Abstract
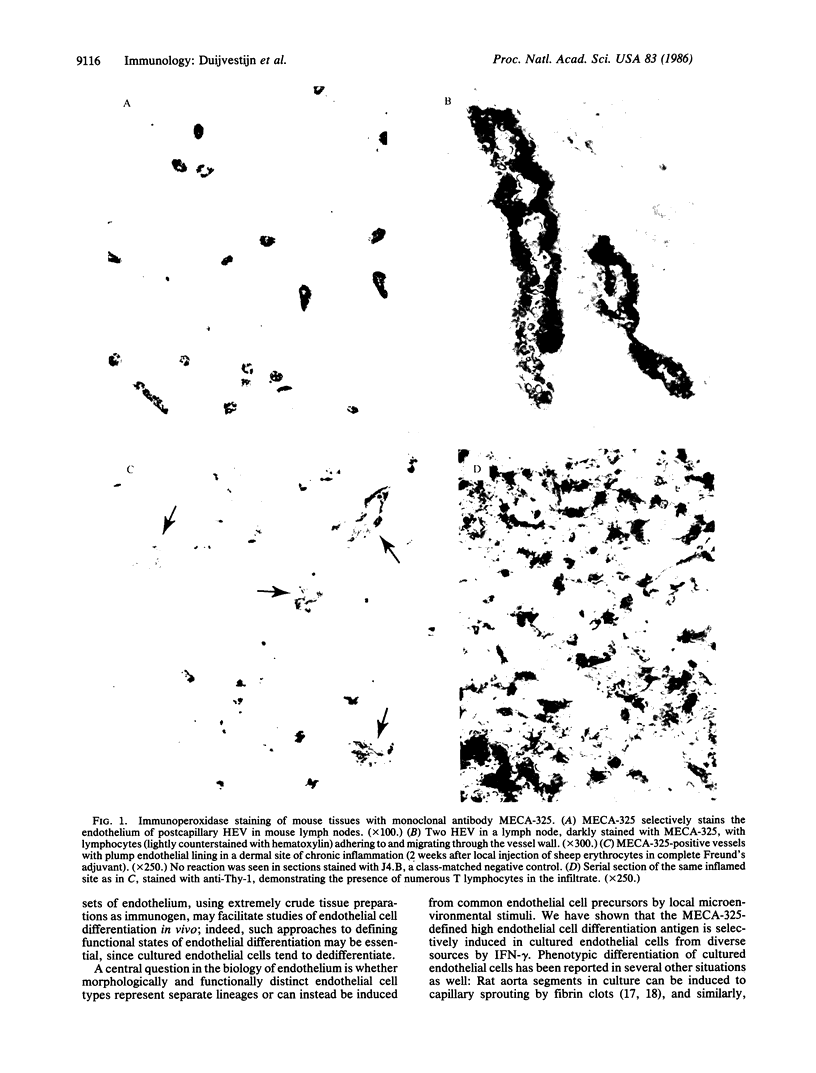
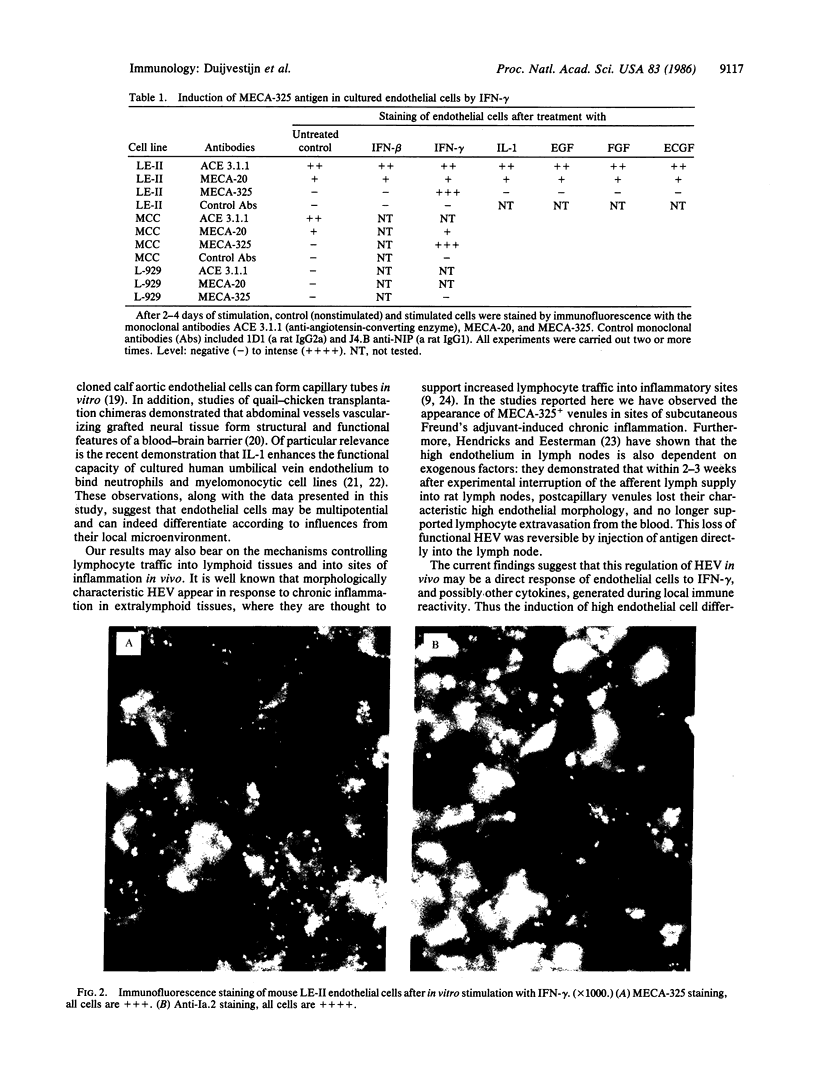
One of the most striking examples of localized vascular differentiation is exhibited by specialized lymphoid organ venules that mediate the extravasation of circulating lymphocytes from the blood. These vessels are characterized by cuboidal or "high" endothelial cell morphology and are unique in their functional capacity to interact with migrating lymphocytes, regulating both the rate and specificity of lymphocyte traffic through particular regions of the body. We describe here a monoclonal antibody, MECA-325, that defines an endothelial cell differentiation antigen selectively expressed on high endothelium in the mouse. Thus an antigen defining a specific functional subset of endothelial cells has been found. Furthermore, we demonstrate that the MECA-325 antigen can be induced in mouse lung or bone marrow-derived endothelial cell lines in vitro by interferon-gamma but not by interferon-beta, interleukin-1, or endothelial cell mitogens. The results define a unique marker associated with differentiated endothelial cells mediating lymphocyte traffic from the blood, and they provide evidence that the specialized phenotype of these high endothelial cells may be induced and controlled by local factors associated with immune activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Alby L., Grieves J., Joseph J., Lindgren C., Morrissey L. W., Sidky Y. A., Tu M., Watt S. L. Monoclonal antibody against angiotensin-converting enzyme: its use as a marker for murine, bovine, and human endothelial cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7891–7895. doi: 10.1073/pnas.79.24.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D. R., Ford D., Vetto R. M., Hamblin A., Goldstein A., Hubbard M., Dumonde D. C. Endothelial cell presentation of antigen to human T cells. Hum Immunol. 1981 Nov;3(3):209–230. doi: 10.1016/0198-8859(81)90019-7. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980 Jul;10(7):556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Chin Y. H., Rasmussen R., Cakiroglu A. G., Woodruff J. J. Lymphocyte recognition of lymph node high endothelium. VI. Evidence of distinct structures mediating binding to high endothelial cells of lymph nodes and Peyer's patches. J Immunol. 1984 Dec;133(6):2961–2965. [PubMed] [Google Scholar]
- Curtis A. S., Renshaw R. M. Lymphocyte-endothelial interactions and histocompatibility restriction. Adv Exp Med Biol. 1982;149:193–198. doi: 10.1007/978-1-4684-9066-4_26. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Galli S. J., Dvorak A. M. Expression of cell-mediated hypersensitivity in vivo--recent advances. Int Rev Exp Pathol. 1980;21:119–194. [PubMed] [Google Scholar]
- Fajardo L. F., Schreiber A. B., Kelly N. I., Hahn G. M. Thermal sensitivity of endothelial cells. Radiat Res. 1985 Aug;103(2):276–285. [PubMed] [Google Scholar]
- Feder J., Marasa J. C., Olander J. V. The formation of capillary-like tubes by calf aortic endothelial cells grown in vitro. J Cell Physiol. 1983 Jul;116(1):1–6. doi: 10.1002/jcp.1041160102. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Jones C. J., Bromley M., Andrews P. Changes in vascular endothelium related to lymphocyte collections in diseased synovia. Arthritis Rheum. 1983 Dec;26(12):1427–1433. doi: 10.1002/art.1780261203. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Ghandour S., Langley K., Gombos G., Hirn M., Hirsch M. R., Goridis C. A surface marker for murine vascular endothelial cells defined by monoclonal antibody. J Histochem Cytochem. 1982 Feb;30(2):165–170. doi: 10.1177/30.2.7061819. [DOI] [PubMed] [Google Scholar]
- Hendriks H. R., Eestermans I. L. Disappearance and reappearance of high endothelial venules and immigrating lymphocytes in lymph nodes deprived of afferent lymphatic vessels: a possible regulatory role of macrophages in lymphocyte migration. Eur J Immunol. 1983 Aug;13(8):663–669. doi: 10.1002/eji.1830130811. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Katz M. E., Tite J. P., Janeway C. A., Jr The immunobiology of T cell responses to Mls-locus-disparate stimulator cells. III. Helper and cytolytic functions of cloned, Mls-reactive T cell lines. J Immunol. 1986 Jan;136(1):1–5. [PubMed] [Google Scholar]
- Nestor J. J., Jr, Newman S. R., DeLustro B., Todaro G. J., Schreiber A. B. A synthetic fragment of rat transforming growth factor alpha with receptor binding and antigenic properties. Biochem Biophys Res Commun. 1985 May 31;129(1):226–232. doi: 10.1016/0006-291x(85)91426-3. [DOI] [PubMed] [Google Scholar]
- Nicosia R. F., Tchao R., Leighton J. Angiogenesis-dependent tumor spread in reinforced fibrin clot culture. Cancer Res. 1983 May;43(5):2159–2166. [PubMed] [Google Scholar]
- Nicosia R. F., Tchao R., Leighton J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro. 1982 Jun;18(6):538–549. doi: 10.1007/BF02810077. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. D., Singer M. S., Yednock T. A., Stoolman L. M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985 May 24;228(4702):1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Wiley M. J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981 May;84(1):183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]