Abstract
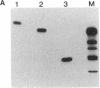
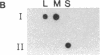
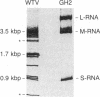
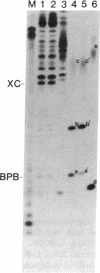
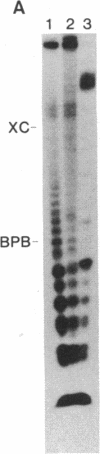
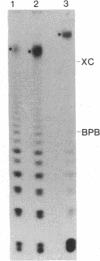
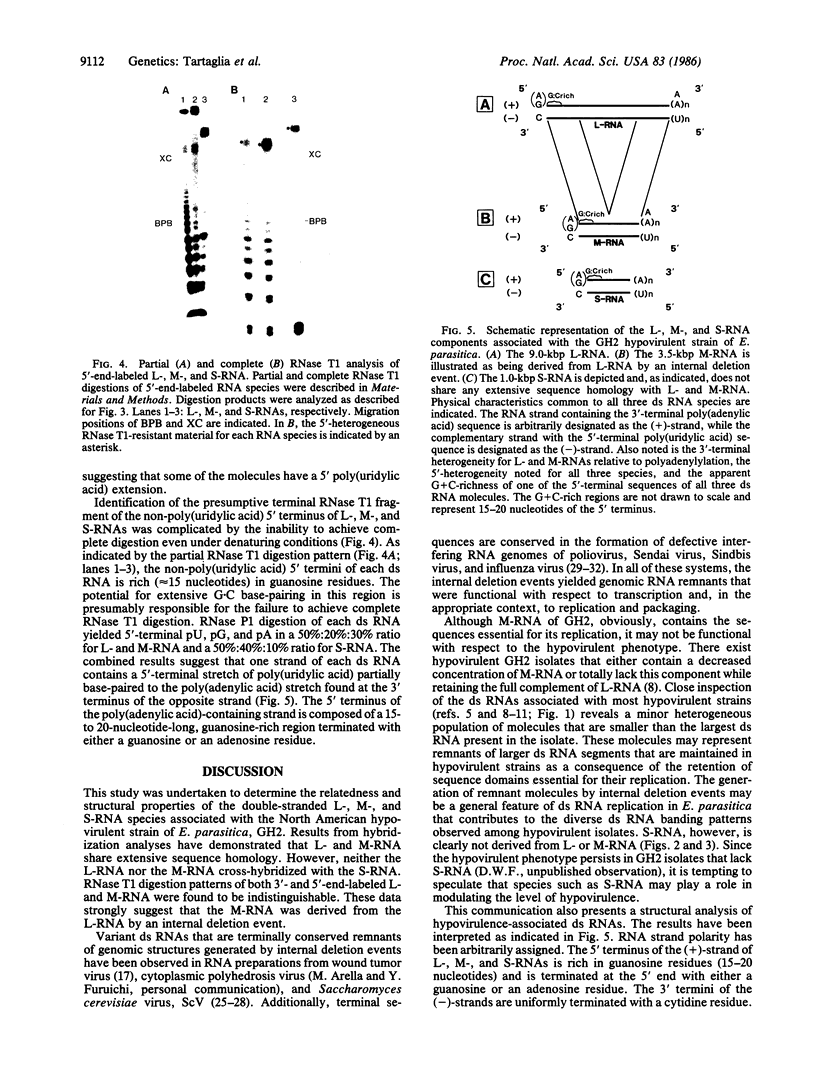
Double-stranded RNAs (ds RNAs) are thought to be the cytoplasmic determinants responsible for the phenomenon of transmissible hypovirulence in the chestnut blight fungus Endothia parasitica [Murr.] Anderson. The three major ds RNA components associated with the North American hypovirulent strain, Grand Haven 2, were characterized with respect to molecular-hybridization specificity and RNase T1-digestion patterns. The large (L-RNA; ≈9 kilobase pairs) and middle-sized (M-RNA; ≈3.5 kilobase pairs) ds RNA components cross-hybridized under stringent conditions and exhibited indistinguishable partial and complete RNase T1 digestion patterns relative to their 5′ and 3′ termini. These results suggest that M-RNA was derived from L-RNA by an internal deletion event. The small (S-RNA; ≈1 kilobase pair) RNA was unrelated to L- and M-RNA by these criteria. However, all three ds RNA components contained RNase T1-resistant oligonucleotides at one 5′ terminus and at the corresponding 3′ terminus of the complementary strand. These RNase T1-resistant species exhibited properties consistent with stretches of poly(uridylic acid) and poly(adenylic acid), respectively. The combined results are discussed in terms of the structural organization of hypovirulence-associated ds RNA molecules and their similarities to “double-stranded” RNA molecules observed in plant and animal cells infected with single-stranded RNA viruses.
Keywords: Endothia parasitica, transmissible hypovirulence, molecular hybridization, terminal-nucleotide analysis, internal deletion
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Asamizu T., Summers D., Motika M. B., Anzola J. V., Nuss D. L. Molecular cloning and characterization of the genome of wound tumor virus: a tumor-inducing plant reovirus. Virology. 1985 Jul 30;144(2):398–409. doi: 10.1016/0042-6822(85)90281-8. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D. IN VITRO SYNTHESIS OF VIRAL RNA BY THE POLIOVIRUS RNA POLYMERASE. Proc Natl Acad Sci U S A. 1964 Mar;51:450–456. doi: 10.1073/pnas.51.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr Hydrolysis of polyadenylic acid by pancreatic ribonuclease. J Biol Chem. 1960 Aug;235:2393–2398. [PubMed] [Google Scholar]
- Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968 Mar 14;32(2):359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Zaitlin M. Replication of tobacco mosiac virus, VI Replicative intermediate and TMV-RNA-related RNAs associated with polyribosomes. Virology. 1975 Jan;63(1):84–97. doi: 10.1016/0042-6822(75)90373-6. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Wang N., Reddy A., Weinberg E., Sofer W. Alcohol dehydrogenase in Drosophila: isolation and characterization of messenger RNA and cDNA clone. Nucleic Acids Res. 1980 Dec 11;8(23):5649–5667. doi: 10.1093/nar/8.23.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Hiti A. L., Nayak D. P. Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):215–219. doi: 10.1073/pnas.77.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M., Pietras D. F., Nemeroff M. E., Corstanje B. J., Field L. J., Bruenn J. A. Conserved regions in defective interfering viral double-stranded RNAs from a yeast virus. J Virol. 1986 May;58(2):402–407. doi: 10.1128/jvi.58.2.402-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Weiss B. G., Tsiang M., Huang H., Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- Mishra N. C. Gene transfer in fungi. Adv Genet. 1985;23:73–178. doi: 10.1016/s0065-2660(08)60512-x. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Summers D. Variant dsRNAs associated with transmission-defective isolates of wound tumor virus represent terminally conserved remnants of genome segments. Virology. 1984 Mar;133(2):276–288. doi: 10.1016/0042-6822(84)90395-7. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re G. G., Morgan E. M., Kingsbury D. W. Nucleotide sequences responsible for generation of internally deleted Sendai virus defective interfering genomes. Virology. 1985 Oct 15;146(1):27–37. doi: 10.1016/0042-6822(85)90050-9. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Isolation and replication of mutant populations of wound tumor virions lacking certain genome segments. Virology. 1977 Jul 15;80(2):336–346. doi: 10.1016/s0042-6822(77)80009-3. [DOI] [PubMed] [Google Scholar]
- Saha B. K., Strelow S., Schlessinger D. Electrophoretic elution of nucleic acids from acrylamide and agarose gels. J Biochem Biophys Methods. 1983 Jul;7(4):277–284. doi: 10.1016/0165-022x(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA IV. Poly(U) in replicative intermediate and double-stranded RNA. Virology. 1975 Oct;67(2):498–505. doi: 10.1016/0042-6822(75)90450-x. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA. II. poly(A) on intracellular RNAs. J Virol. 1975 Jun;15(6):1418–1431. doi: 10.1128/jvi.15.6.1418-1431.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Genome structure and expression of a defective interfering mutant of the killer virus of yeast. Virology. 1984 Aug;137(1):20–31. doi: 10.1016/0042-6822(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Teng M. H., Wimmer E. Poly(U) in poliovirus minus RNA is 5'-terminal. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1101–1109. doi: 10.1016/s0006-291x(74)80397-9. [DOI] [PubMed] [Google Scholar]