Abstract
Objectives
The aim of this study was to investigate the effect of cortisol on growth-related genes in the ovine placenta.
Study design
Ewes carrying singleton pregnancies were operated on between 112 and 116 days of gestation (115 ± 0.4, term=147 days) and randomly assigned into three groups: six control animals, five ewes that were administered cortisol by continuous intravenous infusion (1 mg/kg/day) (high cortisol), and five ewes that were adrenalectomized and replaced with 0.5–0.6 mg cortisol/kg/day and 3 μg aldosterone/kg/day to produce cortisol concentrations equivalent to pre-pregnancy values (low cortisol). At necropsy (130 ± 0.2 days of gestation), placental tissue was frozen and stored at −80°C for mRNA analysis.
Main outcome measures
To assess potential molecular mechanisms by which cortisol alters placental structure and function and fetal growth.
Results
Cortisol levels did not significantly affect 11β-hydroxysteroid dehydrogenase 1 and 2 enzymes, glucocorticoid receptor, mineralocorticoid receptor and angiotensin II receptor, type 1 (AT1R) expression levels. Gene expression levels of AT2R were increased in the high cortisol group for type B placentomes. There was little effect of cortisol on the insulin-like growth factor (IGF) axis. There was significantly more IGF-I mRNA in B versus A type and more IGFBP-2 mRNA in B and C type versus A type placentomes regardless of treatment (p<0.05).
Conclusions
These data suggest that cortisol affects AT2R expression at high concentrations. Cortisol had little effect on the IGF axis in the placenta. However, the IGF axis might be involved in remodeling the fetal zone of placentomes during normal development.
Keywords: cortisol, placentomes, insulin-like growth factor, angiotensin receptor
1. Introduction
Ruminants, such as sheep and cows, have a cotyledonary type of placentation, which initially consists of interdigitated maternal and fetal villi that serve in the exchange of nutrients between mother and fetus during most of the gestation [3]. The placentomes reach their maximum weight at approximately 80 days, but undergo subsequent changes in vascularity and a structural remodeling which enable the increase in uterine blood flow that is necessary to support nutrient exchange when fetal demand rises towards the end of gestation [2]. In the ovine placenta, there is a pronounced increase in capillary density in the fetal zone, which exceeds that of the maternal zone in late gestation [4]. Based on their morphologic appearance, ovine placentomes have been characterized as types A–D [1]. However, despite the impression that the more everted type of placentomes such as type C and D placentomes are more vascular, recent evidence suggests that placentome morphology or size has little effect on capillary vascularity or rate of cell proliferation (ref 5). Placentomes can shift their classification bidirectionally in late gestation. This process of eversion and apparent growth of the fetal zone appears to slow as cortisol levels rise towards the end of pregnancy [2]. The functional significance of these different placentome types remains unclear. Capillary area is slightly greater in the cotyledonary zone of type B and D cotyledons than in type A cotyledons, and vessels in larger cotyledons have a greater response to vasoconstrictors than do those in smaller cotyledons [5].
Previous studies have demonstrated that the normal pregnancy-associated elevation of maternal plasma cortisol levels is important for fetal development and homeostasis [6–8]. The fetus can be adversely affected when cortisol levels are either elevated or reduced relative to the endogenously-regulated physiological level. When maternal cortisol levels are low, fetuses are slower growing, with an increased incidence of relative hypotension and hypoxia [6]. Moreover, when maternal cortisol levels are high, fetuses also have reduced growth rates and have increased blood pressure [8, 9]. In addition, uterine and placental blood flow, as well as gross morphology of ovine placentomes, are altered by high or low maternal cortisol levels [8, 9]. Overall, there is a shift in the placentome types in the ewe, with an increased proportion of type B placentomes, and reduced fetal placental blood flow with either increased or decreased cortisol levels. Furthermore, type B placentomes are lighter with both low [8] and high maternal cortisol [9]. All these changes in fetal growth and placental structure were due to modest, physiologically relevant, alterations in maternal cortisol. Reduced fetal growth and increased blood pressure have also been produced by treatment with much higher doses of synthetic glucocorticoids. [10–12] Fetal cortisol levels have also been shown to influence the ontogenetic shift in placentome classification and distribution of placentome types in late gestation [2]; the proportion of D type placentomes decreases and the proportion of A type cotyledons increases. Direct fetal cortisol administration also reduces the proportion of everted placentomes [2]. The mechanisms by which altered maternal and/or fetal cortisol levels produce structural or functional variations in the placenta are unknown. Specifically, it is not known whether either reduced or elevated maternal cortisol alters the local expression of the elements of the IGF system, and it is unknown whether these changes in cortisol alter the abundance of the relevant steroid receptors or biotransforming enzymes.
In the current study, we examined gene expression in placentas (in the different placentome types) subjected to either low or high maternal cortisol concentrations. Tissues were taken from a previous cohort of animals that has already been reported (see above –ref#8)We tested the hypothesis that cortisol acts on the placenta to produce a pattern of gene expression that is consistent with increased cotyledonary and fetal growth and vascular development and reduced responsiveness to cortisol. Quantitative real-time PCR was used to test for genes that mediate cortisol action, including mineralocorticoid receptor (MR), glucocorticoid receptor (GR), and the 11β-hydroxysteroid dehydrogenases (11 β-HSD1 and 11β-HSD2), as well as genes that might be involved in growth such as the insulin-like growth factors (IGFs), their receptors and binding proteins, and the angiotensin receptors (AT1R and AT2R).
2. Materials and Methods
2.1. Experimental design
Pregnant ewes of mixed breed (mean body weight: 56 ± 2 kg, range: 43–66 kg) carrying singleton pregnancies were studied. All animal use conformed to the Guidelines of the NIH and was approved by the University of Florida Institutional Animal Care and Use Committee. Ewes and their fetuses were operated on between 112–116 (115 ± 0.4) days gestation. Animals were randomly assigned into three groups before surgery. The first group consisted of six control animals, the second consisted of five ewes that were administered cortisol (Solu-cortef; Upjohn company, Kalamazoo, MI) by continuous intravenous infusion (1 mg/kg/day) (high cortisol), and the third consisted of five ewes that were adrenalectomized and replaced with 0.5–0.6 mg cortisol/kg/day and 3 μg aldosterone/kg/day in order to produce cortisol concentrations similar to endogenous concentrations in intact nonpregnant animals (low cortisol). Cortisol replacement in this group of adrenalectomized animals was provided by implanting three to four pellets containing cortisol hemisuccinate (200 mg, released over 21 days; Innovative Research, Sarasota FL). Aldosterone replacement was achieved by intravenous infusion of aldosterone hemisuccinate in saline (Steraloids; Wilton, NH). The low cortisol dose was chosen based on previous studies showing altered maternal ACTH and altered fetal blood pressure, lung liquid production rate and urine production at this dose [6, 7, 13]. The high cortisol dose was chosen based on our previous studies, which altered placentome type and blood flow, and infusion rates that produced concentrations similar to that seen with mild maternal stressors [8, 9, 14, 15].
2.2. Surgical procedures
Ewes were anesthetized during surgery with halothane (1.5–2.5%) in oxygen and fetal arterial, venous and amniotic catheters were placed as described previously [6]. Bilateral adrenalectomy was only performed in the low cortisol group, with the ewe in sternal recumbency using bilateral flank incisions; following adrenalectomy the pellets of cortisol hemissucinate were implanted subcutaneously [13].
After recovery from anesthesia, ewes were returned to their pen. In adrenalectomized ewes, 1 liter of 0.9% sodium chloride and 1.5 mg/kg/day cortisol and aldosterone (3 μg/kg/day) were infused intravenously for the first 16–20 hours post-operatively. For the following day, 1 mg/kg/day cortisol and aldosterone were infused. The fluid and steroid therapy was designed to assure recovery of adrenalectomized ewes from the surgical procedures. On postoperative day three until the end of the study, only aldosterone was infused in the adrenalectomized animals, as the subcutaneous implants supplied the total cortisol dose (0.5 mg/kg/d). The infusion of cortisol in saline in the ewes in the high cortisol group commenced on the day after surgery to allow for recovery from the surgery-induced increase in maternal cortisol secretion.
The ewes were housed in individual pens with access to food, water, and salt blocks ad libitum. Ampicillin (750 mg im bid) was administered (Polyflex, Fort Dodge Animal Health, Fort Dodge, Iowa) for five days postoperatively.
2.3. Experimental protocol
Samples for hormones and measurement of maternal and fetal blood pressure, uterine blood flow and cotyledonary blood flow were made in these animals at 120 and 130 days of gestation. Fetal growth was evaluated by measurement of changes in girth from the day of surgery to the day of sacrifice at approximately 130 days. These data for these animals has been previously reported [8].
At 130 (± 0.2) days of gestation, the ewe was killed with an overdose of pentobarbital and placentomes were separated from the maternal tissue and categorized by type (A, B and C; Fig. 1). This tissue was removed as quickly as possible, frozen in liquid nitrogen and stored at −80°C for later RNA analysis. No type D placentomes were found. As previously reported [8], it was found that the number of B type cotyledons was increased in the low cortisol and high cortisol groups, and that while overall placentome weight was unchanged, type B placentomes were lighter in the low cortisol group than those in controls.
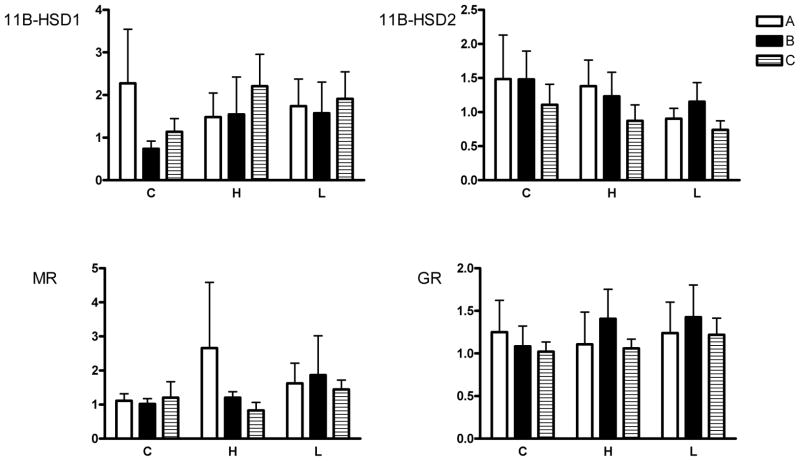
Figure 1.
Expression of mRNA for mineralocorticoid receptor (MR), glucocorticoid receptor (GR), 11β-hydroxysteroid dehydrogenase-1 (11β-HSD1) and 11β-HSD2 in A, B and C type placentomes.
Fold changes with respect to A type placentomes in the control group are shown. No significant changes were found. C=controls, H=high cortisol, L=low cortisol.
2.4. Real-time PCR
Quantitative real-time PCR was utilized to measure gene expression using an ABI Prism 7000 Sequence detection system (Applied Biosystems Inc; Foster City, CA). RNA was extracted from tissue using Trizol reagent (GIBCO/BRL, Grand Island, NY), and treated to remove genomic DNA (Turbo DNA free kit; Ambion Inc). cDNA was prepared using a high capacity cDNA kit (Applied Biosystems). Reactions were carried out using cDNA template (20 or 100 ng). Probes and primers used in the analysis are previously published sequences: IGF-I [16], IGF-II, insulin-like growth factor-I receptor (IGF-IR) and IGF-IIR [17], insulin-like growth factor binding protein-1 (IGF-BP1), -BP2 and -BP3[18], MR and GR [19], AT1R, AT2R, 11β-HSD1 and 2 [20], and β-actin [21]. All samples were run in triplicate with samples from all three experimental groups run on the same plate. Efficiency of amplification of all genes was quantitated to validate use of the delta Ct method. Expression of all genes was normalized to the expression of β-actin and data were analyzed by the delta Ct method [22].
2.5. Data analysis
Changes in gene expression were analyzed using the delta Ct values calculated relative to β-actin. A two-way ANOVA test was used to determine effects of cortisol treatment and placentome type on gene expression. Changes in gene expression between placentome types across all treatment groups were analyzed also by one way analysis of variance (ANOVA). Differences between individual groups were compared by post-hoc test, Duncan’s multiple range test. A p value of < 0.05 was considered statistically significant. Fold changes of the genes were calculated using the expression 2−ΔΔCt with respect to the mean value of delta Ct in the control group and the mean ± standard error of the mean fo the fold date was used for graphical purposes.
3. Results
Fetal weight, growth rate, maternal and fetal cortisol, ACTH, mean arterial blood pressure, and blood gases for the groups of animals used in this study have been previously reported (ref#8). Plasma cortisol concentrations at the end of the study were actually higher in the fetuses in the low cortisol group (11± 3 ng/ml) as compared to those in the high cortisol group (7.4±1.0 ng/ml) and control fetuses (5.7±1.0 0.9) because plasma ACTH values were elevated in this group by 130 days (204±1.0 54 as compared to 103±1.0 13 in the control and 80±1.0 7 in the high cortisol groups) (ref#8).
3.1. 11β-HSD1 and 2
There were no significant effect of either treatment group or placentome type on expression of 11β-HSD1 and 2 (Fig. 1). In addition, expression of 11β-HSD1 was similar to that of 11β-HSD2.
3.2. GR and MR
There were no significant of either treatment group or placentome type on expression of GR or MR among the treatment groups (Fig. 1).
3.3. AT1R and AT2R
There was no significant effect of treatment on the expression of AT1R in placenta, but treatment significantly altered AT2R expression in placenta (Fig. 2). Expression of AT2R mRNA was significantly increased overall in the high cortisol group (Figure 2, p=0.02); expression of AT2R was increased in B type placentomes in the high cortisol group compared with controls. There was reduced expression overall (regardless of group) of AT1R in type C cotyledons compared with type A (p=0.03). In all placentomes, expression of AT2R was 1.5- to 2-fold lower than that of AT1R. However, the ratio of AT2R to AT1R was significantly different across the placentome types (p<0.01), with a greater ratio of AT1R to AT2R expression in type A cotyledons compared with B and C types.
Figure 2.
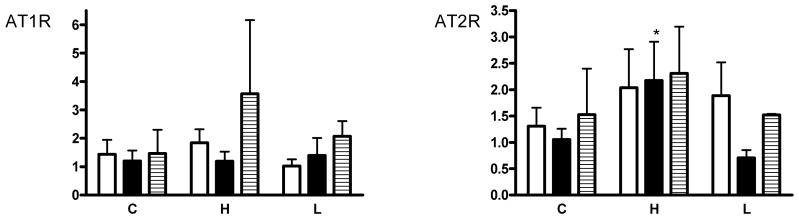
Expression of mRNA for the angiotensin receptors (AT1R and AT2R) in A, B and C type placentomes.
Fold changes with respect to A type placentomes in the control group are shown. C=controls, H=high cortisol, L=low cortisol. *p<0.05 compared with control B type.
3.4. The IGF axis
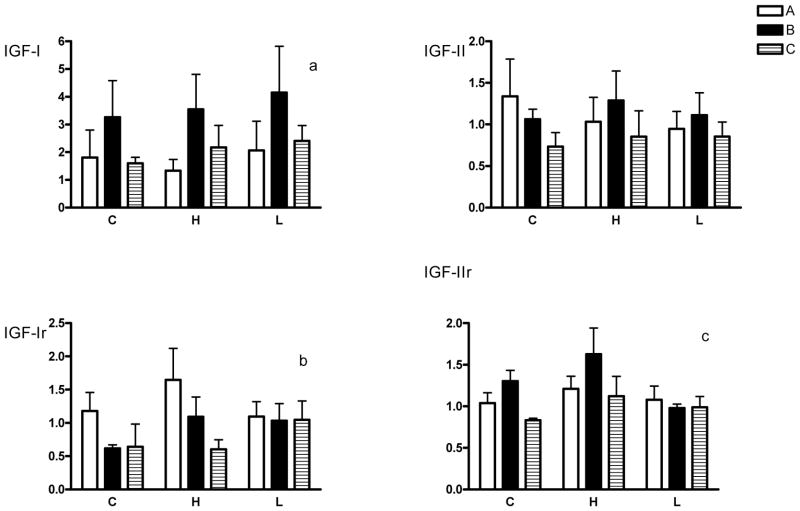
There were no differences in IGF-I, IGF-II, IGR-IR or IGF-IR expression among the treatment groups, although there were differences in expression among the placentome types. There was significantly more IGF-I mRNA in B versus A type placentomes overall (Fig. 3, p<0.01). There were no significant differences in IGF-II mRNA for placentome type or group. There was significantly less IGF-IR expression in B and C type placentomes compared with A types overall (p<0.05) and significantly less IGF-IIR RNA in C versus B type placentomes overall (p<0.05). There was a trend (p=0.06) for the high cortisol group to have higher IGF-IIR expression compared with controls regardless of placentome type, and a trend for IGF-IIR expression to be lower in B placentomes in the low cortisol group compared with controls (p=0.06). There were no significant differences in IGFBP-1 mRNA among placentome types or experimental groups (Fig. 4). There was significantly more IGFBP-2 mRNA in B and C type placentomes compared with A types regardless of group (p<0.05). In addition, IGFBP-2 mRNA was significantly higher in C placentomes in the high cortisol group compared with controls (p<0.05).
Figure 3.
Expression of mRNA for the insulin-like growth factors (IGF-I and IGF-II) and their receptors (IGF-IR and IGF-IIR) in A, B and C type placentomes.
Fold changes with respect to A type placentomes in the control group are shown. C=controls, H=high cortisol, L=low cortisol. Overall IGF expression in placentome types regardless of group: ap<0.05, B type placentomes compared with A type; bp<0.05, B and C type placentomes compared with A type; cp<0.05, C type placentomes compared with B type.
Figure 4.
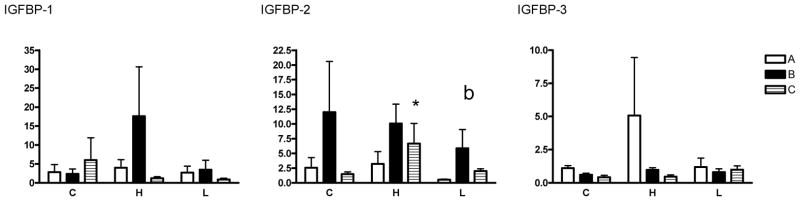
Expression of mRNA for the insulin-like growth factor binding proteins (IGFBP1-3) in A, B and C type placentomes.
Fold changes with respect to A type placentomes in the control group are shown. C=controls, H=high cortisol, L=low cortisol. *p<0.05 compared with control C type.
4. Discussion
Chronic cortisol level alterations during late pregnancy modulate placental and fetal growth, as well as uterine blood flow, which could have an effect on the growing nutrient demand of the fetus by the end of pregnancy. Previous studies in our lab have shown in late gestation fetal sheep that cortisol levels that are below normal [8] or above normal [8, 9] during pregnancy alter the distribution of placentome types. The same group of animals that were used for gene analysis in the current study had more type B placentomes in the high- and low-cortisol groups and individual B types were lighter in the low cortisol group than in the other two groups [8]. In addition, blood flow to the fetal zone of the placentomes was lower in the high- and low cortisol groups than in controls, although there were no significant changes in blood flow to different placentome types within any of the three treatment groups. In this cohort of animals we had also previously found that the rate of fetal growth was reduced with both high and low maternal cortisol levels (ref#8). Therefore, in the current study, we investigated the expression of genes in placentomes that could potentially be affected by cortisol to effect changes in placental and/or fetal growth and morphology.
We found that alteration of maternal cortisol concentrations did not have a significant effect on the expression of mineralocorticoid or glucocorticoid receptors or of 11β-HSD1 and 2 enzymes in the placenta. Both MR and GR were expressed in the ovine placenta, although expression of GR exceeded that of MR by 20–40 fold. Previous studies have suggested that 11β-HSD isoforms in placenta are altered by changes in maternal or fetal cortisol. In the human placenta, Murphy et al. showed a decrease in 11β-HSD2 in late gestation along with an increase in 11β-HSD1 gene expression during labor [23]. They suggested that in humans and other species, 11β-HSD2 is a key factor in determining fetal growth via its role in preventing excess maternal cortisol from crossing the placenta to the fetus. A previous study in sheep has also demonstrated that placental 11β-HSD2 decreases with increasing gestational age [24]. Clarke et al. showed that the decrease in 11β-HSD2 activity in ovine placentomes was associated with the rise in fetal plasma cortisol concentrations towards term, suggesting that cortisol may suppress 11β-HSD2 activity in late gestation [24]. In ewes, maternal treatment with dexamethasone also reduces placental 11β-HSD2 expression [25]. In contrast, expression of 11β-HSD1 in human chorionic trophoblasts was increased by incubation with 0.1–1 μM cortisol (REF: Li et al 2006 Endo.) Our data show that physiologic changes in maternal cortisol levels does not influence 11β-HSD2 or 11β-HSD1 expression in the late gestation placenta. Although at the time of sacrifice fetal plasma cortisol levels were not significantly changed by the changes in maternal cortisol, the changes in fetal ACTH (increased in the low cortisol group and decreased in the high cortisol group) suggested that there were small chronic changes in fetal cortisol concentrations. This result suggests the possibility that the magnitude of change in concentration of cortisol in maternal or fetal plasma required to alter 11β-HSD2 or 11β-HSD1 expression is greater than is accomplished with normal, physiological changes in maternal plasma cortisol concentration. Consistent with this latter possibility, we did not find any evidence of autoregulation of either GR or MR abundance in response to the induced changes in maternal plasma cortisol concentration.
The renin-angiotensin system has been implicated in normal vascularization of the placenta and placental blood flow [26, 27] The AT1 and AT2 receptors are also involved in fetal tissue development and cell differentiation [20, 28]. AT2Rs are widely expressed in the fetus and the neonate and rapidly decrease in expression at birth [28]. Consistent with our results, other investigators [20] have found that in the sheep cotyledonary tissue AT2R gene expression is more abundant than AT1R at all gestational ages. AT1R expression is greatest in early gestation at the time of maximal placental growth. Despite the differences in gene expression, the number of AT1R and AT2R binding sites in placental membranes is similar at 130 days. Both AT1R and AT2R mRNA have been found to be primarily localized to maternal tissue surrounding the fetal villi and in blood vessels. However, immunohistochemistry has suggested that AT1Rs are also localized in fetal binucleate and trophoblast cells and placental microcirculation [27]. In our study, maternal cortisol infusion significantly increased the expression of AT2R mRNA in the placenta. AT2R expression levels were significantly increased, particularly in B type placentomes, in the high cortisol group compared with those in controls or the low cortisol group. The significance of this finding is unclear as the role of the AT2R is poorly understood in fetal life.
We did not find any change in the expression of AT1R in the placenta with maternal cortisol treatment. Previous studies in other fetal organs have found a decrease in gene expression of AT1R in the kidney and liver and an increase [29] or decrease [17] in the heart as a result of cortisol treatment. We found that there was a significant increase in the ratio of AT2 to AT1 receptor expression in the B and C type cotyledons compared with A types, independent of treatment group. These findings are interesting since AT1R mediates effects such as vasoconstriction, smooth muscle cell proliferation or angiogenesis, while AT2R is believed to have “anti-AT1R effects” such as inhibition of cell growth, fetal tissue development, modulation of the extracellular matrix, apoptosis, cellular differentiation and vasodilation [20, 28]. Thus, the higher AT2R to AT1R ratio in type B and C cotyledons might reflect remodeling in these placentomes. AT1R is thought to have a critical role in mediating angiotensin II-induced endothelial cell proliferation [27].
The IGF axis plays an important role in placental development [30, 31] via IGF-I and IGF-II, which are capable of stimulating proliferation and differentiation in a variety of cell types [32]. Many of the components of the IGF axis have been found in the ovine placenta [33]. In our study, we found limited changes in the IGF axis in the placenta with varying maternal cortisol concentrations. We found an increase in IGFBP-2 expression in C type placentomes in the high cortisol group compared with controls. IGFBP-2 is thought to inhibit the actions of the IGFs by reducing their bioavailability [32]. The significance of these changes in the IGF axis is unclear.
A striking outcome in these experiments is the increased expression of IGF-I, IGF-II, and IGF-IIR and decreased expression of IGF-IR in the type B cotyledons compared with the other cotyledon types. We have previously reported that the high and low cortisol groups had an increased proportion of B relative to other types of placentomes [8]. Recent reports have indicated that IGF-I has a strong effect on angiogenesis, representing the formation of neovasculature from the endothelium of preexisting vessels [34, 35]. Therefore, it is possible that local IGF-I expression could have been involved in the increased proportion of the larger, B type placentomes, which may be more vascular. IGF-II has also been shown to be an angiogenic factor in uterine vascular adaptation during pregnancy [36]. We also found that IGF-IR expression was decreased in B and C type placentomes, perhaps suggesting downregulation of this receptor in response to the increased ligand expression. A decrease in IGF receptors could reflect a counter-regulatory mechanism of the increase in IGF-I [37].
In conclusion, these data suggest that genes that mediate cortisol action (11β-HSD1 and 2, GR and MR) in the placenta are unaffected by modest, yet physiologically relevant, changes in maternal cortisol concentrations. The lack of effect of the changes in maternal cortisol on expression of the components of the IGF system indicates that the outcome in fetal growth is not mediated by placental IGF expression. However, association of altered expression of genes of the IGF system with the shift in placentome type to the more everted shapes, especially type B placentomes, suggests that the IGF axis might be involved in angiogenesis during normal development in the fetal zone of the placenta. Changes in the renin-angiotensin system with cortisol infusion or between placentome types may play a role in vascular reactivity. Further studies are required to determine how maternal cortisol levels affect the structure and function of the placenta.
References
- 1.Vatnick I, Schoknecht PA, Darrigrand R, Bell AW. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol. 1991;15:351–6. [PubMed] [Google Scholar]
- 2.Ward JW, Forhead AJ, Wooding FB, Fowden AL. Functional Significance and Cortisol Dependence of the Gross Morphology of Ovine Placentomes During Late Gestation. Biol Reprod. 2005 doi: 10.1095/biolreprod.105.046342. [DOI] [PubMed] [Google Scholar]
- 3.Bell AW, Hay WW, Jr, Ehrhardt RA. Placental transport of nutrients and its implications for fetal growth. Journal of Reproduction and Fertility Supplement. 1999;54:401–10. [PubMed] [Google Scholar]
- 4.Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Redmer DA, et al. Placental angiogenesis in sheep models of compromised pregnancy. J Physiol. 2005;565:43–58. doi: 10.1113/jphysiol.2004.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vonnahme KA, Arndt WJ, Johnson ML, Borowicz PP, Reynolds LP. Effect of morphology on placentome size, vascularity, and vasoreactivity in late pregnant sheep. Biol Reprod. 2008;79:976–82. doi: 10.1095/biolreprod.108.070748. [DOI] [PubMed] [Google Scholar]
- 6.Jensen E, Wood C, Keller-Wood M. The normal increase in adrenal secretion during pregnancy contributes to maternal volume expansion and fetal homeostasis. J Soc Gynecol Investig. 2002;9:362–71. [PubMed] [Google Scholar]
- 7.Jensen E, Wood CE, Keller-Wood M. Alterations in maternal corticosteroid levels influence fetal urine and lung liquid production. J Soc Gynecol Investig. 2003;10:480–9. doi: 10.1016/s1071-5576(03)00153-9. [DOI] [PubMed] [Google Scholar]
- 8.Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol. 2005;288:R54–61. doi: 10.1152/ajpregu.00149.2004. [DOI] [PubMed] [Google Scholar]
- 9.Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late-gestation fetal sheep. J Endocrinol. 2002;174:27–36. doi: 10.1677/joe.0.1740027. [DOI] [PubMed] [Google Scholar]
- 10.Newnham JP, Evans SF, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. J Matern Fetal Med. 1999;8:81–87. doi: 10.1002/(SICI)1520-6661(199905/06)8:3<81::AID-MFM3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Hill KJ, Lumbers ER, Elbourne I. The actions of cortisol on fetal renal function. J Dev Physiol. 1988;10:85–96. [PubMed] [Google Scholar]
- 12.Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Effect of cortisol on blood pressure and vascular reactivity in the ovine fetus. Experimental Physiology. 1992;77:709–717. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- 13.Keller-Wood M, Cudd TA, Norman W, Caldwell SM, Wood CE. Sheep model for study of maternal adrenal gland function during pregnancy. Lab Anim Sci. 1998;48:507–12. [PubMed] [Google Scholar]
- 14.Wood CE. Are fetal adrenocorticotropic hormone and renin secretion suppressed by maternal cortisol secretion? Am J Physiol. 1988;255:R412–R417. doi: 10.1152/ajpregu.1988.255.3.R412. [DOI] [PubMed] [Google Scholar]
- 15.Wood CE, Rudolph AM. Can maternal stress alter fetal adrenocorticotropin secretion? Endocrinology. 1984;115:298–301. doi: 10.1210/endo-115-1-298. [DOI] [PubMed] [Google Scholar]
- 16.Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, Auer JA, et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660–72. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 17.Reini SA, Wood CE, Jensen E, Keller-Wood M. Increased maternal cortisol in late gestation ewes decreases fetal cardiac expression of 11{beta}-HSD2 mRNA and the ratio of AT1 to AT2 receptor mRNA. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1708–R1716. doi: 10.1152/ajpregu.00294.2006. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh S, Bloomfield FH, Bauer MK, Phua HH, Gilmour RS, Harding JE. Amniotic IGF-I supplementation of growth-restricted fetal sheep alters IGF-I and IGF receptor type 1 mRNA and protein levels in placental and fetal tissues. J Endocrinol. 2005;186:145–55. doi: 10.1677/joe.1.06113. [DOI] [PubMed] [Google Scholar]
- 19.Keller-Wood M, Wood CE, Hua Y, Zhang D. Mineralocorticoid receptor expression in late-gestation ovine fetal lung. Journal of the Society for Gynecologic Investigation. 2005;12:84–91. doi: 10.1016/j.jsgi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Koukoulas I, Mustafa T, Douglas-Denton R, Wintour EM. Angiotensin II receptor (type 1 and 2) expression peaks when placental growth is maximal in sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R972–82. doi: 10.1152/ajpregu.00070.2002. [DOI] [PubMed] [Google Scholar]
- 21.Keller-Wood M, von Reitzenstein M, McCartney J. Is the fetal lung a mineralocorticoid receptor target organ? Induction of cortisol-regulated genes in the ovine fetal lung, kidney and small intestine. Neonatology. 2009;95:47–60. doi: 10.1159/000151755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–44. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 24.Clarke KA, Ward JW, Forhead AJ, Giussani DA, Fowden AL. Regulation of 11 beta-hydroxysteroid dehydrogenase type 2 activity in ovine placenta by fetal cortisol. J Endocrinol. 2002;172:527–34. doi: 10.1677/joe.0.1720527. [DOI] [PubMed] [Google Scholar]
- 25.Kerzner LS, Stonestreet BS, Wu KY, Sadowska G, Malee MP. Antenatal dexamethasone: effect on ovine placental 11beta-hydroxysteroid dehydrogenase type 2 expression and fetal growth. Pediatr Res. 2002;52:706–12. doi: 10.1203/00006450-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Cox BE, Williams CE, Rosenfeld CR. Angiotensin II indirectly vasoconstricts the ovine uterine circulation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R337–44. doi: 10.1152/ajpregu.2000.278.2.R337. [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Bird IM, Chen DB, Magness RR. Angiotensin II regulation of ovine fetoplacental artery endothelial functions: interactions with nitric oxide. J Physiol. 2005;565:59–69. doi: 10.1113/jphysiol.2004.082420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucius R, Gallinat S, Busche S, Rosenstiel P, Unger T. Beyond blood pressure: new roles for angiotensin II. Cellular and Molecular Life Sciences. 1999;56:1008–19. doi: 10.1007/s000180050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segar JL, Bedell K, Page WV, Mazursky JE, Nuyt AM, Robillard JE. Effect of cortisol on gene expression of the renin-angiotensin system in fetal sheep. Pediatr Res. 1995;37:741–746. doi: 10.1203/00006450-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 31.Wathes DC, Reynolds TS, Robinson RS, Stevenson KR. Role of the insulin-like growth factor system in uterine function and placental development in ruminants. Journal of Dairy Science. 1998;81:1778–89. doi: 10.3168/jds.S0022-0302(98)75747-9. [DOI] [PubMed] [Google Scholar]
- 32.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds TS, Stevenson KR, Wathes DC. Pregnancy-specific alterations in the expression of the insulin-like growth factor system during early placental development in the ewe. Endocrinology. 1997;138:886–97. doi: 10.1210/endo.138.3.4983. [DOI] [PubMed] [Google Scholar]
- 34.Dobrucki LW, Tsutsumi Y, Kalinowski L, Dean J, Gavin M, Sen S, et al. Analysis of angiogenesis induced by local IGF-1 expression after myocardial infarction using microSPECT-CT imaging. Journal of Molecular and Cellular Cardiology. 2009 doi: 10.1016/j.yjmcc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9833–8. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herr F, Liang OD, Herrero J, Lang U, Preissner KT, Han VK, et al. Possible angiogenic roles of insulin-like growth factor II and its receptors in uterine vascular adaptation to pregnancy. Journal of Clinical Endocrinology and Metabolism. 2003;88:4811–7. doi: 10.1210/jc.2003-030243. [DOI] [PubMed] [Google Scholar]
- 37.Rubini M, Hongo A, D’Ambrosio C, Baserga R. The IGF-I receptor in mitogenesis and transformation of mouse embryo cells: role of receptor number. Experimental Cell Research. 1997;230:284–92. doi: 10.1006/excr.1996.3430. [DOI] [PubMed] [Google Scholar]