Abstract
Previous studies indicated that B7-H4, the youngest B7 family, negatively regulates T cell-mediated immunity and is significantly overexpressed in many human tumors. Tumor stem cells are purported to play a role in tumor renewal and resistance to radiation and chemotherapy. However, the link between B7-H4 and tumor stem cells is unclear. In this study, we investigated B7-H4 expression in the medium of human glioma U251 cell cultures. Immunofluorescence results showed that U251 cells cultured in serum-free medium (supplemented with 2% B27, 20 ng/mL epidermal growth factor, 20 ng/mL basic fibroblast growth factor) maintained stem-like cell characteristics, including expression of stem cell marker CD133 and the neural progenitor cell markers nestin and SOX2. In contrast, U251 cells cultured in serum-containing medium highly expressed differentiation marker glial fibrillary acidic protein. Flow cytometry analysis showed serum-free medium-cultured U251 cells expressed higher intracellular B7-H4 than serum-containing medium-cultured U251 cells (24%–35% vs. 8%–11%, P < 0.001). Immunofluorescence in purified monocytes from normal human peripheral blood mononuclear cells revealed moderate expression of B7-H4 after stimulation with conditioned medium from U251 cells cultured in serum-containing medium. Moreover, conditioned medium from U251 stem-like cells had a significant stimulation effect on B7-H4 expression compared with serum-containing conditioned medium (P < 0.01). Negative costimulatory molecule B7-H4 was preferentially expressed in U251 stem-like cells, and conditioned medium from these cells more effectively induced monocytes to express B7-H4 than conditioned medium from U251 cells cultured in the presence of serum. Our results show that U251 stem-like cells may play a more crucial role in tumor immunoloregulation with high expression of B7-H4.
Keywords: U251 cells, brain tumor stem cells, B7-H4, monocytes
Glioblastoma multiforme (GBM), the most common primary malignant tumors of the brain, are aggressive, highly invasive, neuro-logically destructive, and almost always fatal[1]. Despite advances in surgery, radiotherapy, and chemotherapy, the prognosis for GBM patients remains dismal[2]–[5]. Molecular studies revealed that malig-nant brain tumor masses contain stem-like cells (CD133+)[6]–[10] that express specific neural progenitor proteins, such as nestin, SOX2, OCT4, and Musashi[11]–[13]. Increasing evidence suggests that these cells are responsible for tumor invasion, angiogenesis, recurrence[14]–[16], and resistance to radiotherapy and chemo-therapy[17]–[19].
B7-H4 (also known as B7-S1 and B7x), a newly discovered member of the B7 family, exerts both costimulatory and negative immune regulatory functions[20]–[23]. However, recent studies showed that B7-H4 is aberrantly expressed in human tumors of the lung[24], breast[25], [26], kidney[27]–[29], ovary[26],[30], and brain[31], and that it strongly inhibits immune responses and contributes to tumor escape from immune surveillance[32],[33]. B7-H4 may negatively regulate T cell–mediated immunity by delivering inhibitory signals to T cells, though it is currently recognized as an orphan ligand[34]. Taken together, B7-H4 seems to be a new pivotal molecule that shapes immune response to tumors.
Since tumor stem cells may account for tumor invasion, angiogenesis, and recurrence and B7-H4 as the new negative costimulatory molecule related with tumor immunosuppression, we hence explored whether there are any connections between them. In this article, we investigated the expression of B7-H4 in U251 stem-like cells and in monocytes in the peripheral circulation stimulated with conditioned medium from U251 cells.
Materials and Methods
Tumor cell culture
The human glioma U251 cell line was purchased from the Chinese Academy of Sciences Cell Bank and was cultured at 37°C in an incubator with a 5% CO2 atmosphere. Cells grown in the presence of serum were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) and were routinely passaged every 3 days. Cells grown in serum-free conditions were cultured in a medium DMEM/F12 (Gibco) supplemented with 2% B27 (Gibco), 20 ng/mL epidermal growth factor (EGF; PeproTech), and human recombinant basic fibroblast growth factor (b-FGF; PeproTech). New complete medium was added every 2–3 days. U251 tumor spheres were cultured in 6-well plates on a nonadhesive substrate. Adhesive serum-free U251 cells were cultured on poly-D-lysine (PDL; Millipore) and laminin (Sigma) pretreated coverslips. Tumor spheres were passaged by 1 mg/mL papain (Sigma) dissociation when the center of spheres became dark. All U251 cell culture supernatants were separately collected as serum conditioned medium and serum-free conditioned medium.
Isolation and stimulation of human peripheral blood mononuclear cells
Peripheral blood mononuclear cells were isolated from healthy donor blood using standard Ficoll-Hypaque (Sigma-Aldrich) density gradient centrifugation[35]. Monocytes were purified using CD11b microbeads (Miltenyi Biotec) according to the manufacturer's instructions. Monocytes were cultured for 3 days in RPMI-1640 medium (Gibco) supplemented with 10% FBS, and with or without U251 cell conditioned medium, at 37°C in an incubator with 5% CO2.
Immunocytochemistry analysis
After centrifugation, U251 tumor spheres were suspended in PBS and smeared on slides by cytocentrifugation. Adhesive U251 cells were plated onto coverslips precoated with PDL and laminin and cultured in serum-free medium for 12 h. To detect differentiation, U251 tumor spheres were dissociated and cultured in DMEM medium with 10% FBS on coverslips for 48 h. After 30 min of fixation with 4% paraformaldehyde, cells were incubated with 0.2% Triton X-100 for 30 min, with 2% bovine serum albumin (BSA) for 2 h at room temperature, and then with primary antibodies [mouse anti-nestin (Millipore, 1:200); rabbit anti-SOX2 (Millipore, 1:250); rabbit anti-glial fibrillary acidic protein (GFAP; Millipore, 1:250); and mouse anti-CD133 (Miltenyi Biotec, 1:50)] overnight at 4°C. After washing with PBS, cells were incubated in goat anti-mouse IgG Alexa Fluor®488 (Invitrogen, 1:500) or goat anti-rabbit IgG Alexa Fluor®594 (Invitrogen, 1:500) for 2 h at room temperature. Monocytes stimulated with conditioned medium were collected, incubated with primary anti-CD11b (ICR44, Ebioscience, 1:100) and anti–B7-H4 antibody (clone 9, Ebioscience, 1:200), and then incubated with corresponding Alexa Fluor®-labeled secondary antibodies. 40-6-Diamidino-2-phenylindole (DAPI,Sigma) was used to counterstain nuclei. Fluorescence signals were detected with a Nikon fluorescent microscope at excitation/emission wavelengths of 470/505 (Alexa Fluor®488, green) and 535/565 nm (Alexa Fluor®594, red). Results were recorded with a digital camera. Controls included omitting or preabsorbing primary antibody or omitting secondary antibody.
Positive cells were quantified by counting all stained cells within 20 randomly selected microscopic fields under 400× magnification and calculating a percentage based on the total number of nuclei counted.
Flow cytometry analysis
Adhesive U251 cells cultured in serum were dissociated using a solution of 0.25% trypsogen containing 0.02% EDTA, whereas nonadhesive tumor spheres cultured in serum-free medium were dissociated with papain (1 mg/mL). Cells were analyzed by flow cytometry as described previously[31]. Briefly, after dissociation into single cell suspension, 1 × 106 cells were placed in flow cytometry tubes and treated with Fix and Perm® cell permeabilization reagents (Caltag Laboratories) according to the manufacturer's instructions. Tumor cells were incubated for 20 min at 4°C with 2 µL PE-conjugated anti-B7-H4 antibody (clone 9, Ebioscience) or with 2 µL isotype control antibody (PE-mouse IgG1; Ebioscience). Cells were washed twice with flow cytometry buffer and then resuspended in 200 µL PBS. Analysis was performed on a FACSCalibur system (Becton Dickinson Immunocytometry Systems). In addition, 1 × 105 cells were collected and analyzed with WinMDI 2.9 software[31]. All flow cytometry evaluations were separately performed three times.
Statistical analysis
Parametric data are presented as mean ± standard deviation. Data were analyzed with SPSS10.0 statistical software. When two groups were compared, the unpaired, dependent-samples t-test was used. A value of P < 0.05 was considered statistically significant.
Results
Serum deprivation in U251 cells induced tumor sphere formation
U251 cells were adhesively grown in serum-containing medium, whereas after being cultured in serum-free medium supplemented with B27, bFGF, and EGF for 12 h, U251 tumor cells became nonadhesive and formed tumor spheres. After an additional 48 h, the tumor spheres expanded to contain more than 200 cells and showed a sharp edge (Figure 1A). When dissociated U251 tumor cells were seeded on coverslips precoated with PDL and laminin in serum-free medium for 12 h, most cells migrated out from the small tumor spheres and became adhesive (Figure 1B).
Figure 1. Serum deprivation in U251 cells induced tumor sphere formation.

A, cells became nonadherent and formed tumor spheres when switched from serum-containing medium to serum-free medium, which favors the growth of stem-like cells. B, when seeded on pretreated coverslips and cultured in serum-free medium for 12 h, tumor spheres became adherent and most cells migrated out from the small spheres.
Characteristics of U251 cells cultured in serum-free medium
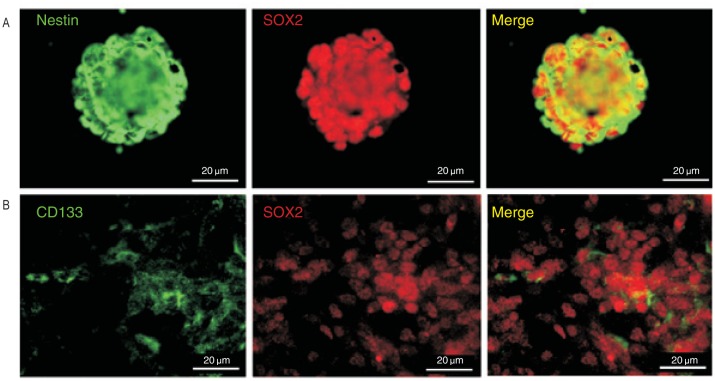
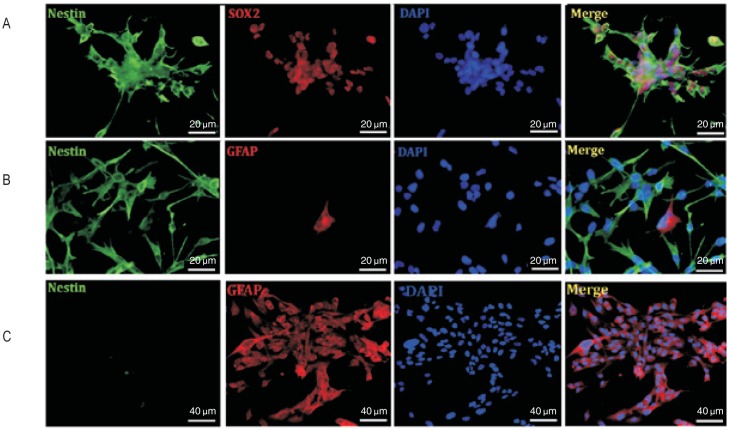
We stained U251 cells cultured in serum-free medium with neural stem cell markers including CD133, nestin, and SOX2. All U251 cells in tumor spheres expressed both nestin and SOX2 (Figure 2A). Among adhesive U251 cells cultured in serum-free medium, CD133+ cells were present in both tumor spheres and migrating cells (Figure 2B). All adhesive U251 cells expressed both nestin and SOX2 (Figure 3A). To investigate the differentiation of adhesive U251 cells cultured in serum-free medium, differentiation marker GFAP was used and staining results showed a few cells expressed GFAP; however, these cells were also moderately nestin-positive (Figure 3B). To analyze cell differentiation, dissociated tumor sphere cells were cultured in DMEM containing 10% FBS on coverslips for 48 h. The serum-cultured U251 cells expressed a high level of GFAP and were scarcely nestin- positive compared with adhesive serum-free cultured cells (Figure 3C). These results indicate that both U251 tumor spheres and adhesive U251 cells cultured in serum-free medium exhibited stem-like precursor cell properties.
Figure 2. Stem cell marker expression in U251 cells.
A, cells in tumor spheres all expressed neural precursor cell markers nestin (green, left panel) and SOX2 (red, middle panel). B, CD133-positive cells (green, left panel) exist both in tumor spheres and in migrating U251 cells with expression of SOX2 (red, middle panel). All right panels show merge images.
Figure 3. Characteristics of U251 cells cultured in different mediums.
A, all cells expressed the neural precursor cell markers nestin (green, first panel) and SOX2 (red, second panel) when tumor spheres were seeded on pretreated coverslips. B, cells that migrated out from tumor spheres cultured in serum-free medium were all nestin-positive (green, first panel), and a little cells coexpressed GFAP (red, second panel). C, when tumor spheres were cultured in serum-containing medium for 48 h, nestin was undetectable (green, first panel) but GFAP was highly expressed (red, second, panel). DAPI-counterstained nuclei are shown in blue. All right panels show merge images.
U251 cells cultured in serum-free medium express higher levels of cytoplasmic B7-H4
To explore the expression of B7-H4 in U251 stem-like cells, we cultured U251 cells, both nonadhesive and adhesive, in medium conditions favoring the growth of stem cells. To determine whether B7-H4 was expressed on U251 tumor cell membranes, we performed flow cytometry using anti-B7-H4 antibody without first treating the cells with Fix and Perm® cell permeabilization reagents. Little B7-H4 was detected on the membrane of cells cultured in either serum-containing medium (0.5%, data not shown) or serum-free medium (1%, data not shown). Then we explored cytoplasmic B7-H4 expression and found that 24%–35% of cells cultured in serum-free medium (Figure 4A) and 8%–11% of cells cultured in serum-containing medium (Figure 4B) were positive for B7-H4. As shown in Figure 4C, the expression of cytoplasmic B7-H4 was significantly higher in U251 cells cultured in serum-free medium than in U251 cells cultured in serum-containing medium (P < 0.01).
Figure 4. U251 cells express cytoplasmic B7-H4.
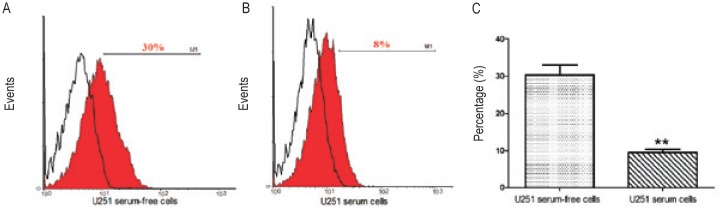
A, cells cultured in serum-free medium express cytoplasmic B7-H4 (isotype as control: open histogram 0.5% and B7-H4-pE: red filled histogram 30%). B, cells cultured in medium with serum also expressed cytoplasmic B7-H4 (isotype as control: open histogram 0.5% and B7-H4-pE: red filled histogram 8%). C, cells cultured in serum-free medium expressed higher levels of cytoplasmic B7-H4 than cells cultured in serum-containing medium (**, P < 0.01).
Phenotype of monocytes cultured with U251 cell conditioned medium
Monocytes were purified using CD11b microbeads and confirmed by flow cytometry (CD11b+ purity >95%). Purified CD11b+ monocytes were divided to 5 groups: no stimulation, serum-containing medium (SM), serum-free medium (SFM), serum conditioned medium (SCM), and serum-free conditioned medium (SFCM). After 72 h in culture, monocytes were collected for double immunofluorescence staining with CD11b and B7-H4. SFCM-stimulated monocytes (Figure 5A) had a higher expression of B7-H4 than the SCM-stimulated group (Figure 5B) (P < 0.01). All monocytes stimulated with conditioned medium moderately expressed B7-H4 compared with the no stimulation group (Figure 5C) (P < 0.001).
Figure 5. Monocytes in conditioned medium from U251 cells express B7-H4.
After 72 h of stimulation, monocytes were collected for double immunofluorescence staining with CD11b (green, first panel) and B7-H4 (red, second panel). A, monocytes (green, first panel) in the serum-free conditioned medium group (SFCM) highly expressed B7-H4 (red, second panel). B, monocytes (green, first panel) in the serum conditioned medium (SCM) group moderately expressed B7-H4 (red, second panel). DAPI-counterstained nuclei are shown in blue. All right panels show merge images. C, expression of B7-H4 in the SCM and SFCM groups was significantly up-regulated compared with the no stimulation group (black***, P < 0.001). Moreover, B7-H4 was expressed in a higher percentage of monocytes in the SFCM group compared with the SCM group (red**, P < 0.01). SM, serum-containing medium; SFM, serum-free medium.
Discussion
In this article, we focused on the expression of the negative costimulatory molecule B7-H4 in U251 cells cultured in different mediums. Interestingly, serum-free cultured U251 stem-like cells expressed higher levels of cytoplasmic B7-H4 compared with serum-containing cultured U251 cells. Stimulation with conditioned medium from U251 cells cultured in the presence of serum for 72 h induced monocytes to moderately express B7-H4, whereas conditioned medium from U251 stem-like cells showed a more significant stimulatory effect. Although we are still not sure which factor in the conditioned medium accounts for B7-H4 up-regulation, increasing evidence suggests that B7-H4 is crucial to tumor immunity. The cross-talk between tumor cells, especially stem-like cells, and immunocytes may be an important target and a useful new strategy for immunologic tumor therapy.
GBM (World Health Organization grade IV) tumors are the most common and lethal primary intracranial tumor composed of a heterogeneous cellular composition[1]. In spite of aggressive surgery, radiotherapy, and chemotherapy, the prognosis for GBM patients remains dismal, with a median survival of 12–15 months[2]–[5]. Previous studies show that the tumor mass contains malignant stem-like cells (CD133+) that may be responsible for tumor renewal and resistance to radiation and chemotherapy[17]–[19]. These stem-like cells share many characteristics with normal neural stem cells, including self-renewal and multipotency[13]. These stem-like cells also express specific neural progenitor proteins, including nestin, SOX2, OCT4, and Musashi[11],[12]. To detect stem-like cells in the established glioma U251 cell line, we analyzed the phenotype of U251 cells in different culture mediums. Double immunofluorescence staining results show that both U251 tumor spheres (Figure 2A) and adhesive U251 cells (Figure 3A) cultured in serum-free medium expressed the neural progenitor proteins nestin and SOX2, whereas differentiation marker GFAP was nearly negative (Figure 3B). CD133-positive cells were present in both U251 spheres and adhesive U251 cells cultured in stem proliferation medium (Figure 2B). However, when U251 cells cultured in serum-free medium were seeded in serum-containing medium for 48 h, almost all expressed GFAP while nearly none expressed nestin (Figure 3C). These results reveal that established U251 cells contain a population of brain tumor stem cells. Furthermore, U251 cells maintain stem cell properties when cultured in stem cell proliferation medium, as observed in a previous study[36].
B7-H4, the youngest B7 family, was identified by searching the NCBI database for genes with homology to other B7 extracellular Ig domains[20]–[22],[37]. B7-H4 protein expression is undetectable in normal tissues and organs[20],[37]; however, it is significantly overexpressed in many human tumors, including lung tumors[24], breast tumors[25],[26], ovarian tumors[26],[30],[38], kidney tumors[27]–[29], and gliomas[31]. Krambeck et al.[29] found that 59.1% of renal cell carcinomas express B7-H4, and B7-H4 expression relates to patient prognosis. Kryczek et al.[38] found that 0.5% of primary ovarian tumor cells express surface B7-H4 and 91% express intracellular B7-H4. Moreover, B7-H4 expression can be induced on tumor-associated macrophages in the tumor microenvironment. Chen et al.[39] found that tumor-associated macrophages induce up-regulation of B7-H4 expression on the surface of lung cancer cells. Previous studies indicate that B7-H4 participates in negative regulation of T cell-mediated immunity[20]–[23],[34]. These findings strongly suggest that there is cross-talk between tumor cells and the tumor microenvironment. Our previous study showed that 8.12%–80.59% of cells in primary astrocytomas and 20.12%–45.07% in primary medulloblastomas were B7-H4–positive[31]. Moreover, double immunofluorescence staining results revealed B7-H4 was predominantly expressed in quiescent tumor cells and in a subset of brain tumor stem-like cells. These findings indicate that B7-H4–positive tumor stem cells may account for tumor recurrence and T-cell inhibition.
In this study, we explored the relationship between the negative co-stimulatory molecule B7-H4 and U251 stem-like cells. Flow analysis showed that cytoplasmic B7-H4 was expressed in U251 cells cultured in either serum-free medium or serum-containing medium, whereas surface B7-H4 was difficult to detect in these two groups (data not shown). Moreover, serum-free cultured U251 stem-like cells showed a higher expression of cytoplasmic B7-H4 than cells cultured in serum-containing medium (Figure 4C, P < 0.01). However, we observed that U251 glioma cells expressed a lower level of B7-H4 than primary glioma cells, which is consistent with a previous study that showed glioma stem cells become less immunosuppressive with sequential passages[40]. We also found that conditioned medium from U251 cells induced monocytes to express B7-H4 (Figure 5). In addition, B7-H4 was highly expressed in the U251 stem cell-like group (Figure 5, P < 0.01). Our data support the standpoint that there is cross-talk between tumor cells and the tumor microenvironment. Further, tumor stem cells may play an important role in tumor immune escape by expressing and inducing immunocytes to express negative costimulatory molecules. Kryczek et al.[38] suggests that interleukein-10 and interleukin-6 in the tumor microenvironment induce tumor-associated macrophages to express B7-H4 and strongly suppress T-cell immunity. Although several studies suggest B7-H4 delivers an inhibitory signal to T cells, B7-H4 remains an orphan ligand[34] and the mechanism of its effects remains unknown.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81272797 to Y.Y.), Innovation Program of Shanghai Municipal Education Commission (No. 13ZZ010 to Y.Y.), and Shanghai Talents Development Funds (No. 2011063 to Y.Y.).
References
- 1.Davis FG, Frees S, Grutsch J, et al. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis base on surveillance, epidemiology, end results (SEER) data 1973-1991. J Neurosurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 2.Kelly KA, Kirkwood JM, Kapp DS. Glioblastoma multiforme: pathology, natural history and treatment. Cancer Treat. 1984;11:1–26. doi: 10.1016/0305-7372(84)90014-8. [DOI] [PubMed] [Google Scholar]
- 3.Surawicz TS, Davis F, Frells S, et al. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 5.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasm. 2007;9:882–892. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez Castillo A, Aguilar-Morante D, Morales-Garcia JA, et al. Cancer stem cell and brain tumors. Clin Transl Oncol. 2008;10:262–267. doi: 10.1007/s12094-008-0195-8. [DOI] [PubMed] [Google Scholar]
- 7.Piccirillo SG, Combi R, Cajola L, et al. Distinct pools of cancer stem-like cells coexist within human glioblastoma and display different tumorigenicity and independent genomic evolution. Oncogene. 2009;28:1807–1811. doi: 10.1038/onc.2009.27. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto OK, Oba-Shinjo SM, Lopes L, et al. Expression of HOXC9 and E2F2 are upregulated in CD133+ cells isolated from human astrocytomas and associated with transformation of human astrocytes. Biochim Biophys Acta. 2007;1769:437–442. doi: 10.1016/j.bbaexp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Oka N, Soeda A, Noda S, et al. Brain tumor stem cell from an adenoid glioblastoma multiforme. Neurol Med Chir. 2009;49:146–151. doi: 10.2176/nmc.49.146. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleau AM, Howard BM, Taylor LA, et al. New strategy for the analysis of phenotypic marker antigens in brain tumor-derived neurospheres in mice and humans. Neurosurg Focus. 2008;24:E28. doi: 10.3171/FOC/2008/24/3-4/E27. [DOI] [PubMed] [Google Scholar]
- 12.Chinnayan P, Wang M, Rojiani AM, et al. The prognostic value of nestin expression in newly diagnosed glioblastoma: report from the radiation therapy oncology group. Radiat Oncol. 2008;3:32. doi: 10.1186/1748-717X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X, Salford LG, Widegren B. Glioma stem cell: evidence and limitation. Semin. Cancer Biol. 2007;17:214–218. doi: 10.1016/j.semcancer.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 15.Molina JR, Hayashi Y, Stephens C, et al. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12:453–463. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao XH, Ping YF, Chen JH, et al. Glioblastoma stem cells produce vascular endothelial growth factor by activation of a G-protein coupled formylpeptide receptor FPR. J Pathol. 2008;215:369–376. doi: 10.1002/path.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao SD, Wu QL, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 18.Bao SD, Wu QL, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 19.Porta CL, Alessandri G, Marras C, et al. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia. 2006;54:850–860. doi: 10.1002/glia.20414. [DOI] [PubMed] [Google Scholar]
- 20.Sica GL, Choi IH, Zhu G, et al. B7-H4, a molecule of the B7 family negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 21.Prasad DV, Richards S, Mai XM, et al. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 22.Zang X, Loke P, Kim J, et al. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichikawa M, Chen L. Role of B7-H1 and B7-H4 molecules in down-regulating effector phase of T-cell immunity: novel cancer escaping mechanisms. Front Biosci. 2005;10:2856–2860. doi: 10.2741/1742. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Wang Y, Zhao J, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Salceda S, Tang T, Kmet M, et al. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Tringler B, Zhuo S, Pilkington G, et al. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 27.Crispen PL, Boorjian SA, Lohse CM, et al. Predicting disease progression after nephrectomy for localized renal cell carcinoma: the utility of prognostic models and molecular biomarkers. Cancer. 2008;113:450–460. doi: 10.1002/cncr.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ficarra V, Galfano A, Novara G, et al. Risk stratification and prognostication of renal cell carcinoma. World J Urol. 2008;26:115–125. doi: 10.1007/s00345-008-0259-y. [DOI] [PubMed] [Google Scholar]
- 29.Krambeck AE, Thompson RH, Dong H, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salceda S, Tang T, Kmet M, et al. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Yao Y, Wang X, Jin K, et al. B7-H4 is preferentially expressed in non-dividing brain tumor cells and in a subset of brain tumor stem-like cells. J Neurooncol. 2008;89:121–129. doi: 10.1007/s11060-008-9601-x. [DOI] [PubMed] [Google Scholar]
- 32.Miyatake T, Tringler B, Liu W, et al. B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely correlated with tumor T-cell infiltration. Gynecol Oncol. 2007;106:119–127. doi: 10.1016/j.ygyno.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 33.Lu B, Chen L, Liu L, et al. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50:269–275. doi: 10.1007/s12026-011-8227-9. [DOI] [PubMed] [Google Scholar]
- 34.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 35.Graham JM. Isolation of peripheral blood mononuclear cells from macaques on a density barrier. ScientificWorldJournal. 2002;2:1654–1656. doi: 10.1100/tsw.2002.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollard SM, Yoshikawa K, Clarke ID, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Choi IH, Zhu G, Sica GL, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 38.Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identi-es a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C, Qu QX, Shen Y, et al. Induced expression of B7-H4 on the surface of lung cancer cell by the tumor-associated macrophages: a potential mechanism of immune escape. Cancer Lett. 2012;317:99–105. doi: 10.1016/j.canlet.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]