Abstract
BACKGROUND
The independent prognostic value of prehypertension for incident coronary heart disease (CHD) remains unsettled. We examined associations between prehypertension (systolic blood pressure of 130–139.9 and/or diastolic blood pressure of 80–89mm Hg) and incident acute CHD and cardiovascular disease (CVD) death.
METHODS
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study includes 30,239 black and white community-dwelling adults aged ≥45 years recruited from 2003 to 2007. Endpoints were centrally adjudicated by experts and included incident nonfatal myocardial infarction (MI), acute CHD (nonfatal and fatal MI), and a composite of nonfatal MI or CVD death. Cox proportional hazards models estimated the hazard ratios (HRs) for these endpoints by blood pressure (BP) categories adjusting for sociodemographics and CHD risk factors.
RESULTS
The 24,388 participants free of CHD at baseline (mean age = 64.1±9.3 years; 58% women; 42% blacks) were followed for a mean of 4.2±1.5 years. The unadjusted HR for incident acute CHD was 1.23 (95% confidence interval (CI) = 0.93–1.64) for prehypertension and 2.28 (95% CI = 1.71–3.04) for hypertension. With full adjustment, the HR for prehypertension remained nonsignificant. The HR for nonfatal MI and for acute CHD death was also nonsignificant. For the combined endpoint (incident fatal and nonfatal MI or CVD death), the unadjusted HR was 1.29 (95% CI = 1.02–1.64) but the adjusted HR was 1.15 (95% CI = 0.91–1.47). Finally, after adjustment for other CHD risk factors, there was no significant interaction of BP with race.
CONCLUSIONS
In this sample, prehypertension was not associated with incident acute CHD.
Keywords: blood pressure, coronary heart disease, hypertension, myocardial infarction, prehypertension.
It is well known that blood pressure (BP) in the prehypertensive range, defined by the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) as a systolic BP (SBP) of 120–139mm Hg and/or a diastolic BP (DBP) of 80–89mm Hg, is associated with the future development of frank hypertension (HTN).1–5 But what is less well understood are the cardiovascular risks conferred by prehypertension, before frank HTN is manifest. It remains controversial whether prehypertension alone or higher BP within the prehypertensive range is independently associated with coronary heart disease (CHD) risk or whether any observed increased risk is the result of associated risk factors. In 1 report, 64% of prehypertensive subjects had >1 cardiovascular disease (CVD) risk factor.6
Several studies have reported on the CHD risk of prehypertension. Manious et al. analyzed participants in the first National Health and Nutrition Examination Survey (1971–1975) and ascertained major CVD events over the next 18 years.7 They divided prehypertension into categories, “low prehypertension” (120–129/80–84mm Hg) and “high prehypertension” (130–139/80–89mm Hg), and found that unadjusted analysis demonstrated risk for both groups but adjustment attenuated the statistical significance of the risk in the low prehypertension (unadjusted hazard ratio (HR) = 1.56, 95% confidence interval (CI) = 1.23–1.98; adjusted HR = 1.24, 95% CI = 0.96–1.59) but not in the high prehypertension group (unadjusted HR = 2.12, 95% CI = 1.64–2.76; adjusted HR = 1.42, 95% CI = 1.09–1.84).7 In the Framingham Heart Study, BP levels of 130–139/85–89mm Hg were associated with twice the risk of CVD compared with BP levels <120/80mm Hg.4 In the past decade, overall CVD events have significantly declined in the general population, but the CVD risks conferred by prehypertension are unclear.
To examine the association between prehypertension and CHD and CVD outcomes in the modern era, we used data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, an ongoing epidemiologic cohort that includes black and white community dwellers from all over the United States. Specifically we wanted to examine association of prehypertension with acute CHD events and CVD mortality and whether these associations were explained by co-occurring risk factors.
METHODS
Study population
REGARDS is a national, population-based, biracial, longitudinal cohort study designed to examine underlying causes for racial and regional differences in stroke and CHD. The study oversampled blacks and residents of the Stroke Belt region of the United States, an area that has stroke mortality rates higher than the rest of the country. Between January 2003 and October 2007, 30,239 individuals were enrolled, including 42% blacks, 58% whites, 45% men, and 55% women. The sample includes 21% of participants from the Stroke Buckle (coastal plain region of North Carolina, South Carolina, and Georgia), 35% from the remaining areas of the Stroke Belt states (remainder of North Carolina, South Carolina, and Georgia, plus Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), and 44% from the other 40 contiguous states (referred to as non-Belt). REGARDS participants were selected from commercially available lists (Genesys). A letter and brochure informed participants of the study and an upcoming phone call. During that call, verbal consent was obtained and a 45-minute questionnaire was administered. Including an estimate of eligibility among participants not reached, the telephone response rate was 33%; the cooperation rate among those with confirmed eligibility was 49% (similar to the Multi-Ethnic Study of Atherosclerosis, which had a 39.8% participation rate among those contacted and to whom the study was explained).8
A participant was considered enrolled in the study if they completed the 45-minute telephone questionnaire and the in-person physical examination. Using a computer-assisted telephone interview (CATI), demographic information and medical history were obtained by trained interviewers. Consent was obtained verbally by telephone and subsequently in writing during a follow-up in-home visit. Three to four weeks after the CATI, a brief physical exam was conducted in the home and included anthropometric and BP measurements, blood sample collection, and recording of an electrocardiogram (ECG). A medication inventory was also conducted by pill bottle review at the time of the in-home visit. Self-administered questionnaires were left with the participant to gather additional information.
Participants were followed by telephone at 6-month intervals for surveillance of medical events. Report of a potential event triggered medical retrieval, and reports of death triggered interviews with the next-of-kin or other proxies in addition to retrieval of any hospital records that corresponded to a hospitalization near the time of death. The National Death Index was also queried for the cause of death. Study methods were reviewed and approved by all involved institutional review boards. Additional methodological details are provided elsewhere.9
In this analysis, the 5,314 individuals with self-reported CHD at baseline (myocardial infarction (MI) or coronary intervention) or evidence of MI on the study ECG were initially excluded because the focus was on primary prevention. This analysis includes follow-up through 31 December 2009, a mean of 4.2 years.
Main exposure
As recommended by JNC 7, “at least 2 measurements should be made and the average recorded”; and this was the protocol used by REGARDS.1 SBP and DBP were defined as the average of 2 measurements taken by a trained technician using a standard protocol and regularly tested aneroid sphygmomanometer, measured in the fasting state (except that there was no prohibition of coffee) after the participant was seated for 5 minutes with both feet on the floor. BP quality control was monitored by central examination of digit preference, and retraining of technicians took place as necessary.
The primary independent variable was BP, which was categorized as no HTN (BP ≤130/80mm Hg and not on antihypertensive medication); prehypertension (SBP = 130–139.9 and/or DBP = 80–89.9mm Hg and not on antihypertensive medication); and HTN (BP ≥140/90mm Hg or treated with antihypertensive medication). HTN was classified as controlled if SBP was <140mm Hg or DBP was <90 mmHg and the subject was eceiving antihypertensive therapy and as uncontrolled if BP was ≥140/90mm Hg regardless of antihypertensive therapy. The group with no HTN was used as the referent for analysis.
Endpoints
The primary dependent variables were incident MI (definite or probable); incident acute CHD (nonfatal and fatal MI), and acute CHD plus CVD death, the definitions of which were based on international consensus.10 MI was diagnosed if there was a biomarker (almost always troponin) rising or falling pattern with the peak greater than twice the lowest listed upper limit of normal, plus symptoms or signs suggestive of ischemia or ECG changes consistent with acute ischemia. If there were diagnostic ECG changes and ischemic signs or symptoms present but biomarkers were either unavailable or equivocal, the event was classified as probable MI. Only definite or probable MIs were included in this analysis.
Acute CHD death was defined as definite fatal MI if death was within 28 days of hospital admission or postmortem findings were consistent with MI within 28 days of death; probable fatal MI was defined as death within 28 days of hospital admission with cardiac symptoms and/or signs when other confirmatory data (biomarkers, ECG) were absent or not diagnostic. Cardiovascular death was defined as fatal MI, fatal stroke, fatal heart failure, and other fatal cardiovascular-related deaths.
Additional covariables
Demographic factors included age (defined in 10-year strata starting with age 45), race, and sex. Measures of socioeconomic status included annual household income and education (defined in strata, see Table 1). Cardiovascular risk factors included diabetes (fasting glucose >126mg/dl or nonfasting glucose >200mg/dl or self-reported use of diabetes medications); dyslipidemia (total cholesterol ≥240mg/dl, low-density lipoprotein cholesterol ≥160mg/dl, high-density lipoprotein cholesterol <40mg/dl, or self-reported use of lipid-lowering medications); high-sensitivity C-reactive protein; smoking status (never, past, or current); report of engaging in no physical activity that worked up a sweat in the past week; alcohol use (never, past, or current); and baseline self-report of having had a stroke or transient ischemic attack in the past.
Table 1.
Baseline characteristics of REGARDS participants free of coronary heart disease at baseline, by blood pressure category
| Characteristic | Total (n = 24,388) | Normotensive SBP <130mm Hg and DBP <80mm Hg (n = 6,791) | Prehypertension SBP = 130–139.9mm Hg and/or DBP = 80–89.9mm Hg (n = 3,860) | Hypertension | |
|---|---|---|---|---|---|
| BP <140/90mm Hg (n = 8,378) | BP ≥140/90mm Hg (n = 5,359) | ||||
| Demographics | |||||
| Age, y, mean (SD) | 64.1 (9.3) | 61.1 (9.3) | 62.9 (9.2) | 65.2 (9.0) | 65.9 (9.3) |
| Female, % | 58.4 | 60.0 | 50.2 | 63.5 | 54.5 |
| Black, % | 42.4 | 26.2 | 36.3 | 50.7 | 54.3 |
| Region of residence, % | |||||
| Stroke Belt | 34.7 | 34.1 | 32.7 | 35.2 | 36.1 |
| Stroke Buckle | 20.9 | 21.6 | 19.1 | 22.5 | 18.9 |
| Non-Belt | 44.4 | 44.3 | 48.2 | 42.4 | 44.9 |
| Education < high school % | 11.6 | 7.0 | 9.3 | 13.3 | 16.2 |
| Annual income <$20,000, % | 19.6 | 13.9 | 15.7 | 22.0 | 25.8 |
| Framingham risk factors | |||||
| LDL cholesterol, mg/dl, mean (SD) | 116.5 (34.4) | 118.7 (33.6) | 122.2 (34.3) | 110.7 (33.4) | 118.4 (35.6) |
| HDL cholesterol, mg/dl, mean (SD) | 52.8 (16.3) | 54.7 (16.5) | 52.4 (15.9) | 51.7 (16.0) | 52.1 (16.4) |
| Statin use, % | 28.3 | 20.0 | 19.0 | 39.5 | 28.2 |
| Current smoking, % | 14.2 | 14.9 | 13.7 | 12.5 | 16.3 |
| Diabetes,% | 19.5 | 9.5 | 11.5 | 26.9 | 26.6 |
| Non-Framingham risk factors | |||||
| hsCRP, mg/L, median [25th–75th percentile] | 2.2 (0.9–5.0) | 1.5 (0.7–3.7) | 2.0 (0.9–4.4) | 2.7 (1.1–6.0) | 2.7 (1.2–5.9) |
| Current alcohol use, % | 52.6 | 58.9 | 58.2 | 47.2 | 48.9 |
| Body mass index, kg/m2, mean (SD) | 29.3 (6.2) | 26.9 (5.1) | 29.0 (5.6) | 30.5 (6.4) | 30.7 (6.8) |
| No exercise, % | 33.5 | 29.0 | 28.4 | 36.9 | 37.7 |
| Self-reported stroke or TIA, % | 8.2 | 4.4 | 5.0 | 11.3 | 10.7 |
Abbreviations: HDL; high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein, TIA, transient ischemic attack.
Statistical analysis
Descriptive statistics for the BP categories were obtained by using unadjusted χ2 tests for the categorical characteristics and analysis of variance for continuous characteristics. Sequentially adjusted Cox proportional hazards models were fitted to examine HRs among different categories of BP for the 3 endpoints of incident nonfatal MI, incident acute CHD, and the composite of acute CHD or CVD death. Initial unadjusted Cox proportional hazards model included only the BP categories. Model 1 adjusted for age, race, sex, region, educational level, and income. Model 2 added Framingham CHD risk factors (low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking status, and diabetes status) and use of statins to the Model 1 covariables. Model 3 added body mass index, physical activity level, alcohol consumption, high-sensitivity C-reactive protein level, and self-reported history of stroke or transient ischemic attack to the covariables in Model 2. Blood tests were missing for low-density lipoprotein cholesterol (n = 1,439), total cholesterol (n = 1,021), and high-sensitivity C-reactive protein (n = 1,529); thus, we used multivariable multiple imputation by chained equations with 5 datasets in STATA version 12 (StataCorp, College Station, TX) to impute missing covariables.
We conducted 3 additional analyses. First, because prehypertension is reportedly more common in women than men (29% vs. 21%), we stratified the analysis on sex. Second, as a sensitivity analysis, we conducted an analysis that excluded individuals treated with hypertensive medication, exactly analogous to the main analysis described above, to better contrast any observed risk with untreated individuals with HTN. Third, to examine whether prehypertension might confer differential risks for fatal vs. nonfatal CHD, we tested the interaction between category of BP and fatal vs. nonfatal incident CHD events. Also, because in the REGARDS study we have found a differential effect of HTN on the risk of stroke among blacks compared with whites, we examined an interaction of BP categories and race.11 Possible interactions of BP with sex, obesity, and diabetes status at baseline were examined. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC) and STATA version 12.
RESULTS
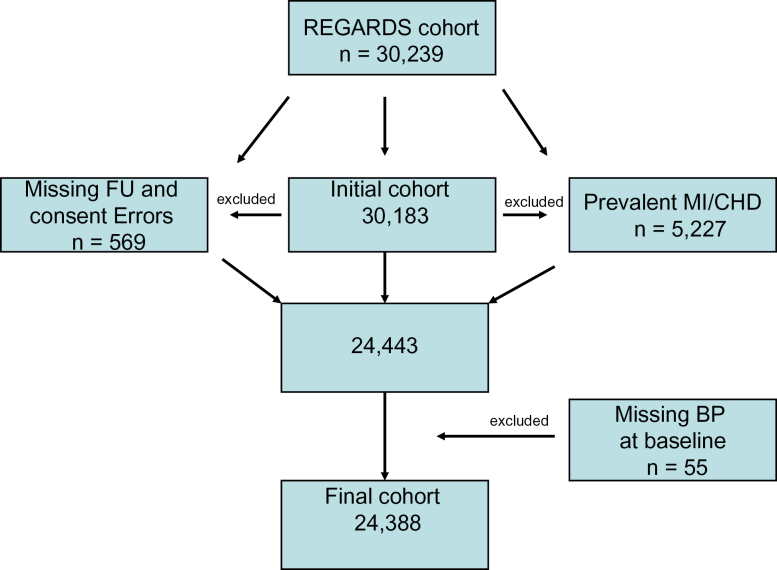
The exclusionary cascade is shown in Figure 1. The 24,388 participants free of CHD at baseline were followed for a mean of 4.2±1.5 years with a total of 442 nonfatal MIs, 657 acute CHD events, and 961 nonfatal MIs or CVD deaths. Mean age was 64.1±9.3 years; 58% of subjects were women, and 42% were blacks. The baseline characteristics of the cohort are shown in Table 1, grouped by BP category; 27.8% had normal BP, 15.8% had prehypertension, and 56.3% had HTN with 60.0% of hypertensive individuals controlled. As expected, those with prehypertension were younger than hypertensive subjects (62.9 vs. 65.9 years) but fewer were black. Prehypertension was slightly less frequent in the Stroke Belt and Stroke Buckle, and low-density lipoprotein cholesterol was slightly higher in the prehypertension group compared with the referent and HTN groups.
Figure 1.
Exclusionary cascade. Abbreviations: CHD, coronary heart disease; DBP, diastolic blood pressure; FU, follow-up; MI, myocardial infarction; SBP, systolic blood pressure.
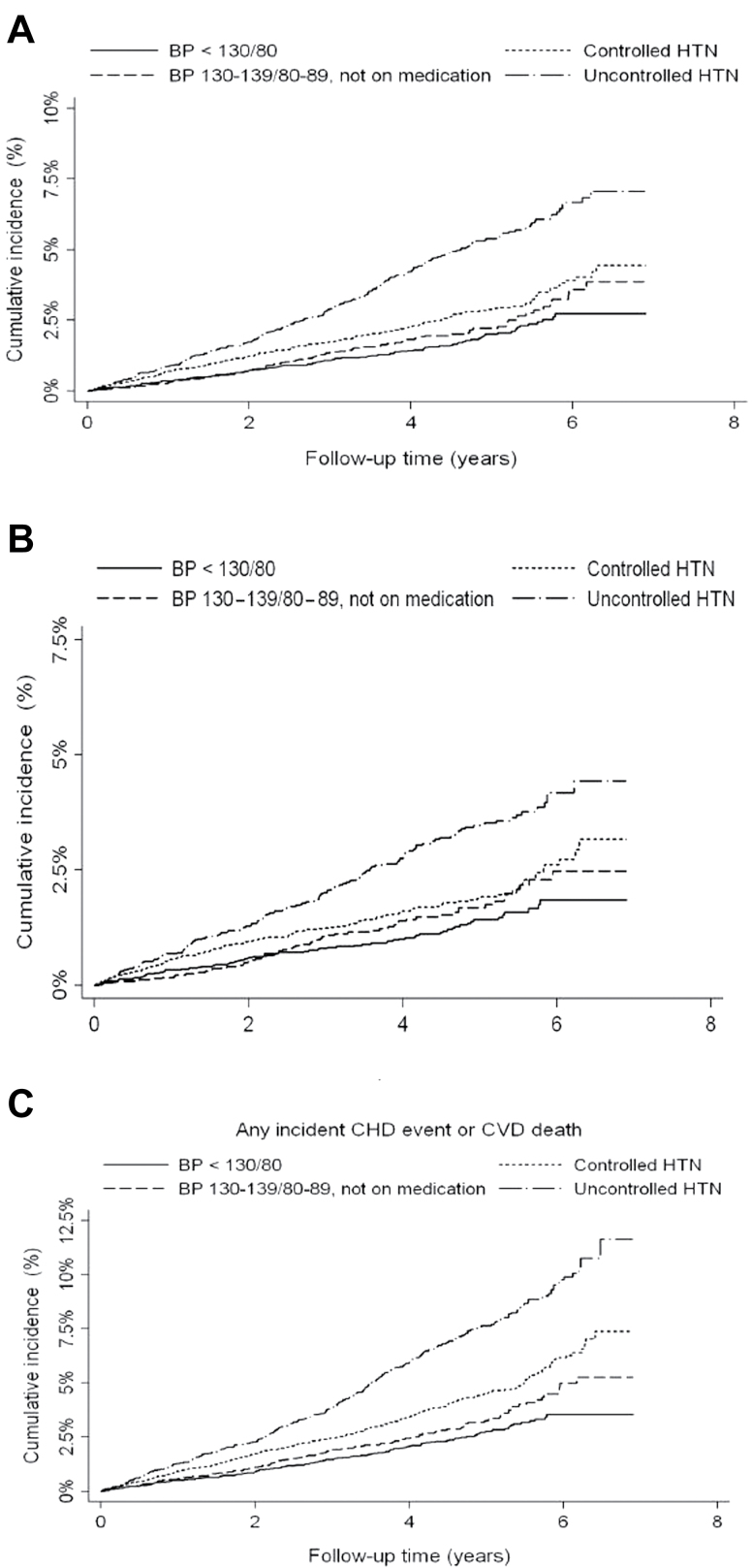
Table 2 presents the HRs for incident nonfatal MI, acute CHD, and the composite of acute CHD or CVD death. Figure 2 presents Kaplan–Meier plots depicting the BP categories for each dependent outcome. These plots demonstrate the unadjusted graded association of BP and outcome. For nonfatal MI, the HR for prehypertension vs. no HTN was 1.29 (95% CI = 0.92–1.80) in the unadjusted analysis; this relationship r24emained nonsignificant with full adjustment. For acute CHD, the HR for prehypertension vs. no HTN was 1.23 (95% CI = 0.93–1.65) in the unadjusted analysis; this relationship also remained nonsignificant with full adjustment. For the composite of acute CHD or CVD death, the HR for prehypertension vs. no HTN was 1.29 (95% CI = 1.02–1.64) in the unadjusted analysis, but the HR in the fully adjusted model was 1.15 (95% CI = 0.91–1.47). As expected, the HR for uncontrolled HTN was strongly associated with each of the 3 endpoints in both unadjusted and fully adjusted analyses. However, the HR for controlled and treated HTN were significant in unadjusted analyses but became nonsignificant with adjustment.
Table 2.
Association of incident cardiac events with blood pressure in participants free of coronary heart disease at baseline
| Categories of BP | No. | Events | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) |
|---|---|---|---|---|---|---|
| First nonfatal definite/probable myocardial infarction (n = 442) | ||||||
| Normotensive (referent) | 6,791 | 79 | 1.00 | 1.00 | 1.00 | 1.00 |
| Prehypertension | 3,860 | 60 | 1.29 (0.92–1.80) | 1.20 (0.86–1.68) | 1.18 (0.84–1.65) | 1.18 (0.84–1.65) |
| Hypertension 1 | 8,378 | 144 | 1.49 (1.13–1.96) | 1.42 (1.08–1.88) | 1.32 (1.00–1.76) | 1.25 (0.94–1.66) |
| Hypertension 2 | 5,359 | 159 | 2.49 (1.90–3.26) | 2.19 (1.66–2.89) | 1.98 (1.50–2.61) | 1.88 (1.41–2.50) |
| Acute coronary heart disease (fatal/nonfatal myocardial infarction) (n = 657) | ||||||
| Normotensive (referent) | 6,791 | 113 | 1.00 | 1.00 | 1.00 | 1.00 |
| Prehypertension | 3,860 | 83 | 1.23 (0.93–1.65) | 1.12 (0.85–1.49) | 1.11 (0.84–1.48) | 1.11 (0.83–1.47) |
| Hypertension 1 | 8,378 | 212 | 1.53 (1.22–1.93) | 1.39 (1.08–1.72) | 1.30 (1.03–1.65) | 1.21 (0.95–1.53) |
| Hypertension 2 | 5,359 | 249 | 2.72 (2.17–3.39) | 2.19 (1.74–2.75) | 2.00 (1.59–2.52) | 1.88 (1.49–2.37) |
| Acute coronary heart disease (fatal/nonfatal myocardial infarction) + cardiovascular death (n = 961) | ||||||
| Normotensive (referent) | 6,791 | 154 | 1.00 | 1.00 | 1.00 | 1.00 |
| Prehypertension | 3,860 | 118 | 1.29 (1.02–1.64) | 1.15 (0.91–1.47) | 1.15 (0.91–1.47) | 1.15 (0.91–1.47) |
| Hypertension 1 | 8,378 | 327 | 1.74 (1.44–2.11) | 1.42 (1.17–1.73) | 1.37 (1.12–1.67) | 1.25 (1.02–1.52) |
| Hypertension 2 | 5,359 | 362 | 2.89 (2.39–3.49) | 2.13 (1.75–2.58) | 1.98 (1.63–2.41) | 1.83 (1.50–2.22) |
Model1 adjusts for age, race, sex, region, education, and income. Model 2 adjusts for model 1 covariables plus low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, statin use, smoking, and diabetes. Model 3 adjusts for model 2 +body mass index, physical activity, alcohol consumption, high-sensitivity C-reactive protein, and baseline history of stroke or transient ischemic attack. Normotensive was defined as blood pressure (BP) <130/80mm Hg. Prehypertension was defined as BP of 130–139.9/80–89.9mm Hg. Hypertension 1 was defined as BP <140/90mm Hg. Hypertension 2 was defined as BP ≥140/90mm Hg.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Bolded numbers represent statistical significance.
Figure 2.
Kaplan–Meier curves of blood pressure categories and outcomes. (a) Any incident coronary heart disease event. (b) Incident nonfatal myocardial infarction. (c) Any incident coronary heart disease event or cardiovascular disease death. Abbreviations: BP, blood pressure; HTN, hypertension.
The sex-stratified analyses demonstrated no differences by sex. The analysis restricted to individuals not treated with antihypertensive agents included 1,952 subjects with untreated and uncontrolled HTN in addition to those with no HTN and those with prehypertension. Among those with uncontrolled and untreated HTN vs. those without HTN, both unadjusted and adjusted HRs were significantly associated with each of the 3 endpoints (nonfatal MI: adjusted HR = 1.65, 95% CI = 1.20–2.19; acute CHD: adjusted HR = 1.62, 95% CI = 1.33–2.470; composite: adjusted HR = 1.64, 95% CI = 1.27–2.12).
The P value for the interaction between BP category and fatal vs. nonfatal MI was 0.25, indicating little evidence of a difference in risk. Similarly, the P value for the interaction between BP categories and sex predicting any incident CHD was 0.31, and the P value for the interaction between BP categories and race predicting any incident CHD event was 0.27, indicating similar effects for men and women and for blacks and whites. Finally, although there was an interaction between BP categories and diabetes predicting events (P = 0.006), there were too few events and resultant wide confidence limits to make meaningful conclusions
DISCUSSION
In our study of a large, biracial, community-based cohort we found no association of prehypertension and incident nonfatal MI, acute CHD, or a composite of nonfatal MI or CVD death. Only the composite endpoint demonstrated an association in unadjusted analyses, but when adjustment was made for CVD risk factors, this association became nonsignificant. This study does not support an independent risk of prehypertension for several CHD endpoints over a mean of 4.2 years.
Our findings support prior reports that found that risks in individuals with prehypertension were attributable to the clustering of CVD risk factors; however not all studies have had similar findings.5,6,12 For example, the Woman’s Health Initiative evaluated the CVD risk at 7.7 years of follow-up and found an increased risk of MI, stroke, CVD death, and other outcomes in the prehypertensive group, but another study found that after adjustment for risk factors, prehypertension was not associated with all-cause or CVD mortality.13 Yet, a year later the same authors did report a residual risk of morbidity in prehypertensives subjects after adjustment.14 The question of whether prehypertension is an independent risk factor for CVD outcomes or whether excess risks are attributable to co-occurring CVD risk factors remains to be answered definitively. Based upon the available evidence and lifetime risk of HTN, JNC 7 recommended that in prehypertensive individuals, lifestyle modifications to prevent the progressive increase in BP and CVD should be considered.1
The possibility of excess CVD risk in subjects with prehypertension has pathophysiologic plausibility. Several alterations in cardiovascular structure and function have been reported to precede the finding of frank HTN. These include left ventricular hypertrophy in children and young adults of hypertensive parents, diastolic filling abnormalities in normotensive individuals predisposed to HTN, endothelial dysfunction as a precursor to the finding of HTN and increased arterial stiffness in normotensive subjects predisposed to develop HTN.15 In recent studies of subjects with confirmed prehypertension, carotid intimal-medial thickness was increased compared with subjects who were normotensive.16 If changes in vascular integrity precede the development of HTN, prehypertension could well fit into a continuum in which prehypertension is the first manifestation of a pathologic process with isolated systolic HTN as a later manifestation.
A challenge for studies of prehypertension is that the definition of prehypertension has varied in the literature. JNC 7 defined prehypertension as an SBP of 120–139mm Hg and/or DBP of 80–89mm Hg. However, the World Health Organization and the International Society of Hypertension defined high-normal BP as a SBP of 130–139mm Hg or a DBP of 85–89mm Hg.17 In the Trial of Preventing Hypertension (TROPHY), the definition used was SBP of 130–139mm Hg and DBP ≤89mm Hg or SBP ≤139mm Hg and DBP of 85–89mm Hg.18 In our study, there were relatively few outcomes among subjects with BPs in the 120–129 range, precluding robust analyses of this group or use of this group for comparisons. Because of the relatively short time horizon of this study, we were primarily interested in risks associated with the high-normal range of BPs, therefore we defined prehypertension as SBP of 130–139mm Hg and/or DBP of 80–89mm Hg.
The strength of our study is that we used a large, nationally distributed sample of community dwellers that included individuals not receiving regular healthcare. We also had available a relatively high number of events with rigorous central adjudication of endpoints and excellent follow-up. The high number of women and blacks in our study population is another strength. Our study’s limitations include the relatively short time frame and its observational design, which warrants caution when drawing causal inferences. The self-report of some variables (such as prior CHD) is common to most epidemiologic studies but has known limitations. Among US adults, the prevalence of prehypertension, as defined here, is approximately 31%, yet we observed a prevalence of 15.8%; our lower prevalence may in part be explained by our more restricted definition and also the high proportion of blacks, who are more likely to have frank HTN at older ages.9
In conclusion, we found no evidence of a risk for incident nonfatal MI, acute CHD, or a composite of acute CHD plus CVD death associated with prehypertension over 4.2 years of follow-up.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health , Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. We thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
REFERENCES
- 1. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung Blood Institute Joint National Committee on Prevention, Detection Evaluation Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on the prevention, detection, evaluation, and treatment of high blood pressure, the JNC 7 report. JAMA 2003; 289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 2. Qureshi A, Fareed M, Suri K, Kirmani JF, Divani AA, Mohammad Y. Prehypertension triples heart attack risk. ScienceDaily, published online 6 August 2005. http://www.sciencedaily.com/releases/2005/08/050805110759.htm [Google Scholar]
- 3. Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among U.S. adults: the third National Health and Nutrition Examination Survey mortality follow-up study. Ann Epidemiol 2008; 18:302–309 [DOI] [PubMed] [Google Scholar]
- 4. Vassan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 345:1291–1297 [DOI] [PubMed] [Google Scholar]
- 5. Lee J, Heng D, Ma S, Chew SK, Hughes K, Tai ES. Influence of prehypertesnion on all-cause mortality and cardiovascular mortality: the Singapore Cardiovascular Cohort Study. Int J of Cardiol 2009; 135:331–337 [DOI] [PubMed] [Google Scholar]
- 6. Greenland P, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med 2004; 164:2113–2118 [DOI] [PubMed] [Google Scholar]
- 7. Manious AG, Everett CJ, Liszka HA, King DE, Egan BM. Prehypertension and mortality in a nationally representative cohort. Am J Cardiol. 2004; 94:1496–1500 [DOI] [PubMed] [Google Scholar]
- 8. Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol 2006; 163:197–203 [DOI] [PubMed] [Google Scholar]
- 9. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study: objectives and design. Neuroepidemiology 2005; 25:135–143 [DOI] [PubMed] [Google Scholar]
- 10. Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosmond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003; 108:2543–2549 [DOI] [PubMed] [Google Scholar]
- 11. Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. Arch Intern Med 2013; 173:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glasser SP, Judd S, Basile J, Lackland D, Halanych J, Cushman M, Prineas R, Howard V, Howard G. Prehypertension, racial prevalence and its association with risk factors: analysis of the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. Am J Hypertens 2011; 24:194–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsia J, Margolis KL, Eaton CB, Wenger NK, Allison M, Wu L, LaCrox AZ, Black HR, Women’s Health Initiative Investigators Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation 2007; 115:855–860 [DOI] [PubMed] [Google Scholar]
- 14. Lizka HA, Mainous AG, 3rd, King DE, Everett CJ, Egan BM. Prehypertension and cardiovascular morbidity. Ann Fam Med 2005; 3:294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glasser SP, Arnett DK. Vascular stiffness and the “chicken-or-the-egg” question. Hypertension 2008; 51:177. [DOI] [PubMed] [Google Scholar]
- 16. Femia R, Kozakova M, Nannipieri M, Gonzales-Villalpando C, Stern MP, Haffner SM, Ferrannini E. Carotid intima-media thickness in confirmed prehypertensive subjects: predictors and progression. Arterioscler Thromb Vasc Biol 2007; 27:2244–2249 [DOI] [PubMed] [Google Scholar]
- 17. Whitworth JA, World Health Organization, International Society of Hypertension Writing Group World Health Organization (WHO)/International Society of Hypertension (ISH) Statement on management of hypertension. J Hypertension 2003; 21:1983–1992 [DOI] [PubMed] [Google Scholar]
- 18. Duprez DA, Alves B, Grandits G, Nesbit SD, Egan BM, Julius S, Cohn JN. Small artery elasticity predicts development of hypertension in prehypertensive patients: results from a TROPHY substudy. JACC 2008; 51:A370 [Google Scholar]