed with medulloblastoma (MB). However, these therapies are associated with significant long-term toxicities. Furthermore, patients with high-risk disease or recurrent tumor face a paucity of effective therapies. Preclinical studies have revealed the potential for treatment of MB with molecularly targeted therapies. This review synthesizes the preclinical and clinical data to date that support the use of targeted therapies as a novel treatment strategy in patients with high-risk or recurrent MB.
MB is a tumor of still-uncertain etiology that arises in the posterior fossa.1 It is the most common malignant brain tumor in children aged <4 years, and comprises approximately 12% of all childhood brain and central nervous system (CNS) tumors.2 According to the World Health Organization (WHO), there are 4 major histologic variants of MB: classic, desmoplastic/nodular, MB with extensive nodularity, and anaplastic/large-cell. Each is associated with a distinct morphology, age of onset, and prognosis. Other histologic features present in multiple variants can include MB with myogenic differentiation and MB with melanotic differentiation.3,4 More recent data suggest that MBs are comprised of at least 4 distinct subgroups based on gene expression.5
The current standard of care for patients with MB aged ≥3 years involves surgery followed by craniospinal radiation and chemotherapy.6,7 Various combination chemotherapy regimens, administered with or following treatment with craniospinal radiotherapy, have proven effective in patients with newly diagnosed MB.1 In the recurrent setting, a combination of surgery, reirradiation, and/or chemotherapy with or without autologous stem-cell rescue have demonstrated efficacy.6 Treatment regimens for recurrent disease include high-dose chemotherapy, bevacizumab, irinotecan, temozolomide (TMZ), and/or etoposide, metronomic chemotherapy, and molecularly targeted agents (reviewed in Aguilera et al.8).
In infants and young children, radiation therapy is rarely used because of the risk of long-term neurocognitive deficits, the severity of which inversely correlates with patient age at the time of treatment.7 Postoperative multiagent chemotherapy followed by intraventricular methotrexate has proven effective in children aged <3 years9 and <4 years,10 particularly in patients with nonmetastatic disease and in patients with desmoplastic/nodular MB.9,10 Furthermore, neurocognitive function appeared to be less affected in children treated with this chemotherapy regimen, compared with children treated with radiotherapy following standard chemotherapy (ie, without intraventricular methotrexate).9,11 Radiotherapy is used as a salvage regimen in patients who relapse following chemotherapy.9,10
Five-year event-free survival rates for patients with high-risk MB are >60% and can be >80% in patients with standard-risk disease.1,2 However, patients at a high risk of recurrence (aged <3 years, with significant residual disease following surgery, large-cell/anaplastic MB, or metastatic disease) have lower survival rates.1,12–14 In addition, long-term control in patients with recurrent disease is difficult to achieve.1,12 Neurocognitive sequelae in MB survivors is one of the most devastating side effects of current treatments. This is most significant in young patients who are treated with craniospinal radiation.1,7 Considering the lack of a salvage therapy that is clearly effective and durable for patients with recurrent disease, it is clear that novel therapies are needed for patients with MB.
Signaling Pathways in MB
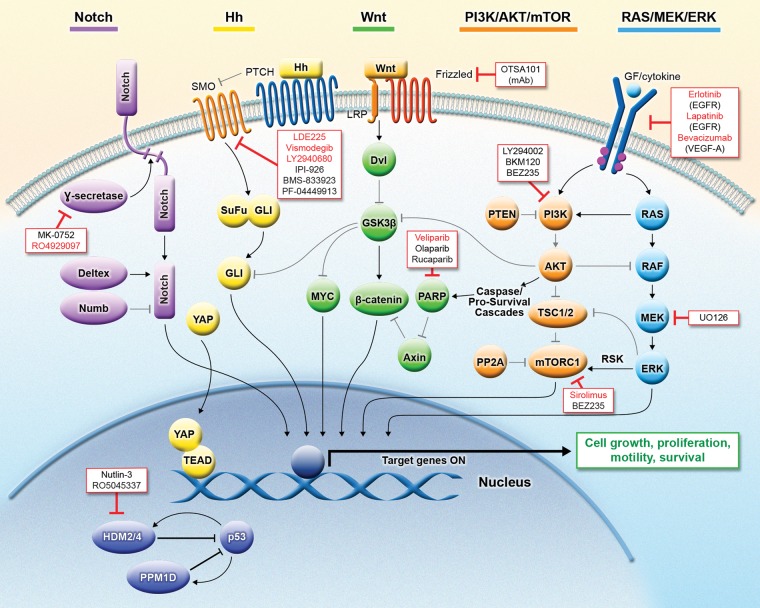
Based on data from numerous transcriptional profiling studies,15–18 a consensus was determined that described at least 4 distinct molecular subgroups of MB.5 The 4 groups: WNT [group 1], sonic hedgehog [Hh; group 2], group 3, and group 4, are distinguished by demographics, histology, DNA copy-number aberrations, and outcome.5 Molecular profiling and independent studies have identified the hedgehog (Hh) and WNT pathways, among others, as potential molecular targets in MB19,20 (Fig. 1) and have sparked numerous preclinical studies of molecularly targeted therapies in models of MB (Table 1). The molecular pathogenesis of groups 3 and 4 MBs is not well understood. Further studies are required to elucidate the key signaling pathways involved in their pathogenesis.
Fig. 1.
Molecular signaling pathways implicated in MB and targeted therapies under investigation for the treatment of MB. Several key signaling pathways—including Notch, Hh, WNT, PI3K/AKT/mTOR, RAS/MEK/ERK, and p53—have been implicated in the tumorigenesis and/or maintenance of MB. Numerous agents that target these pathways are being developed and investigated in clinical trials. A subset of these agents (shown in red) is currently being investigated in clinical studies of MB. Abbreviations: AKT, Ak mouse thymoma; Dvl, disheveled; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; GF, growth factor; Gli, glioma-associated oncogene; GSK3b, glycogen synthase kinase 3 beta; Hh, hedgehog; mAb, monoclonal antibody; MB, medulloblastoma; HDM2/4, p53 E3 ubiquitin protein ligase (human homologue of MDM2/4); MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; MYC, v-myc myelocytomatosis viral oncogene homolog (avian); PARP, poly (ADP-ribose) polymerase; PI3K, phosphoinositide 3-kinase; PP2A, protein phosphatase 2A; PPM1D, p53-induced protein phosphatase, Mg2+/Mn2+ dependent, 1D; PTCH, patched; PTEN, phosphatase and tensin homolog; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma; RSK, ribosomal protein S6 kinase; SMO, smoothened; SuFu, suppressor of fused; TEAD, transcription enhancer and activator domain; TSC1/2, tuberous sclerosis 1/2; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; Wnt, Wingless and int; YAP, Yes-associated protein.
Table 1.
Preclinical evidence for inhibition of key signaling pathways implicated in medulloblastoma
| Signaling Pathway | Neoplastic Effects in MB | Inhibitor (target) | Preclinical Effects of Signaling Inhibition in MB | References |
|---|---|---|---|---|
| Hedgehog/SMO |
|
HhAntag (SMO); GDC-0449 (SMO); LDE225 (SMO); IPI-926 (SMO); PF-5274857 (SMO) |
|
5,24-29 |
| WNT/β-catenin/PARP |
|
Rucaparib (PARP) |
|
35–37,40 |
| PI3K/AKT/mTOR |
|
LY294002 (PI3K); |
|
46 |
| YM024 (PI3K p110α); PIK-75 (PI3K p110α); |
|
43 | ||
| AS-252424 (PI3K p110γ) |
|
44 | ||
| mTOR |
|
BEZ235 (PI3K-mTOR); BKM120 (PI3K); RAD001 (mTOR); |
|
27,51,54 |
| Sorafenib (multitargeted inhibitor of mTOR) + valproate or vorinostat (HDAC inhibitors); |
|
107 | ||
| Rapamycin (mTOR); PP242 (mTORC1/2); |
|
108 | ||
| OSU-03012 (PDK1) alone or in combination with temsirolimus (mTOR); |
|
109 | ||
| OSU-03012 (PDK1) ± LY294002 (PI3K) |
|
109 | ||
| RAS/MEK/ERK |
|
U0126 (MEK) |
|
60, 61 |
| PDGFR |
|
Imatinib (PDGFR); |
|
110 |
| Sunitinib (PDGFR); |
|
111 | ||
| Tandutinib (PDGFRA) |
|
112 | ||
| IGF-1R |
|
NVP-ADW742 (IGF-1R) + TMZ |
|
113 |
| EGFR/HER2 |
|
AEE788 (HER1/2 and VEGFR1/2); |
|
114 |
| Gefitinib (EGFR) |
|
115 | ||
| VEGF/VEGFR |
|
Commercially available inhibitor VEGF V1 (VEGF-dependent angiogenesis via NRP-1 inhibition) |
|
116 |
| p53/PPM1D/HDM2 |
|
Nutlin-3a (HDM2); CCT007093 (PPM1D) |
|
71 |
| Notch |
|
γ-secretase inhibitor (Notch) |
|
74,75 |
| COX-2 |
|
Celecoxib (COX-2) |
|
82,83 |
Abbreviations: CCND1, cyclin D1; COX-2, cyclooxygenase-2; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; HDAC, histone deacetylase; HDM2/4, p53 E3 ubiquitin protein ligase (human homologue of MDM2/4); HER2, human epidermal growth factor receptor 2; IGF-1R, insulin-like growth factor 1 receptor; MB, medulloblastoma; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; NRP-1, neuropilin-1; PAK1, p21-activated kinase 1; PARP, poly(ADP-ribose) polymerase; PDGFR, platelet-derived growth factor receptor; PDK1, phosphoinositide-dependent kinase 1; PI3K, phosphoinositide 3-kinase; PPM1D, p53-induced protein phosphatase, Mg2+/Mn2+ dependent, 1D; SMO, smoothened; TMZ, temozolomide; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Hh/Smoothened
The Hh pathway is critical for cell proliferation, differentiation, and patterning during early embryonic development and for tissue homeostasis in adults.21,22 Together with insulin-like growth factor signaling, Hh signaling can drive formation of MBs in vivo.23 Several Hh pathway inhibitors that target the transmembrane receptor smoothened (SMO) have demonstrated antitumor activity in MB in vivo. The SMO inhibitors HhAntag, vismodegib (GDC-0449), LDE225, IPI-926, and PF-5274857 each reduced tumor growth significantly and increased survival in several mouse models of MB,24–29 suggesting that the Hh pathway is required for maintenance of MB tumor growth.
Recent work suggests that components of the Hh signaling pathway may cross talk with other pathways, such as Hippo, to promote MB growth and/or treatment resistance.30,31 The Hippo pathway plays an important role in the control of organ development.32 Its downstream effector, Yes-associated protein (YAP), is an oncoprotein, which is normally inactivated by Hippo signaling.33 Conversely, Hh signaling promotes expression and activation of YAP. In fact, in the absence of Hh, ectopic YAP promotes proliferation of cerebellar granule neuron precursors, one of the cells of origin of MB. Furthermore, expression of YAP was shown to be amplified in 3% of human MB samples.30 Evidence from mouse models further suggests that YAP promotes resistance to radiation therapy.31 This suggests an important mechanism of treatment resistance in YAP high-expressing, Hh-activated MBs.
WNT/β-Catenin
The WNT/β-catenin pathway is involved in cell proliferation, differentiation, cell polarity, and migration during embryogenesis and in tissue homeostasis in adults.34 WNT pathway genes and β-catenin, the main effector of the WNT pathway, are overexpressed in MB and are associated with favorable patient prognosis.35,36 Similarly, expression of the poly (ADP-ribose) polymerase (PARP) enzyme has been observed in tumor samples from patients with MB compared with those with normal brain tissue; however, PARP expression is associated with poor prognosis.37 Thus, the majority of WNT pathway inhibitors developed to date target PARP and lead to the destruction of β-catenin.38,39 The PARP inhibitor rucaparib (AG-014699) enhanced TMZ-induced tumor growth delay in human MB xenografts.40 Dickkopf homolog 1 (DKK1), which negatively regulates the WNT pathway, was found to be downregulated in MB patient samples and primary MB cell cultures. In vitro expression of DKK1 in MB cells suppressed tumor growth and induced apoptosis. In addition, DKK1 upregulation was observed following treatment with a histone deacetylase (HDAC) inhibitor,41 suggesting that DKK1 is silenced during MB tumorigenesis.
PI3K/AKT/mTOR
The phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is involved in functions such as cell growth, motility, survival, and angiogenesis,42 and several PI3K isoforms are upregulated in MB tumors.43–45 Mutations and allelic loss in phosphatase and tensin homolog (PTEN), a negative regulator of the PI3K pathway, have been identified in MBs; reduced PTEN expression (sometimes associated with promoter hypermethylation) is common in MB cell lines, mouse models of MB, and tumor samples.46–48 In addition, activation of receptor tyrosine kinases such as insulin-like growth factor 1 receptor and human epidermal growth factor receptor 2 (HER2)/ERBB2, which both lie upstream of and activate PI3K, has been observed in MB.49
Treatment with LY294002, a PI3K small-molecule inhibitor, caused a significant reduction in cell growth of MB cell lines, which was reversed upon expression of a constitutively activated form of AKT.46 Similarly, RNA interference–mediated downregulation of p110α reduced growth, increased apoptosis, and inhibited migration of MB cells.43 In addition to its role in driving neoplastic growth in vitro, PI3K signaling is upregulated in MB tumors resistant to SMO inhibitors in vivo. In a mouse model of MB, inhibition of PI3K signaling with the PI3K inhibitor BKM120 or the dual PI3K/mTOR inhibitor BEZ235 led to a significant delay in development of resistance to SMO inhibition,27 suggesting that dual inhibition of PI3K and SMO could circumvent or delay MB tumor resistance. Consistent with these findings, PI3K activation drove the survival of MB stem cells following radiation in vivo.48
In addition to canonical signaling, signaling through common downstream targets between pathways appears to play an important neoplastic role in MB. The PI3K/AKT/mTOR, WNT, and Hh pathways can each inactivate glycogen synthase kinase 3 beta (GSK-3β), which in turn induces MYC upregulation and protein stabilization.49,50 Data suggest that any of the PI3K/AKT/mTOR, WNT, or Hh pathways can inactivate GSK-3β, an important negative regulator of MYC, resulting in upregulation and stabilization of MYC protein. Consistent with the neoplastic role of MYC, data from a recent report demonstrated that cerebellar cells overexpressing MYC together with a dominant-negative form of p53 had a similar molecular profile to that of human MB and that these tumors were dependent on PI3K signaling.51 The hepatocyte growth factor (HGF)/scatter factor-c-MET pathway also signals through activation of MYC.52 HGF and its receptor c-MET are strongly expressed in MB, particularly the large-cell MB subtype, and are associated with poor prognosis.53 HGF/c-MET-stimulated MYC signaling is mediated in part by mitogen-activated protein kinase kinase (MEK) and PI3K and results in cell cycle progression and proliferation.52 Together, these data demonstrate that multiple oncogenic signaling pathways can converge on common intracellular molecular effectors, which are excellent candidates for inhibition using molecularly targeted therapies.
RAS/MEK/ERK
Growth factor stimulation of the RAS/MEK/extracellular signal-regulated kinase (ERK) pathway has been observed in MBs, particularly classical MBs. Moreover, expression of ERK is associated with a favorable prognosis.54,55 Activation of ERK was shown to activate mTOR and downregulate protein phosphatase 2A.54,56 Data thus far suggest that ERK is a common downstream target of epidermal growth factor receptor (EGFR), RAF, and the chemokine receptor CXCR4,54,56 which is upregulated in the SHH group of MB tumors.57 In addition, the EGFR family member HER2/neu was found to be overexpressed in a subset of tumors from patients with MB, which has been correlated with poor patient outcome.58,59
Increased ERK and platelet-derived growth factor receptor alpha (PDGFR-α) signaling have been observed in tumor samples from patients with metastatic MB.60 PDGF-dependent MB cell migration was shown to be dependent on ERK-mediated activation of p21-activated kinase 1 (PAK1). Tissue microarray analysis of MB samples demonstrated that PAK1 is overexpressed in over 50% of MB tumor samples and is associated with unfavorable outcomes. Treatment of MB cells with the MEK/ERK inhibitor U0126 abolished PAK1 activation and PAK1-dependent cell migration,61 suggesting a role for ERK in migration of MB cells.
p53
One-third of MBs exhibit gain of the long arm of chromosome 17 (17q) and isochromosome 17q.62,63 p53, on 17p13, is the most frequently inactivated gene in human cancers. However, it is only mutated in approximately 10% of MBs, and its impact on survival is controversial.64–68 Evidence suggests that p53 signaling is abnormal, especially in aggressive histologic subtypes of MB. A recent study identified focal amplification of the p53-inactivating genes PPM1D and MDM4 in SHH MBs.69 We have previously demonstrated increased expression of PPM1D in non-WNT MBs.70,71 Recent publications demonstrate evidence of cross-talk between PPM1D and Hh pathways and suggest a role for targeting PPM1D in Hh-active tumors.72,73
Additional Pathways and Processes
The Notch signaling pathway, which is important for the specification, proliferation, and survival of neural precursors, has also been implicated in MB tumorigenesis.74 In MB cell lines, inhibition of the Notch pathway with γ-secretase inhibitors led to cell cycle exit, apoptosis of stem-like cells, and neuronal differentiation.75 γ-secretase inhibition may also be an effective therapy for patients with MB with spinal metastasis. Inhibition of γ-secretase blocked the proteolytic processing of the p75 neurotrophin receptor, which in turn reduced MB cell migration and invasion in vitro and in vivo.76
Targeting global cellular processes may be another approach for the treatment of MB. For example, because many tumor suppressor genes are epigenetically silenced in MB,77,78 HDAC inhibitors have become an area of interest in MB research.79 In addition, the proteasome inhibitor bortezomib is being evaluated and has demonstrated promising activity in vitro and in vivo.80,81
Therapies using an antiangiogenic approach are currently in various stages of development. Angiogenic targets include vascular endothelial growth factor receptor (VEGFR), PDGFR, and the Notch protein. Similarly, the cyclooxygenase 2 (COX-2) protein is overexpressed in MB and constitutes a potentially valuable therapeutic target, as COX-2 inhibition demonstrates activity against human MB xenograft tumors in vivo.82,83
Clinical Investigation of Targeted Therapies in MB
Hh/SMO
Multiple ongoing clinical trials are evaluating molecularly targeted agents in patients with MB (Table 2), including several trials with SMO inhibitors. To date, data from clinical trials in MB are only available for vismodegib and LDE225. Vismodegib is currently under evaluation in 5 clinical trials in patients with MB, either as monotherapy (NCT01239316, NCT00939484, NCT00822458) or in combination with TMZ (NCT01601184) or maintenance chemotherapy (NCT01878617). Preliminary data from a phase 1 study in pediatric patients with recurrent or refractory MB demonstrated that vismodegib was well tolerated, with 1 grade 3 elevation of γ-glutamyl transpeptidase (GGT) at a vismodegib dose of 170 mg/m2 and no grade 4 toxicities. Efficacy data from this trial are not yet publicly available. However, among 13 patients with treatment-refractory MB and confirmed Hh pathway activation, 1 patient progressed after 6 months of therapy with oral vismodegib. Another patient remained on study and was progression-free after 391 days of follow-up.84 In a phase 1 study of vismodegib in adults with advanced solid tumors, one patient with metastatic MB achieved a partial response (PR), but relapsed after approximately 3 months.85,86
Table 2.
Ongoing clinical trials of targeted agents in MB
| Agent | Target(s) | Phase: Statusa | Study Population (Nplanned) | Regimen | NCT ID |
|---|---|---|---|---|---|
| GDC-0449 (vismodegib) | SMO | 2: ANR | Adult pts with R/R MB (n = 50) | GDC-0449 | NCT00939484 |
| 2: CR | Pts aged 3–21 years with R/R MB (n = 50) | GDC-0449 | NCT01239316 | ||
| 1/2: CR | Adult pts with MB with activated SHH (n = 38) | TMZ ± GDC-0449 | NCT01601184 | ||
| 1: ANR | Pts aged 3–21 years with R/R MB (n = 30) | GDC-0449 | NCT00822458 | ||
| 2: CR | Pts aged 3–21 years with newly diagnosed MB stratified based on clinical risk and molecular subgroup (WNT, SHH, non-WNT/non-SHH) (n = 350) | For SHH group: craniospinal radiation and chemotherapy (cisplatin, vincristine, cyclophosphamide) followed by GDC-0449 + maintenance chemotherapy | NCT01878617 | ||
| LDE225 | SMO | 3: CR | Pediatric or adult pts with Hh-activated, relapsed MB (n = 109) | LDE225 or TMZ | NCT01708174 |
| 1/2: CR | Pediatric or adult pts with R/R MB (n = 91) | LDE225 | NCT01125800 | ||
| 1: ANR | Adult pts with advanced solid tumors, including MB (n = 100) | LDE225 | NCT00880308 | ||
| 1: CR | East Asian adult pts with advanced solid tumors, including MB (n = 44) | LDE225 | NCT01208831 | ||
| LEQ506 | SMO | 1: ANR | Adult pts with advanced solid tumors, including R/R MB (n = 71) | LEQ506 | NCT01106508 |
| Veliparib | PARP | 1: ANR | Pts aged ≤ 21 years with R/R CNS tumors, including MB (n = 24) | ABT-888 + TMZ | NCT00946335 |
| Sirolimus | mTOR | 1: CR | Pediatric or adult pts aged ≤30 years with R/R solid tumors, including MB (n = 24) | Sirolimus + daily celecoxib, plus low-dose etoposide alternating with cyclophosphamide | NCT01331135 |
| Antiangiogenic agents, including bevacizumab | VEGF, VEGFR | 2: CR | Pts aged ≤21 years with R/R MB or CNS primitive neuroectodermal tumors (n = 108) | TMZ + irinotecan hydrochloride ± BEV | NCT01217437 |
| 2: ANR | Pts aged ≤21 years with R/R or progressive malignant gliomas, diffuse/intrinsic brainstem gliomas, MB, ependymomas, or low-grade gliomas (n = 140) | BEV + irinotecan | NCT00381797 | ||
| 2: CR | Pediatric pts with recurrent or progressive MB (n = 40) | BEV + intrathecal etoposide and cytarabine + thalidomide, celecoxib, and fenofibric acid, with alternating cycles of daily low-dose etoposide and cyclophosphamide | NCT01356290 | ||
| 1: CR | Pts aged 18 months to 23 years with R/R CNS tumors, including MB (n = 30) | BEV + irinotecan + TMZ | NCT00876993 | ||
| Vorinostat | HDAC | 1: CR | Pediatric pts aged ≤ 4 years treated with surgery for embryonal CNS tumors, including MB (n = 62) | Vorinostat, isotretinoin + combination chemotherapy | NCT00867178 |
Abbreviations: ANR, active, not recruiting; BEV, bevacizumab; CNS, central nervous system; CR, currently recruiting; GD2, ganglioside G2; HDAC, histone deacetylase; Hh, hedgehog; ID, identifier; mAb, monoclonal antibody; MB, medulloblastoma; mTOR, mammalian target of rapamycin; NCT, national clinical trial; PARP, poly(ADP-ribose) polymerase; pts, patients; R/R, recurrent/refractory; SHH, sonic hedgehog; SMO, smoothened; TMZ, temozolomide; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
aCurrent status of each trial based on a search of ClinicalTrials.gov conducted on July 26, 2013.
LDE225 is currently under investigation as a monotherapy in pediatric and adult patients with recurrent or refractory MB, or other tumors (NCT01125800). LDE225 was well tolerated and showed antitumor activity (complete responses) in 2 of 24 pediatric patients with MB.87 In a phase 1 study of LDE225 in adults with advanced solid tumors, tumor responses were observed in 2 patients with MB (1 PR, 1 metabolic PR).88 All patients from the pediatric and adult studies who responded (complete or partial response) to LDE225 treatment were found to have Hh pathway–activated tumors as determined by a 5-gene Hh signature assay.89 Several additional trials are currently ongoing, including a phase 3 trial of LDE225 in patients with Hh-activated, relapsed MB (NCT01708174) and a phase 1 trial of the SMO inhibitor LEQ506 in adult patients with advanced solid tumors, including MB (NCT01106508).
Resistance to SMO-dependent Hh pathway inhibitors through acquired mutations in SMO, or amplification of the Hh pathway transcription factor glioma-associated oncogene homologue 2 (Gli2) and the Hh target gene CCND1, has been observed in preclinical mouse MB models and was determined to be the cause of relapse in the patient with MB described above who initially responded to vismodegib treatment.27,90,91 The frequency and clinical relevance of this resistance will be realized as results from ongoing trials become available. Several preclinical studies have identified potential mechanisms to overcome this resistance, including combination with PI3K/mTOR inhibitors or arsenic and itraconazole.27,92,93 A phase 1 trial testing the combination of LDE225 and BKM120 in patients with advanced solid tumors is currently recruiting (NCT01576666).
WNT/β-Catenin
Several agents targeting the WNT pathway in pediatric patients with CNS tumors are being evaluated in clinical trials, including the PARP5/tankyrase inhibitors olaparib and veliparib (ABT-888). Veliparib plus TMZ is being evaluated in a phase 1 study in pediatric patients with recurrent or refractory CNS tumors (NCT00946335). A phase 1 study of veliparib plus radiation therapy in adult patients with brain metastasis is currently ongoing (NCT00649207). A phase 1 study of olaparib with TMZ in patients with relapsed glioblastoma is currently recruiting (NCT01390571). Agents targeting additional members of the WNT pathway include the porcupine inhibitor LGK974 (phase 1 trial in patients with WNT ligand–dependent malignancies, NCT01351103) and a radiolabeled monoclonal antibody against frizzled (phase 1 trial in patients with synovial sarcoma, NCT01469975).
PI3K/AKT/mTOR and RAS/MEK/ERK
Although inhibitors of PI3K and MEK are being tested in patients with CNS tumors, no clinical trials are currently evaluating these agents in patients with MB. In contrast, the mTOR inhibitor sirolimus is being evaluated in a phase 1 study in combination with celecoxib plus low-dose etoposide, alternating with cyclophosphamide in patients with relapsed or refractory solid tumors including MB (NCT01331135). Data from this trial have not yet been reported. A second mTOR inhibitor, everolimus, was well tolerated in a phase 1 study in pediatric patients with recurrent or refractory solid tumors; however, no objective responses were observed.94
EGFR
The EGFR inhibitors erlotinib and lapatinib are being investigated in trials in children with CNS tumors, but few results have been published to date. Ongoing trials are testing erlotinib in combination with chemotherapy in young patients with embryonal brain tumors, choroid plexus carcinoma, high-grade glioma, or ependymoma (NCT00602667). Erlotinib is also being tested in combination with radiation in young patients with refractory or relapsed CNS tumors, or in newly diagnosed brainstem glioma (NCT00360854). Data from a phase 1 study demonstrated that erlotinib, followed by combined erlotinib/TMZ, was well tolerated in children with recurrent or refractory solid tumors (NCT00077454).95 Similarly, data from a phase 2 study demonstrated the tolerability of single-agent lapatinib in pediatric patients with refractory/recurrent CNS tumors (NCT00095940). Although no objective responses were reported, disease stabilization was observed in 13 patients, including 1 patient with MB.96
Antiangiogenic Approaches
Angiogenesis inhibitors blocking VEGF or PDGF are also being tested in combination with chemotherapy or other targeted agents in patients with MB. Data from a recent study of patients (n = 16) with recurrent embryonal brain tumors, including MB (n = 7), who were treated with bevacizumab, thalidomide, celecoxib, fenofibrate, etoposide, and cyclophosphamide (NCT01356290) demonstrated favorable rates of event-free survival for patients with MB: 6-month, 12-month, and 24-month event-free survival rates were 100%, 85.7%, and 68.6%, respectively.97 Of the 5 patients with MB with long-term survival (> 12 months), 2 patients received radiotherapy following surgery (n = 1) or in combination with PEI chemotherapy (cisplatin, etoposide, ifosfamide; n = 1) for their most recent relapse before switching to the antiangiogenic regimen described above. Similarly, data from a phase 1 trial of pediatric patients and young adults with refractory solid tumors and leukemia (n = 19) treated with combined bevacizumab, sorafenib, and low-dose cyclophosphamide (NCT00665990) demonstrated that 5 of the 17 patients evaluable for tumor response achieved a PR (including 1 patient with MB), and 9 of 17 achieved stable disease (SD).98
Combined bevacizumab and irinotecan, a topoisomerase I inhibitor, was evaluated in a phase 2 study in young patients with recurrent, progressive, or refractory glioma, MB, ependymoma, or low-grade glioma (NCT00381797). This combination was well tolerated, but no MB efficacy data were reported.99 Early data from 2 patients in a phase 1 study of patients with relapsed/refractory CNS tumors (including MB) treated with bevacizumab plus TMZ and irinotecan (NCT00876993) demonstrated that this combination was well tolerated and was associated with favorable clinical activity: one patient achieved SD > 30 months (ongoing at the time of the report), and the other patient had a near-complete response lasting 18 months.100 A recently published follow-up study reported results in 9 patients treated with bevacizumab and irinotecan with or without TMZ. Six months after the start of salvage therapy, objective response rate was 55%, with 2 patients achieving PR and 3 achieving complete response. Additionally, 1 patient had SD, and 3 had PD. Two patients remain alive and progression-free at 15 and 55 months, and another is alive with stable disease at 20 months.8 Together, these data suggest that antiangiogenic agents such as bevacizumab, in combination with cytotoxic therapies, may provide marked clinical benefit in patients with MB, including patients with recurrent or refractory MB.
Targeting angiogenesis through the Notch pathway via γ-secretase inhibition is under evaluation in pediatric patients with CNS tumors, but limited efficacy data have been reported to date. Safety data from a dose-escalation study of the γ-secretase inhibitor MK-0752 in pediatric patients with refractory or recurrent CNS malignancies (n = 17), including MB (n = 2), demonstrated that although MK-0752 was well tolerated, it was associated with only modest efficacy.101,102 Future clinical development of this agent in MB remains uncertain. A study evaluating the γ-secretase inhibitor RO4929097 in young patients with relapsed/refractory solid tumors, CNS tumors (including MB), lymphoma, or T-cell leukemia is no longer recruiting (NCT01088763). Results have not yet been reported. Several additional trials using antiangiogenic regimens are currently ongoing.
Additional Pathways and Processes
Celecoxib, a COX-2 inhibitor, has demonstrated efficacy when combined with chemotherapy in pediatric patients with MB. In a pilot study of pediatric patients with relapsed MB (n = 4), celecoxib monotherapy or in combination with TMZ was associated with clinically stable disease or better in all 4 patients. One patient (who received the combination regimen) achieved an objective response, as confirmed by magnetic resonance imaging.103 Currently, celecoxib is being tested in combination with antiangiogenic agents in patients with recurrent or progressive MB (NCT01356290).97
The HDAC inhibitor vorinostat has been tested in several clinical trials in children with relapsed/refractory solid tumors. Vorinostat was generally well tolerated, both as a single agent and in combination with isotretinoin, in pediatric patients with recurrent/refractory solid tumors (including MB), lymphoma, or leukemia (NCT00217412).104 Data from 2 phase 1 studies in pediatric patients with relapsed/refractory CNS or solid tumors demonstrated that vorinostat was well tolerated when combined with either TMZ (NCT01076530; n = 19, 2 patients with MB)105 or bortezomib (NCT00994500; n = 23, 1 patient with MB).106 A phase 1 study testing the combination of vorinostat, isotretinoin, and chemotherapy in young patients with previous surgeries for an embryonal tumor is currently recruiting (NCT00867178).
Conclusions
For a subset of patients with MB, particularly the very young and those with recurrent disease, a significant need exists for novel therapies that provide improved clinical benefit with reduced toxicity, compared with existing treatments. Identifying risk status soon after diagnosis may help identify these patients and drive therapeutic decisions.36 Molecular profiling studies of primary tumor samples and preclinical studies using models of MB have identified several key signaling pathways that appear to be involved in the clinical development and maintenance of MB clinically. Inhibitors of these pathways have demonstrated antitumor activity in vitro and in vivo, and several are now being investigated in clinical trials in patients with MB or other CNS tumors. Although only a handful of studies have reported efficacy data in patients with MB, promising clinical activity has been observed with Hh inhibitor monotherapy and with combined antiangiogenic/chemotherapy regimens.
The identification of MB molecular subgroups and advances in molecular profiling techniques have incited a new era in the treatment of MB in which preselection of patients who may derive benefit from a particular targeted therapy is likely possible. Preselection of patients is critical given the toxicities associated with current therapies and potential toxicities associated with novel targeted therapies. Although this new era brings great promise, there are still many hurdles to overcome. In particular, due to the limited number of patients with MB, it may be challenging to conduct prospective studies that are sufficiently powered to determine if molecular profiling data can be used in clinical practice. Nevertheless, the potential for treating MB in the upfront and relapsed settings, using targeted agents based on molecular profiling, is encouraging. Thus, although the molecular mechanisms contributing to the pathogenesis of MB in pediatric and adult patients are not completely known, emerging clinical data from trials investigating these novel targeted agents will help improve the future landscape of available therapies for patients with high-risk and recurrent MB.
Funding
This work was supported by grants from the NIH/NCI (1R01CA172392-01), St. Baldrick's Foundation, CURE Childhood Cancer, Children's Healthcare of Atlanta's Center for Neurosciences Research, and Alex's Lemonade Stand Foundation, all to R.C.C. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
Conflict of interest statement. T.J.M. has acted as an advisor for Novartis Pharmaceuticals Corporation. D.A. and R.C.C. have no conflicts of interest.
Acknowledgments
The authors thank Jillian Brechbiel, PhD, Nicole Parker, PhD, and Karen Miller-Moslin, PhD, for medical editorial assistance with this manuscript. Financial support for editorial assistance was provided by Novartis Pharmaceuticals Corporation.
References
- 1.Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1(3):232–250. doi: 10.1215/15228517-1-3-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CBTRUS. (2012) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008. Available at http://www.cbtrus.org/ . Accessed September 2012.
- 3.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polkinghorn WR, Tarbell NJ. Medulloblastoma: tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat Clin Pract Oncol. 2007;4(5):295–304. doi: 10.1038/ncponc0794. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers. Fort Washington, PA: National Comprehensive Cancer Network; 2012. [Google Scholar]
- 7.Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol. 2008;65(11):1419–1424. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 8.Aguilera D, Mazewski C, Fangusaro J, et al. Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: a multi-institutional experience. Childs Nerv Syst. 2013;29(4):589–596. doi: 10.1007/s00381-012-2013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 10.von Bueren AO, von Hoff K, Pietsch T, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol. 2011;13(6):669–679. doi: 10.1093/neuonc/nor025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutkowski S, Gerber NU, von Hoff K, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol. 2009;11(2):201–210. doi: 10.1215/15228517-2008-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 13.Packer RJ, Sutton LN, Rorke LB, et al. Prognostic importance of cellular differentiation in medulloblastoma of childhood. J Neurosurg. 1984;61(2):296–301. doi: 10.3171/jns.1984.61.2.0296. [DOI] [PubMed] [Google Scholar]
- 14.Caputy AJ, McCullough DC, Manz HJ, Patterson K, Hammock MK. A review of the factors influencing the prognosis of medulloblastoma. The importance of cell differentiation. J Neurosurg. 1987;66(1):80–87. doi: 10.3171/jns.1987.66.1.0080. [DOI] [PubMed] [Google Scholar]
- 15.Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 19.Parsons DW, Li M, Zhang X, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331(6016):435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 22.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805(2):181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Sonic Hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23(36):6156–6162. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 24.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Lee MJ, Hatton BA, Villavicencio EH, et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci U S A. 2012;109(20):7859–7864. doi: 10.1073/pnas.1114718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohner A, Spilker ME, Lam JL, et al. Effective targeting of Hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective Smoothened antagonist that penetrates the blood-brain barrier. Mol Cancer Ther. 2012;11(1):57–65. doi: 10.1158/1535-7163.MCT-11-0691. [DOI] [PubMed] [Google Scholar]
- 27.Buonamici S, Williams J, Morrissey M, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19(19):5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 29.Wong H, Alicke B, West K, et al. PK/PD analysis of vismodegib in preclinical models of mutational and ligand-dependent hedgehog pathway activation. Clin Cancer Res. 2011;17(14):4682–4692. doi: 10.1158/1078-0432.CCR-11-0975. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-L A, Northcott PA, Dalton J, et al. YAP1 is amplified and up-regulated in Hedgehog-associated medulloblastomas and mediates Sonic Hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23(23):2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-L A, Squatrito M, Northcott P, et al. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31(15):1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grumolato L, Liu G, Mong P, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24(22):2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121(3):381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29(11):1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Vuurden DG, Hulleman E, Meijer OL, et al. PARP inhibition sensitizes childhood high grade glioma, medulloblastoma and ependymoma to radiation. Oncotarget. 2011;2(12):984–996. doi: 10.18632/oncotarget.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1(7):563–577. doi: 10.18632/oncotarget.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ClinicalTrials.gov. Available at http://www.clinicaltrials.gov/ . Accessed September 2012.
- 40.Daniel RA, Rozanska AL, Mulligan EA, et al. Central nervous system penetration and enhancement of temozolomide activity in childhood medulloblastoma models by poly(ADP-ribose) polymerase inhibitor AG-014699. Br J Cancer. 2010;103(10):1588–1596. doi: 10.1038/sj.bjc.6605946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vibhakar R, Foltz G, Yoon JG, et al. Dickkopf-1 is an epigenetically silenced candidate tumor suppressor gene in medulloblastoma. Neuro Oncol. 2007;9(2):135–144. doi: 10.1215/15228517-2006-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerreiro AS, Fattet S, Fischer B, et al. Targeting the PI3K p110alpha isoform inhibits medulloblastoma proliferation, chemoresistance, and migration. Clin Cancer Res. 2008;14(21):6761–6769. doi: 10.1158/1078-0432.CCR-08-0385. [DOI] [PubMed] [Google Scholar]
- 44.Guerreiro AS, Fattet S, Kulesza DW, et al. A sensitized RNA interference screen identifies a novel role for the PI3K p110gamma isoform in medulloblastoma cell proliferation and chemoresistance. Mol Cancer Res. 2011;9(7):925–935. doi: 10.1158/1541-7786.MCR-10-0200. [DOI] [PubMed] [Google Scholar]
- 45.Boller D, Doepfner KT, DE Laurentiis A, et al. Targeting PI3KC2beta impairs proliferation and survival in acute leukemia, brain tumours and neuroendocrine tumours. Anticancer Res. 2012;32(8):3015–3027. [PubMed] [Google Scholar]
- 46.Hartmann W, Digon-Sontgerath B, Koch A, et al. Phosphatidylinositol 3′-kinase/AKT signaling is activated in medulloblastoma cell proliferation and is associated with reduced expression of PTEN. Clin Cancer Res. 2006;12(10):3019–3027. doi: 10.1158/1078-0432.CCR-05-2187. [DOI] [PubMed] [Google Scholar]
- 47.Castellino RC, Barwick BG, Schniederjan M, et al. Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma. PLoS One. 2010;5(5):e10849. doi: 10.1371/journal.pone.0010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22(4):436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baryawno N, Sveinbjornsson B, Kogner P, Johnsen JI. Medulloblastoma: a disease with disorganized developmental signaling cascades. Cell Cycle. 2010;9(13):2548–2554. doi: 10.4161/cc.9.13.12170. [DOI] [PubMed] [Google Scholar]
- 50.Kenney AM, Widlund HR, Rowitch DH. Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development. 2004;131(1):217–228. doi: 10.1242/dev.00891. [DOI] [PubMed] [Google Scholar]
- 51.Pei Y, Moore CE, Wang J, et al. An animal model of MYC-driven medulloblastoma. Cancer Cell. 2012;21(2):155–167. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Guessous F, Johnson EB, et al. Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab Invest. 2008;88(2):98–111. doi: 10.1038/labinvest.3700702. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Lal B, Kwon S, et al. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005;65(20):9355–9362. doi: 10.1158/0008-5472.CAN-05-1946. [DOI] [PubMed] [Google Scholar]
- 54.Wlodarski PK, Boszczyk A, Grajkowska W, Roszkowski M, Jozwiak J. Implication of active Erk in the classic type of human medulloblastoma. Folia Neuropathol. 2008;46(2):117–122. [PubMed] [Google Scholar]
- 55.Jozwiak J, Sontowska I, Bikowska B, Grajkowska W, Galus R, Roszkowski M. Favourable prognosis in medulloblastoma with extensive nodularity is associated with mitogen-activated protein kinase upregulation. Folia Neuropathol. 2011;49(4):257–261. [PubMed] [Google Scholar]
- 56.Wlodarski P, Grajkowska W, Lojek M, Rainko K, Jozwiak J. Activation of Akt and Erk pathways in medulloblastoma. Folia Neuropathol. 2006;44(3):214–220. [PubMed] [Google Scholar]
- 57.Sengupta R, Dubuc A, Ward S, et al. CXCR4 activation defines a new subgroup of Sonic Hedgehog-driven medulloblastoma. Cancer Res. 2012;72(1):122–132. doi: 10.1158/0008-5472.CAN-11-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilbertson RJ, Pearson AD, Perry RH, Jaros E, Kelly PJ. Prognostic significance of the c-erbB-2 oncogene product in childhood medulloblastoma. Br J Cancer. 1995;71(3):473–477. doi: 10.1038/bjc.1995.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herms JW, Behnke J, Bergmann M, et al. Potential prognostic value of C-erbB-2 expression in medulloblastomas in very young children. J Pediatr Hematol Oncol. 1997;19(6):510–515. doi: 10.1097/00043426-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald TJ, Brown KM, LaFleur B, et al. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet. 2001;29(2):143–152. doi: 10.1038/ng731. [DOI] [PubMed] [Google Scholar]
- 61.Yuan L, Santi M, Rushing EJ, Cornelison R, MacDonald TJ. ERK activation of p21 activated kinase-1 (Pak1) is critical for medulloblastoma cell migration. Clin Exp Metastasis. 2010;27(7):481–491. doi: 10.1007/s10585-010-9337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biegel JA. Cytogenetics and molecular genetics of childhood brain tumors. Neuro Oncol. 1999;1(2):139–151. doi: 10.1215/15228517-1-2-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellison D. Classifying the medulloblastoma: insights from morphology and molecular genetics. Neuropathol Appl Neurobiol. 2002;28(4):257–282. doi: 10.1046/j.1365-2990.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 64.Saylors RL, 3rd, Sidransky D, Friedman HS, et al. Infrequent p53 gene mutations in medulloblastomas. Cancer Res. 1991;51(17):4721–4723. [PubMed] [Google Scholar]
- 65.Adesina AM, Nalbantoglu J, Cavenee WK. P53 gene mutation and Mdm2 gene amplification are uncommon in medulloblastoma. Cancer Res. 1994;54(21):5649–5651. [PubMed] [Google Scholar]
- 66.Tabori U, Baskin B, Shago M, et al. Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol. 2010;28(8):1345–1350. doi: 10.1200/JCO.2009.23.5952. [DOI] [PubMed] [Google Scholar]
- 67.Gessi M, von Bueren AO, Rutkowski S, Pietsch T. P53 expression predicts dismal outcome for medulloblastoma patients with metastatic disease. J Neurooncol. 2012;106(1):135–141. doi: 10.1007/s11060-011-0648-8. [DOI] [PubMed] [Google Scholar]
- 68.Pfaff E, Remke M, Sturm D, et al. TP53 mutation is frequently associated with CTNNB1 mutation or MYCN amplification and is compatible with long-term survival in medulloblastoma. J Clin Oncol. 2010;28(35):5188–5196. doi: 10.1200/JCO.2010.31.1670. [DOI] [PubMed] [Google Scholar]
- 69.Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castellino RC, De Bortoli M, Lu X, et al. Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J Neurooncol. 2008;86(3):245–256. doi: 10.1007/s11060-007-9470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buss MC, Read TA, Schniederjan MJ, Gandhi K, Castellino RC. HDM2 promotes WIP1-mediated medulloblastoma growth. Neuro Oncol. 2012;14(4):440–458. doi: 10.1093/neuonc/nos001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doucette TA, Yang Y, Pedone C, et al. WIP1 enhances tumor formation in a Sonic Hedgehog-dependent model of medulloblastoma. Neurosurgery. 2012;70(4):1003–1010. doi: 10.1227/NEU.0b013e31823e5332. discussion 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pandolfi S, Montagnani V, Penachioni JY, et al. WIP1 phosphatase modulates the Hedgehog signaling by enhancing GLI1 function. Oncogene. 2012;32(40):4737–4747. doi: 10.1038/onc.2012.502. [DOI] [PubMed] [Google Scholar]
- 74.Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that Notch signaling is critical for the growth and survival of Sonic Hedgehog-induced medulloblastomas. Cancer Res. 2004;64(21):7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 75.Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66(15):7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Cui M, Wang L, Chen X, Xin P. Inhibition of neurotrophin receptor p75 intramembran proteolysis by gamma-secretase inhibitor reduces medulloblastoma spinal metastasis. Biochem Biophys Res Commun. 2010;403(3–4):264–269. doi: 10.1016/j.bbrc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Lindsey JC, Lusher ME, Anderton JA, et al. Identification of tumour-specific epigenetic events in medulloblastoma development by hypermethylation profiling. Carcinogenesis. 2004;25(5):661–668. doi: 10.1093/carcin/bgh055. [DOI] [PubMed] [Google Scholar]
- 78.Briggs KJ, Corcoran-Schwartz IM, Zhang W, et al. Cooperation between the Hic1 and Ptch1 tumor suppressors in medulloblastoma. Genes Dev. 2008;22(6):770–785. doi: 10.1101/gad.1640908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonnemann J, Kumar KS, Heesch S, et al. Histone deacetylase inhibitors induce cell death and enhance the susceptibility to ionizing radiation, etoposide, and TRAIL in medulloblastoma cells. Int J Oncol. 2006;28(3):755–766. [PubMed] [Google Scholar]
- 80.Taniguchi E, Cho MJ, Arenkiel BR, et al. Bortezomib reverses a post-translational mechanism of tumorigenesis for patched1 haploinsufficiency in medulloblastoma. Pediatr Blood Cancer. 2009;53(2):136–144. doi: 10.1002/pbc.21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang F, Jove V, Chang S, et al. Bortezomib induces apoptosis and growth suppression in human medulloblastoma cells, associated with inhibition of AKT and NF-kB signaling, and synergizes with an ERK inhibitor. Cancer Biol Ther. 2012;13(6):349–357. doi: 10.4161/cbt.19239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baryawno N, Sveinbjornsson B, Eksborg S, et al. Tumor-growth-promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro Oncol. 2008;10(5):661–674. doi: 10.1215/15228517-2008-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen KH, Hsu CC, Song WS, et al. Celecoxib enhances radiosensitivity in medulloblastoma-derived CD133-positive cells. Childs Nerv Syst. 2010;26(11):1605–1612. doi: 10.1007/s00381-010-1190-2. [DOI] [PubMed] [Google Scholar]
- 84.Gajjar AJ, Stewart CF, Ellison DW, et al. A phase I pharmacokinetic trial of Sonic Hedgehog (SHH) antagonist GDC-0449 in pediatric patients with recurrent or refractory medulloblastoma: a Pediatric Brain Tumor Consortium study (PBTC 25) [abstract] J Clin Oncol. 2010;28(18_suppl):CRA9501. [Google Scholar]
- 85.Lorusso PM, Rudin CM, Reddy JC, et al. Phase I trial of Hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally-advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(12):2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geoerger B, Aerts I, Casanova M, et al. A Phase I/II study of LDE225, a Smoothened (Smo) antagonist, in pediatric patients with recurrent medulloblastoma (MB) or other solid tumors [abstract] J Clin Oncol. 2012;30(15_suppl):9519. [Google Scholar]
- 88.Tawbi HA, Rodon Ahnert J, Dummer R, et al. Phase I study of LDE225 in advanced solid tumors: updated analysis of safety, preliminary efficacy, and pharmacokinetic-pharmacodynamic correlation [abstract] J Clin Oncol. 2011;29(15_suppl):3062. [Google Scholar]
- 89.Amakye D, Robinson D, Rose K, et al. The predictive value of a 5-gene signature as a patient pre-selection tool in medulloblastoma for Hedgehog pathway inhibitor therapy [abstract] Cancer Res. 2012;72(8, Suppl 1):4818. [Google Scholar]
- 90.Yauch RL, Dijkgraaf GJ, Alicke B, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326(5952):572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dijkgraaf GJ, Alicke B, Weinmann L, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71(2):435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 92.Kim J, Lee JJ, Kim J, Gardner D, Beachy PA. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci U S A. 2010;107(30):13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23(1):23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fouladi M, Laningham F, Wu J, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25(30):4806–4812. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 95.Jakacki RI, Hamilton M, Gilbertson RJ, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium Study. J Clin Oncol. 2008;26(30):4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fouladi M, Stewart CF, Blaney SM, et al. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(27):4221–4227. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peyrl A, Chocholous M, Kieran MW, et al. Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer. 2012;59(3):511–517. doi: 10.1002/pbc.24006. [DOI] [PubMed] [Google Scholar]
- 98.Santana VM, Baker SD, McCarville B, et al. Phase I study of bevacizumab, sorafenib, and low-dose cyclophosphamide (CYC) in children and young adults with refractory solid tumors [abstract] J Clin Oncol. 2011;29(15_suppl):9500. [Google Scholar]
- 99.Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(18):3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aguilera DG, Goldman S, Fangusaro J. Bevacizumab and irinotecan in the treatment of children with recurrent/refractory medulloblastoma. Pediatr Blood Cancer. 2011;56(3):491–494. doi: 10.1002/pbc.22868. [DOI] [PubMed] [Google Scholar]
- 101.Fouladi M, Stewart CF, Olson J, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2011;29(26):3529–3534. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fouladi M, Olson J, Stewart CF, et al. A phase I trial of MK-0752 in children with recurrent or refractory CNS malignancies: a Pediatric Brain Tumor Consortium study [abstract] J Clin Oncol. 2010;28(15_suppl):9502. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milosevic J, Baryawno N, Sveinbjrnsson B, et al. The cyclooxygenase-2 prostaglandin E2 pathway is expressed in childhood medulloblastoma providing novel therapeutic targets as indicated by promising response to Celecoxib-therapy in vitro, in vivo and pilot clinical experience [abstract] Proc Am Assoc Cancer Res. 2009;AACR:3201. [Google Scholar]
- 104.Fouladi M, Park JR, Stewart CF, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children's Oncology Group phase I consortium report. J Clin Oncol. 2010;28(22):3623–3629. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hummel TR, Wagner LM, Ahern CH, et al. A pediatric phase I trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: a Children's Oncology Group Phase I Consortium Study [abstract] J Clin Oncol. 2011;29(15_suppl):9579. doi: 10.1002/pbc.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muscal JA, Thompson PA, Horton TM, et al. A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: A Children's Oncology Group phase I consortium study (ADVL0916) Pediatr Blood Cancer. 2013;60(3):390–395. doi: 10.1002/pbc.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang Y, Yacoub A, Hamed HA, et al. Sorafenib and HDAC inhibitors synergize to kill CNS tumor cells. Cancer Biol Ther. 2012;13(7):567–574. doi: 10.4161/cbt.19771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mohan AL, Friedman MD, Ormond DR, Tobias M, Murali R, Jhanwar-Uniyal M. PI3K/mTOR signaling pathways in medulloblastoma. Anticancer Res. 2012;32(8):3141–3146. [PubMed] [Google Scholar]
- 109.Baryawno N, Sveinbjornsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010;70(1):266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 110.Abouantoun TJ, MacDonald TJ. Imatinib blocks migration and invasion of medulloblastoma cells by concurrently inhibiting activation of platelet-derived growth factor receptor and transactivation of epidermal growth factor receptor. Mol Cancer Ther. 2009;8(5):1137–1147. doi: 10.1158/1535-7163.MCT-08-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abouantoun TJ, Castellino RC, MacDonald TJ. Sunitinib induces PTEN expression and inhibits PDGFR signaling and migration of medulloblastoma cells. J Neurooncol. 2011;101(2):215–226. doi: 10.1007/s11060-010-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ohshima-Hosoyama S, Davare MA, Prajapati SI, et al. Preclinical testing of tandutinib in a transgenic medulloblastoma mouse model. J Pediatr Hematol Oncol. 2012;34(2):116–121. doi: 10.1097/MPH.0b013e3182309fe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou H, Rao J, Lin J, et al. The insulin-like growth factor-I receptor kinase inhibitor NVP-ADW742 sensitizes medulloblastoma to the effects of chemotherapy. Oncol Rep. 2011;25(6):1565–1571. doi: 10.3892/or.2011.1233. [DOI] [PubMed] [Google Scholar]
- 114.Meco D, Servidei T, Zannoni GF, et al. Dual inhibitor AEE788 reduces tumor growth in preclinical models of medulloblastoma. Transl Oncol. 2010;3(5):326–335. doi: 10.1593/tlo.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meco D, Servidei T, Riccardi A, et al. Antitumor effect in medulloblastoma cells by gefitinib: ectopic HER2 overexpression enhances gefitinib effects in vivo. Neuro Oncol. 2009;11(3):250–259. doi: 10.1215/15228517-2008-095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slongo ML, Molena B, Brunati AM, et al. Functional VEGF and VEGF receptors are expressed in human medulloblastomas. Neuro Oncol. 2007;9(4):384–392. doi: 10.1215/15228517-2007-032. [DOI] [PMC free article] [PubMed] [Google Scholar]