Abstract
Purpose
Magnetic resonance imaging (MRI) has been used as an imaging modality to assess pulmonary hypoplasia in congenital diaphragmatic hernias (CDHs). The objective of this study was to determine if there is a correlation between late gestational fetal MRI–derived total lung volumes (TLVs) and CDH outcomes.
Methods
From 2006 to 2009, 44 patients met criteria of an isolated CDH with a late gestational MRI evaluation. The prenatal TLV (in milliliters) was obtained between 32 and 34 weeks gestation. The measured study outcomes included survival, need for extracorporeal membrane oxygenation (ECMO), and length of stay.
Results
There were 39 left and 5 right CDH patients. The average TLV was significantly lower for nonsurvivors (P = .01), and there was a significant association between lower TLV and the need for ECMO (P = .0001). When stratified by TLV, patients with a TLV of greater than 40 mL had a 90% survival vs 35% survival for a TLV of less than 20 mL. Furthermore, patients with a TLV greater than 40 mL had a lower rate of ECMO use (10%) than patients with a TLV of less than 20 mL (86%). Shorter length of stay was found to correlate with increasing TLV (P = .022).
Conclusion
Late gestation fetal MRI–derived TLV significantly correlates with postnatal survival and need for ECMO. Fetal MRI may be useful for the evaluation of patients who present late in gestation with a CDH.
Keywords: Congenital diaphragmatic hernia, ECMO, Fetal MRI, Total lung volume, Pulmonary hypoplasia, Prenatal diagnosis
Congenital diaphragmatic hernia (CDH) is present in 1 of 3000 live births, with a reported mortality of 35% to 40% [1]. The mortality of the CDH patients is mainly caused by 2 pathologic processes: pulmonary hypoplasia and pulmonary hypertension. One of the goals of prenatal prognostic assessment has been to quantify the degree of pulmonary hypoplasia by fetal imaging to better counsel families prenatally, select for fetal intervention for the most severely affected, and anticipate the need for extracorporeal membrane oxygenation (ECMO) postnatally. The most commonly used modality in prenatal diagnosis is ultrasound, and the first and most commonly used prognostic indicator in CDH is lung-to-head circumference ratio (LHR) [2,3]. The use of the LHR has only been validated to accurately predict survival when obtained between 23 and 26 weeks gestation [2–4], and the current literature would suggest that an early gestation LHR of less than 1.0 would identify the infants at highest risk for a poor outcome [5]. However, the measurement of the LHR is challenging, and not all groups using LHR have been able to confirm its reliability [6]. Adding to the controversy, 3 different techniques for measuring LHR have been described, and the Eurofetus group further normalizes the LHR to gestational age expected LHR in normal fetuses to yield the observed-to-expected LHR (O/E LHR). Even if one assumes that the LHR accurately predicts survival, it is only valid when obtained between 23 and 26 weeks gestation precluding its use in counseling patients presenting later in gestation. Prenatal ultrasound also has been used to study the predictive value of prenatal liver herniation in CDH outcomes [5,7]. Mullassery et al [7] recently published a meta-analysis that concluded that liver herniation was associated with a poor prognosis; however, the overall sensitivity and specificity for survival was only 73% and 54%, respectively. Other studies using magnetic resonance imaging (MRI) have also come to similar conclusions that liver herniation may be associated with poor outcomes, but the conclusion from these studies are limited because of the small patient cohorts [8].
Since the first description of the LHR, the prenatal diagnosis and evaluation of patients with a CDH has undergone rapid change with the development of fetal MRI [8–11]. Rypens et al [12] were able to stratify normal fetal lung volumes based on MRI imaging and develop a nomogram for fetal lung volumes (32 week gestation mean fetal lung volume = 72 mL). With this information, the use of this new imaging modality has been used to assess the amount of lung volume in CDH patients, and this value has been used for predicting the postnatal outcomes and the need for ECMO in CDH patients [11,13,14]. The use of fetal MRI to calculate lung volumes in CDH was first reported in 2001 by groups in France [15–17]. Subsequently, Barnewolt et al [14], in 2007, used this technique to derive what they called the percent predictive lung volume (PPLV). In a small series of patients, they showed the PPLV to significantly correlate with mortality and ECMO use. Other recent studies that have examined the use of MRI have further demonstrated a trend toward improved accuracy in predicting patient outcomes using MRI-derived rather than ultrasound-derived observed-to-expected LHR [13].
A second fetal MRI–derived lung volume measurement was recently reported by the group in Mannheim as a predictor of CDH outcomes, the total fetal lung volume. This lung measurement has been found to correlate with both patient survival and ECMO use [11,18]. Our institution has been routinely obtaining this measurement during prenatal evaluation of CDH at 32 to 34 weeks gestation. The purpose of this study was to critically evaluate the predictive value of fetal MRI–derived late gestation total fetal lung volumes for postnatal survival and ECMO use in CDH.
1. Methods and materials
1.1. Patient demographics
Between 2006 and 2009, there were 136 consecutive patients who were evaluated at the Fetal Care Center of Cincinnati at Cincinnati Children’s Hospital for CDH. Patients were excluded from the study if there was an associated congenital cardiac defect or chromosomal abnormality. Of the 136 patients with a diagnosis of CDH evaluated during the study period, 44 had an isolated CDH that had a late gestation (32–34 weeks) fetal MRI for total lung volume (TLV) and who also were treated on the CDH Team Service at Cincinnati Children’s Hospital. This study is a retrospective review of data that have been prospectively collected in accordance with all Health Insurance Portability and Accountability Act (HIPAA) regulations and approval by the Cincinnati Children’s Hospital Institutional Review Board (no. 2008-0894).
1.2. Fetal MRI technique and measurements
Fetal MRI examinations were performed on a 1.5-T Signa magnet (General Electric Medical Systems, Milwaukee, WI). No maternal sedation was administered. Mothers were placed in a lateral decubitus position to avoid vasovagal stimulation. A torso phased array coil was wrapped around the mother’s pelvis, centered at the area of the fetus. Localization of the fetus was obtained with a 3-plane single-shot fast spin-echo sequence followed by a 2-dimensional fast imaging employing steady-state acquisition imaging anatomic to the pregnancy. The technique is one 90° pulse with multiple 180° refocusing echo pulses, obtaining slightly greater than half the phase-encoding lines. These single-shot fast spin-echo sequences with a TR of 4000 and TE of 88, slice thickness of 3 to 5 mm with no gap, field of view of 30 to 32, and matrix of 224 × 192 were performed anatomic to the fetus. No breath holding was necessary for the examination because each image was completed in 3 seconds. The longer acquisition time is caused by a longer echo train for higher signal and better resolution. A coronal sequence of the fetal chest was obtained to render the lung volumes. Therefore, fetal imaging was obtained to insure that minimal fetal motion was present on this sequence.
The images were transferred to a Vitrea workstation (Vital Images Inc, Minnetonka, MN). On each coronal image, the tissue of the right and left lung, excluding hernia contents and mediastinal structures, was measured. The volumes of the right and left lung were calculated by the summation of these measurements multiplied by the thickness of the slice. Total lung volume was then obtained via the summation of the right and left lung volumes (in milliliters).
1.3. Postnatal course
Patients were delivered at the level III obstetric hospitals (University Hospital or Good Samaritan Hospital) that are part of the Fetal Care Center of Cincinnati and near Cincinnati Children’s Hospital. The only exceptions to this were patients selected because of the severity of CDH for delivery at Children’s Hospital by cesarean section with ECMO standby or Ex-Utero Intrapartum Therapy (EXIT)-to-ECMO. The patients’ ventilatory management in the delivery room was aimed to limit any ventilator-induced barotraumas or volutrauma (gentilation) and immediate initiation of inhaled nitric oxide to limit acute pulmonary hypertensive reactions.
Once admitted to the CDH Team Service in the Regional Center for Newborn Intensive Care at Cincinnati Children’s Hospital, all patients were treated by the same group of CDH Team physicians (TMC, FYL, SGK, JSF, BH, and PSK) by standardized treatment protocols and management algorithms. For patient’s ventilatory management, when conventional ventilation was used, peak inspiratory pressure was limited to 24 cm H2O with the goal of keeping the PCO2 less than 65, pH greater than 7.25, and preductal saturation greater than 90%. High-frequency oscillatory ventilation was used only when PCO2 levels increased to greater than 100 despite reaching upper setting limits of conventional ventilation. BiVent settings, when used, were pressure high of less than 20 and pressure low of less than 3, with times high and low adjusted to achieve desired ventilatory rate. Extracorporeal membrane oxygenation support was initiated only when the CDH Team attending physician and the ECMO surgeon agreed that the patient was refractory to medical management. An objective criterion for CDH repair was used in which the patient’s pulmonary pressure was demonstrated to be less than 80% systemic blood pressure estimated by echocardiography. The type of repair was a transversus abdominis muscle flap, prosthetic Gore-Tex patch (Gore Medical, Flagstaff, AZ, USA), or a primary repair. A small subset of patients who had pulmonary pressures less than 50% systemic was repaired by a thoracoscopic approach. The technique of operative repair was at the discretion of the operating surgeon and the size of the defect.
1.4. Study design
This retrospective review of prospectively acquired data had a primary end point of correlating late gestation (32–34 weeks) multiplanar fetal MRI–based TLV with infant survival. Survivalwasdefined, for thepurposes of this study, as 60 days of age. Secondary outcome variables also examined included the need for ECMO support, the length of hospital stay, side of the defect, liver herniation, gestational age at delivery, and LHR obtained at between 23 and 26 weeks.
1.5. Statistical analyses
Results are shown as mean value within a range.Astandard deviation was also calculated for certain mean values. A χ2 test or Fisher’s Exact test was performed for comparison of categorical data, and a 2-tailed Student’s t test was used to compare ratio and interval variables. A bivariate correlation was calculated using TLV and length of stay. Using receiver operating characteristic (ROC) curves, the prognostic value of late gestational fetal MRI was also assessed. A P-value of less than .05 was considered statistically significant. All calculations were compiled and performed using Microsoft Excel (Microsoft Corporation, Redmond, WA) or SPSS version 11.0 software (SPSS Corp, Chicago, IL).
2. Results
2.1. Study population
The study consisted of 44 consecutive patients with an isolated CDH who met selection criteria. The patient demographics are listed in Table 1. There were 39 left CDH and 5 right CDH patients identified during the study period. The average gestational age at birth was 37 6/7 weeks (range, 34–39 5/7 weeks). Liver herniation was present in 59% of the study population. The mean TLV for the entire cohort was 27.7 ± 11.8 mL. Lung-to-head circumference ratio data were available for a subset of the cohort (n = 20), where the mean LHR was 1.12 ± 0.32.
Table 1.
Patient demographics
| Demographics | Values |
|---|---|
| Patients (n) | 44 |
| CDH side | 39 left/5 right |
| Gestational age at delivery | 37 6/7 wk (34–39 5/7 wk) |
| Liver herniation | 26 patients (59%) |
| TLV | 27.7 ± 11.8 mL |
| LHR (n = 20)a | 1.12 ± 0.32 |
Only 20 patients in this cohort were seen between 23 and 26 weeks gestation and had a valid LHR for comparison.
2.2. Patient outcomes
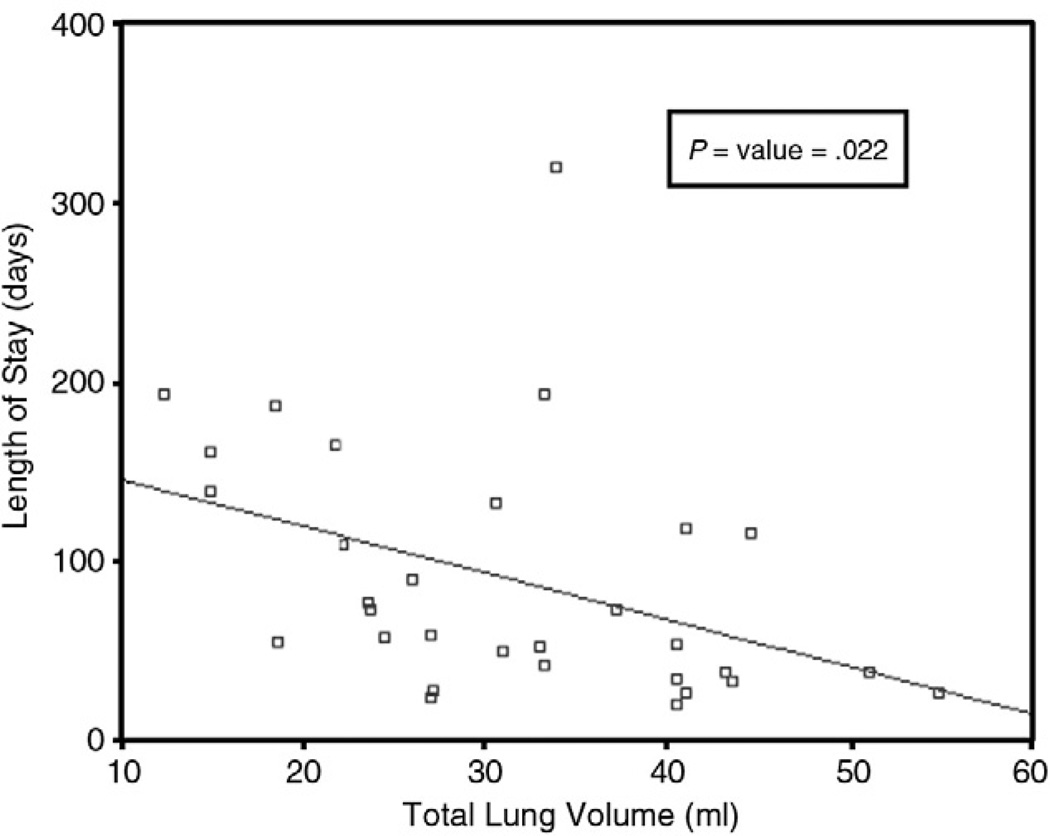
The mean late gestation TLV for survivors was 31.3 ± 11 mL vs 21.8 ± 10.4 mL for patients who died (P = .01). There were 18 patients who required ECMO, and there was a significant correlation between lower lung volume (20.7 ± 9.5 mL) and the need for ECMO support (P = .0001). When analyzing the association between TLV and length of stay (Fig. 1), a significant correlation was found between increasing TLV and decreased length of stay when analyzed by a bivariate correlation (P = .022).
Fig. 1.
A scatterplot of length of stay vs TLV. A lower TLV was correlated with an increased length of stay (P = .022).
2.3. Prognostic accuracy of late gestational total fetal lung volume
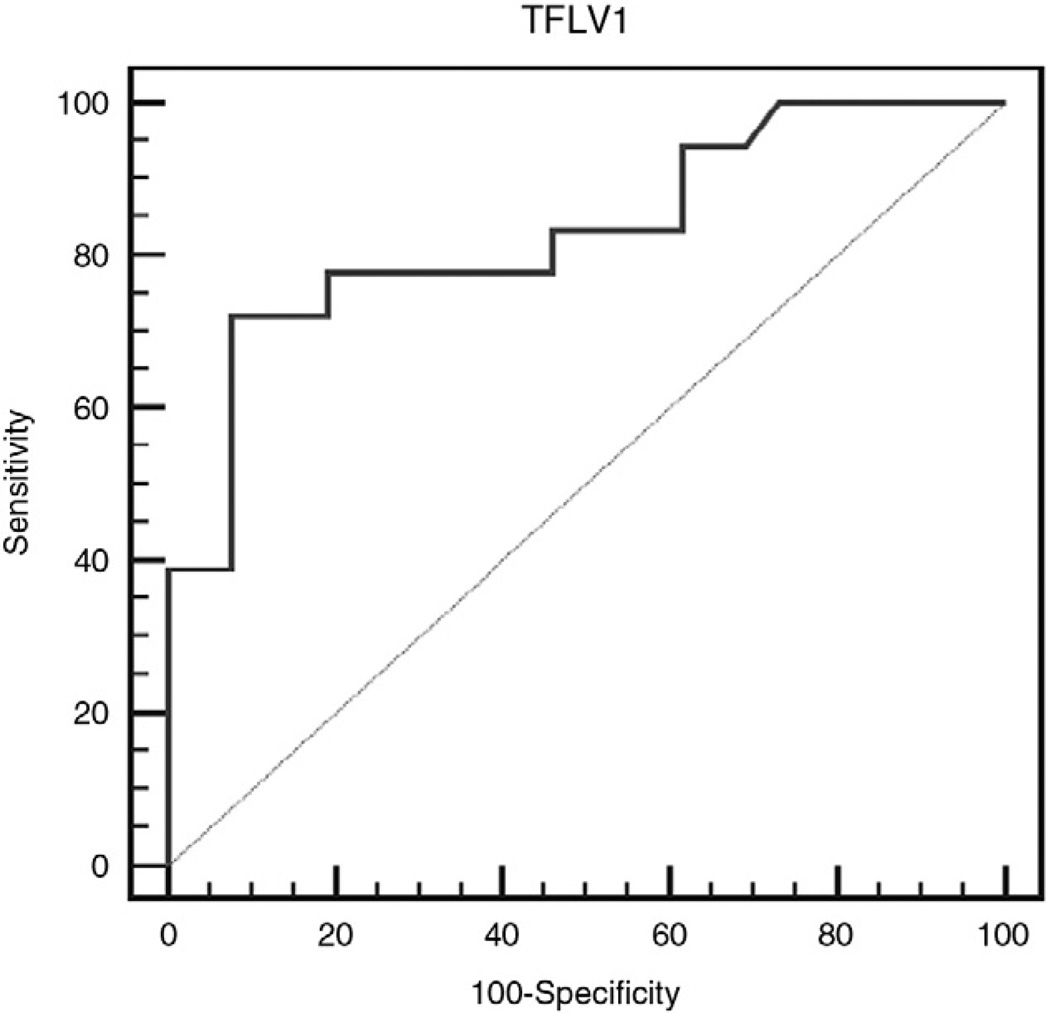
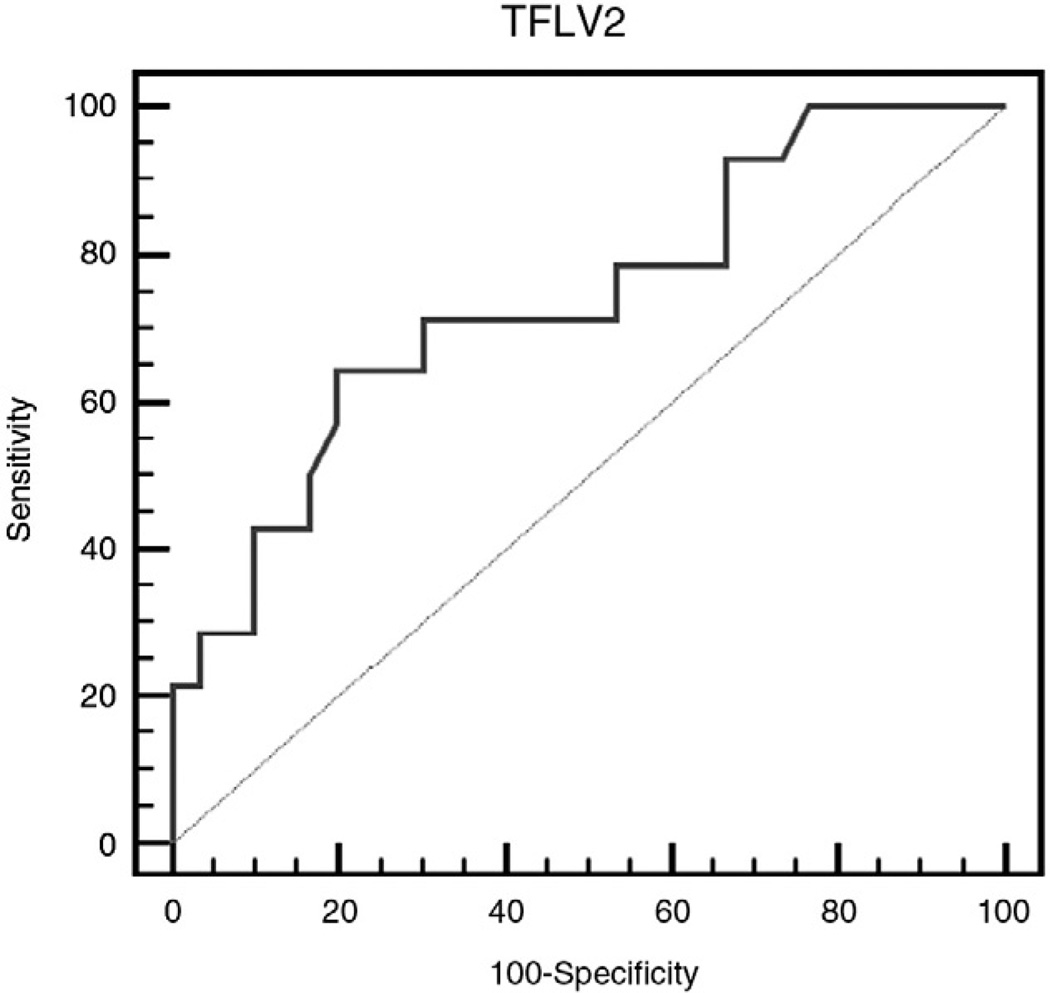
Receiver operating characteristic curves were generated for late gestational fetal MRI TLVs to determine the accuracy in predicting need for ECMO and patient survival. The ROC curve for patients who needed ECMO demonstrated an area under the curve of 0.83, with a sensitivity and specificity of 72% and 92%, respectively (Fig. 2). In regard to survival, the ROC curve demonstrated an area under the curve of 0.74 with a sensitivity of 64% and specificity of 80% (Fig. 3). For both ROC curves, the cutoff point that demonstrated the highest accuracy was at a TLV of 21.2 mL. Therefore, the stratification for outcomes by TLV was based on this cutoff point.
Fig. 2.
Receiver operating characteristic curve analysis (need for ECMO) that demonstrates an area under the curve of 0.83 with a sensitivity and specificity of 72% and 92%, respectively.
Fig. 3.
Receiver operating characteristic curve analysis (survival) that demonstrates an area under the curve of 0.74 with a sensitivity and specificity of 64% and 80%, respectively.
2.4. Outcomes based on TLV
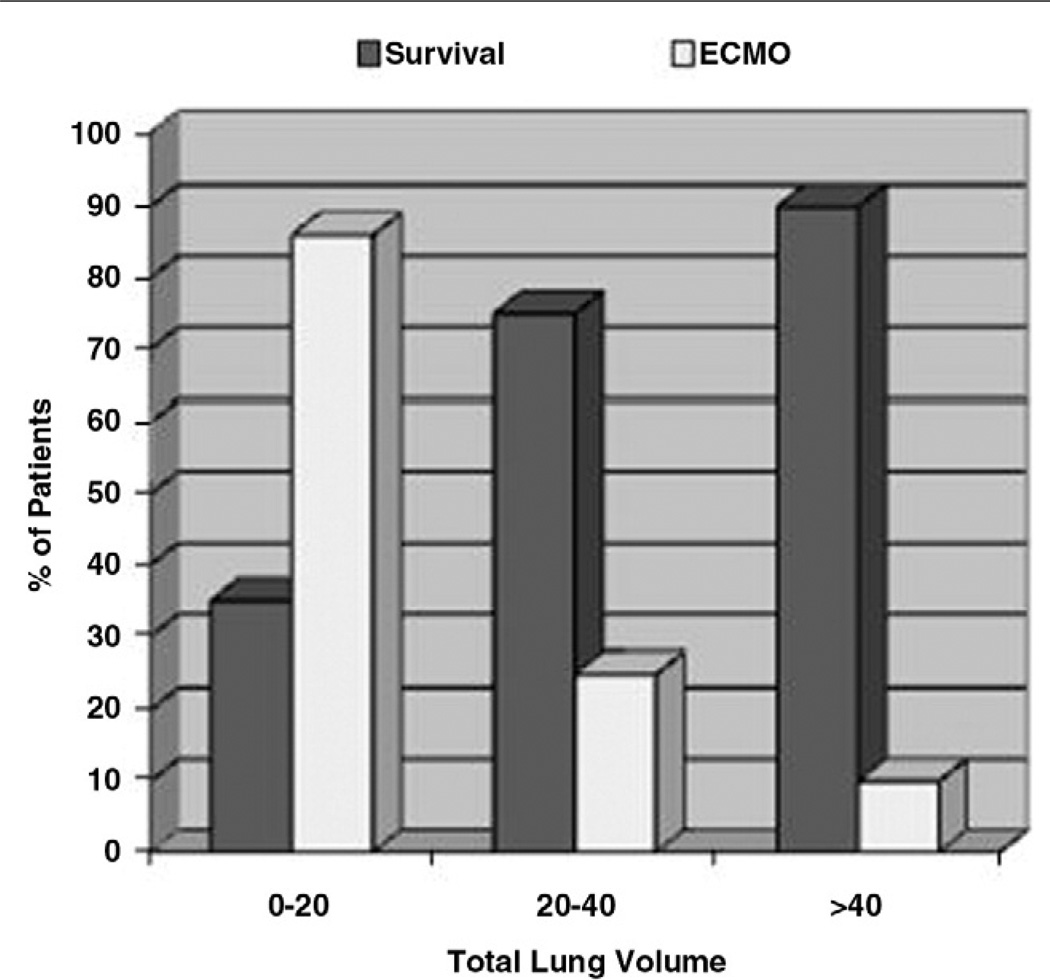
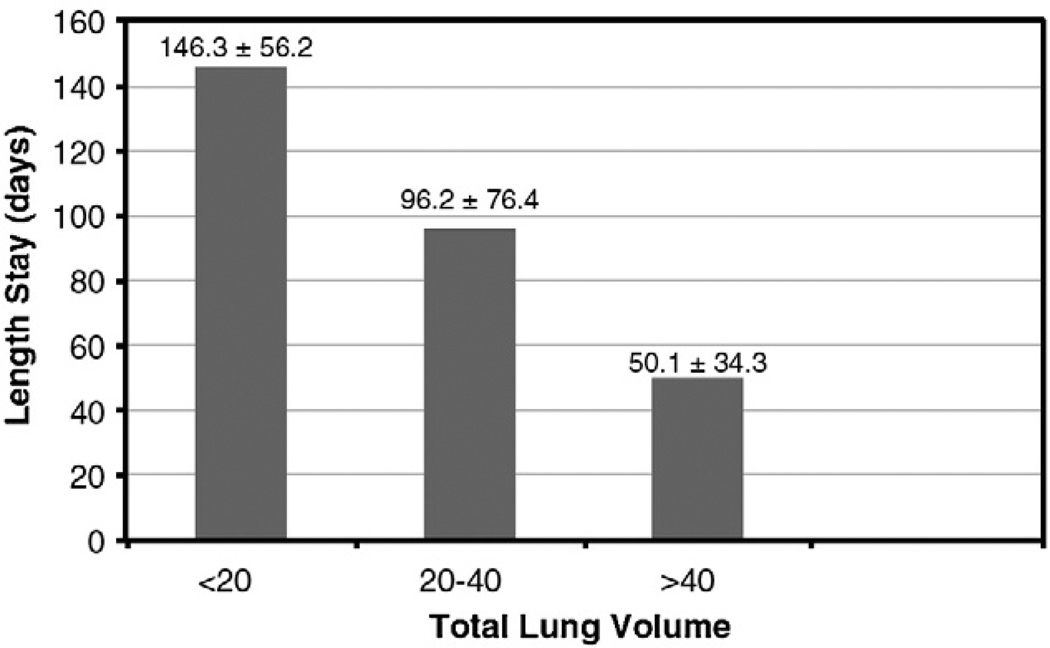
When stratifying patients by TLV, patients with higher lung volumes had better survival and less need for ECMO support (Fig. 4). Patients with a TLV of greater than 40 mL had a 90% survival, whereas patients with TLV of less than 20 mL had a 35% survival. In regard to the need for ECMO support, patients with a TLV of greater than 40 mL have a much lower rate of ECMO support (10% need for ECMO) than patients who have TLV of less than 20 mL (86% need for ECMO). With this same type of analysis, there is also a significant difference in the TLV and the length of stay (Fig. 5). Patients with a TLV of less than 20 mL have an average stay of 146.3 ± 56.2 days. Patients with greater TLV (20–40 mL) have a shorter length of stay (96.2 ± 76.4 days); however, there was no significant statistical difference between the TLV less than 20 and the TLV 20 to 40 mL groups (P = .1). In the final group, patients with a TLV of greater than 40 had a mean stay of 50.1 ± 34.3 days, and this is a significantly shorter stay (P ≤ .05) when compared with the other stratified groups.
Fig. 4.
A bar graph depicting ECMO use and survival after TLV stratification. Patients with a TLV of greater than 40 mL had a 90% survival and only 10% need for ECMO. In contrast, patients with a TLV of less than 20 mL had a 35% survival and 86% need for ECMO.
Fig. 5.
A bar graph depicting the length of stay and TLV stratification. There was a significantly lower length of stay for patients who had a TLV greater than 40 mL when compared with the lower groups (P = .05). There was no significant difference between the TLV less than 20 mL and the TLV 20 to 40 mL groups.
3. Discussion
This study demonstrates that late gestation fetal MRI–derived TLV may provide useful information for the counseling of patients who have a fetus with an isolated CDH. In this study, a clear association was observed between lower TLVs at 32 to 34 weeks gestation and the need for ECMO support and an increased postnatal mortality. These findings may have clinical benefit, especially in those patients with no previous workup or a workup from an outside facility without the capability of obtaining prognostic measurements such as LHR or PPLV. Although ultrasound-based LHR has been a standard means for assessing the severity of a CDH, studies on LHR have not been performed in late gestation, and the validity of LHR in late gestation has not been established. Lung-to-head circumference ratio may be less helpful in predicting outcomes when obtained in patients presenting later in gestation [2–4,19]. Furthermore, there has been controversy over the accuracy of LHR in assessing the degree of pulmonary hypoplasia and its reliability in serving as a predictive biometric parameter for patient outcomes [6,19].
Fetal MRI has developed an increasing role in the prenatal evaluation for isolated CDHs. The initial description of the use of fetal MRI–derived lung volumes in CDH was reported by Mahieu-Caputo et al [15]. Subsequently, Barnewolt et al [14] coined the term percent predicted lung volume (PPLV) and correlated the PPLV with ECMO use and postnatal mortality. The PPLV was calculated by obtaining the predicted lung volume (subtracting the mediastinal volume from the total thoracic volume) and then dividing the total calculated lung volume (right and left) by the predicted lung volume. The study demonstrated that patients with a PPLV of less than 15% had an increased need for ECMO support and a higher mortality. Although this is an elegant way to assess pulmonary hypoplasia, it relies on multiple measurements of mediastinal structures, total thoracic lung volume, as well as individual lung volume, whereas other studies have focused specifically on TLV to predict postnatal outcomes [11,18,20]. Kilian et al [11] and Busing et al [18] have reported a significant association between TLV and postnatal survival. Busing et al [18] further concluded that the absolute TLV was an accurate predictor of ECMO use and postnatal survival.
The results of the present study support the findings of these previous studies. In this study, we used an absolute TLV without any further calculations to correct to normative data or adjust for gestational age. The ROC curves demonstrated that the sensitivity and specificity of this absolute TLV is acceptable for allowing prognostic accuracy in determining survival and ECMO use. The TLV was shown to have a significant association between lower TLVs and increased mortality. Survivors had a significantly higher TLV than nonsurvivors, and non-ECMO patients also had a significantly higher TLV than ECMO patients. To better discriminate patient survival based on TLV, we also stratified patients based on TLV ROC curve analysis, which identified the most accurate cutoff TLV at 21 mL. Patients with a TLV of less than 20 mL had a 65% mortality rate, whereas patients with a TLV of greater than 40 mL had a 90% survival. This same trend was also seen with the need for ECMO. This would support the concept that the TLV may reflect the severity of pulmonary hypoplasia and in turn play a role in CDH outcomes and mortality. The final variable that was studied was the correlation between the length of stay and the TLV. The results showed a significant association between the TLV and length of stay (P = .022). When these patients were once again stratified, the group with the TLV greater than 40 mL had the shortest hospital stay with an average of 7 to 8 weeks.
The value of this data and its analysis is the ability to appropriately counsel expectant mothers at our institution in regard to the anticipated duration of the postnatal hospital course and postnatal outcomes. As has been observed with other predictors of survival in CDH, the correlation of TLV with specific rates of survival, need for ECMO, and duration of hospital stay may be institution specific, but general trends should translate to other institutions. The utility of late gestational TLV MRI in our institution is that as a referral center, we often see patients later in gestation without the benefit of testing earlier in gestation (ie, LHR at 24–26 weeks). The use of fetal MRI–derived TLV in late gestation is a tool on which we rely not only in assessing the severity of a CDH and then appropriately counseling new patients but also in confirming the degree of severity of patients who were evaluated earlier in gestation. It has been our clinical impression that late gestation assessment of fetal lung volume has an advantage over earlier assessment because it reflects the degree of lung growth that should have occurred during the third trimester. Congenital diaphragmatic hernia prevents the normal lung growth that should occur during the third trimester; and the more severe the herniation, the less lung growth occurs.
Although this study supports the current body of literature, the results should be examined with an understanding that these are institution-specific findings with potential inherent biases. There are 3 full-time radiologists working in Fetal Imaging who review all fetal MRIs and a single technologist who generates the lung volumes for these studies. This allows for a level of consistency in the generation of TLV. In addition, this is a retrospective study of a quaternary referral patient population, which allows for observational biases, and the sample size is relatively small. Our findings in late gestation fetal MRI–derived TLV should be validated in other centers with fetal MRI capabilities or a multicenter collaboration where larger numbers can be obtained in a prospective fashion. This prospective manner would also allow for direct comparisons of the predictive values of TLV with LHR and PPLV.
In conclusion, the results of this study demonstrate a significant association between late gestation fetal MRI–derived TLV and patient outcomes in cases of isolated CDH. These results may allow for personalized predictive prenatal counseling for expectant mothers whose child has an isolated CDH. In particular, TLV may be used for more accurate counseling in patients who present later in gestation from an outside facility or who have had no previous imaging and allow patients to accurately plan for the duration of the postnatal hospitalization.
References
- 1.Langham MR, Jr, Kays DW, Ledbetter DJ, et al. Congenital diaphragmatic hernia: epidemiology and outcome. Clin Perinatol. 1996;23:671–688. [PubMed] [Google Scholar]
- 2.Lipshutz GS, Albanese CT, Feldstein VA, et al. Prospective analysis of lung-to-head ratio predicts survival for patients with prenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 1997;32:1634–1636. doi: 10.1016/s0022-3468(97)90471-1. [DOI] [PubMed] [Google Scholar]
- 3.Metkus AP, Filly RA, Stringer MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. Pediatr Surg. 1996;31:148–151. doi: 10.1016/s0022-3468(96)90338-3. [DOI] [PubMed] [Google Scholar]
- 4.Yang SH, Nobuhara KK, Keller RL, et al. Reliability of the lung-to-head ratio as a predictor of outcome in fetuses with isolated left congenital diaphragmatic hernia at gestation outside 24–26 weeks. Am J Obstet Gynecol. 2007;197:30.e1–30.e7. doi: 10.1016/j.ajog.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Hedrick HL, Danzer E, Merchant A, et al. Liver position and lung-to-head ratio for prediction of extracorporeal membrane oxygenation and survival in isolated left congenital diaphragmatic hernia. Am J Obstet Gynecol. 2007;197:422.e1–422.e4. doi: 10.1016/j.ajog.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Heling KS, Wauer RR, Hammer H, et al. Reliability of the lung-to-head ratio in predicting outcome and neonatal ventilation parameters in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2005;25:112–118. doi: 10.1002/uog.1837. [DOI] [PubMed] [Google Scholar]
- 7.Mullassery D, Ba’ath ME, Jesudason EC, et al. Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2010;35:609–614. doi: 10.1002/uog.7586. [DOI] [PubMed] [Google Scholar]
- 8.Worley KC, Dashe JS, Barber RG, et al. Fetal magnetic resonance imaging in isolated diaphragmatic hernia: volume of herniated liver and neonatal outcome. Am J Obstet Gynecol. 2009;200:318.e1–318.e6. doi: 10.1016/j.ajog.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Vuletin JF, Lim FY, Cnota J, et al. Prenatal pulmonary hypertension index: novel prenatal predictor of severe postnatal pulmonary artery hypertension in antenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 2010;45:703–708. doi: 10.1016/j.jpedsurg.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balassy C, Kasprian G, Brugger PC, et al. Assessment of lung development in isolated congenital diaphragmatic hernia using signal intensity ratios on fetal MR imaging. Eur Radiol. 2010;20:829–837. doi: 10.1007/s00330-009-1633-x. [DOI] [PubMed] [Google Scholar]
- 11.Kilian AK, Schaible T, Hofmann V, et al. Congenital diaphragmatic hernia: predictive value of MRI relative lung-to-head ratio compared with MRI fetal lung volume and sonographic lung-to-head ratio. AJR Am J Roentgenol. 2009;192:153–158. doi: 10.2214/AJR.08.1082. [DOI] [PubMed] [Google Scholar]
- 12.Rypens F, Metens T, Rocourt N, et al. Fetal lung volume: estimation at MR imaging-initial results. Radiology. 2001;219:236–241. doi: 10.1148/radiology.219.1.r01ap18236. [DOI] [PubMed] [Google Scholar]
- 13.Jani J, Cannie M, Sonigo P, et al. Value of prenatal magnetic resonance imaging in the prediction of postnatal outcome in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol. 2008;32:793–799. doi: 10.1002/uog.6234. [DOI] [PubMed] [Google Scholar]
- 14.Barnewolt CE, Kunisaki SM, Fauza DO, et al. Percent predicted lung volumes as measured on fetal magnetic resonance imaging: a useful biometric parameter for risk stratification in congenital diaphragmatic hernia. J Pediatr Surg. 2007;42:193–197. doi: 10.1016/j.jpedsurg.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Mahieu-Caputo D, Sonigo P, Dommergues M, et al. Fetal lung volume measurement by magnetic resonance imaging in congenital diaphragmatic hernia. BJOG. 2001;108:863–868. doi: 10.1111/j.1471-0528.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- 16.Paek BW, Coakley FV, Lu Y, et al. Congenital diaphragmatic hernia: prenatal evaluation with MR lung volumetry—preliminary experience. Radiology. 2001;220:63–67. doi: 10.1148/radiology.220.1.r01jl4163. [DOI] [PubMed] [Google Scholar]
- 17.Gorincour G, Bouvenot J, Mourot MG, et al. Prenatal prognosis of congenital diaphragmatic hernia using magnetic resonance imaging measurement of fetal lung volume. Ultrasound Obstet Gynecol. 2005;26:738–744. doi: 10.1002/uog.2618. [DOI] [PubMed] [Google Scholar]
- 18.Büsing KA, Kilian AK, Schaible T, et al. MR relative fetal lung volume in congenital diaphragmatic hernia: survival and need for extracorporeal membrane oxygenation. Radiology. 2008;248:240–246. doi: 10.1148/radiol.2481070952. [DOI] [PubMed] [Google Scholar]
- 19.Ba’ath ME, Jesudason EC, Losty PD. How useful is the lung-to-head ratio in predicting outcome in the fetus with congenital diaphragmatic hernia? A systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2007;30:897–906. doi: 10.1002/uog.5164. [DOI] [PubMed] [Google Scholar]
- 20.Neff KW, Kilian AK, Schaible T, et al. Prediction of mortality and need for neonatal extracorporeal membrane oxygenation in fetuses with congenital diaphragmatic hernia: logistic regression analysis based on MRI fetal lung volume measurements. AJR Am J Roentgenol. 2007;189:1307–1311. doi: 10.2214/AJR.07.2434. [DOI] [PubMed] [Google Scholar]