Abstract
Among the now pandemic sexually transmitted infections (STIs), Chlamydia trachomatis (C. trachomatis) is the predominant bacterial pathogen and human immunodeficiency virus type 1 (HIV-1) is the most lethal of the viral pathogens. The female genital tract is the primary site for heterosexual transmission of both C. trachomatis and HIV-1. Infection with C. trachomatis, and with a variety of other STIs, increases the risk for transmission of HIV-1, although the mechanisms for this finding remain unclear. We have used in vitro modeling to assess the mechanisms by which infection with genital C. trachomatis serovars might increase the transmission of HIV-1 across the female genital tract. C. trachomatis infection of an immortalized endocervical epithelial cell line (A2EN) increases the cell surface expression of the HIV-1 alternative primary receptor, galactosyl ceramide (GalCer), and of the HIV-1 co-receptors, CXCR4 and CCR5. C. trachomatis infection also increases the binding of HIV-1 to A2EN cells, and, subsequently, increases levels of virus in co-cultures of HIV-exposed A2EN and susceptible MT4-R5 T cells. Finally, in vivo endocervical cell sampling reveals a dramatic increase in the number of CD4+, CXCR4 and/or CCR5 positive T cell targets in the endocervix of C. trachomatis positive women when compared to those who are C. trachomatis negative. This combination of in vitro and in vivo results suggests several mechanisms for increased transmission of HIV-1 across the endocervices of C. trachomatis-infected women.
Keywords: C. trachomatis, HIV-1, endocervix, epithelia
INTRODUCTION
The worldwide burden of Chlamydia-associated morbidity is great. Chlamydia trachomatis (C. trachomatis) infections represent the most common preventable cause of blindness worldwide and the predominant cause of bacterial sexually transmissible infections (STIs) [1]. There exist a wide variety of clinical pathologies associated with chlamydial infections. C. trachomatis serovars D-K cause the most prevalent bacterial STI in the United States [2–3]. In 2009, there were 409.2 cases of C. trachomatis infection reported per 100,000 people in the United States alone [2] and, despite worldwide detection and treatment programs, the incidence of C. trachomatis infection continues to increase [4]. Infection in females is often asymptomatic but can lead to chronic disease and significant morbidity, including ascending infection, co-infection with other pathogens, pelvic inflammatory disease, chronic and debilitating pelvic pain and tubal infertility. The remarkable ability of C. trachomatis to infect the female genital tract in the absence of significant local or systemic symptoms allows the pathogen to go untreated for extended periods of time. This characteristic increases the likelihood of secondary pathogen introduction into a C. trachomatis-infected genital tract.
Sexually transmitted infections by C. trachomatis
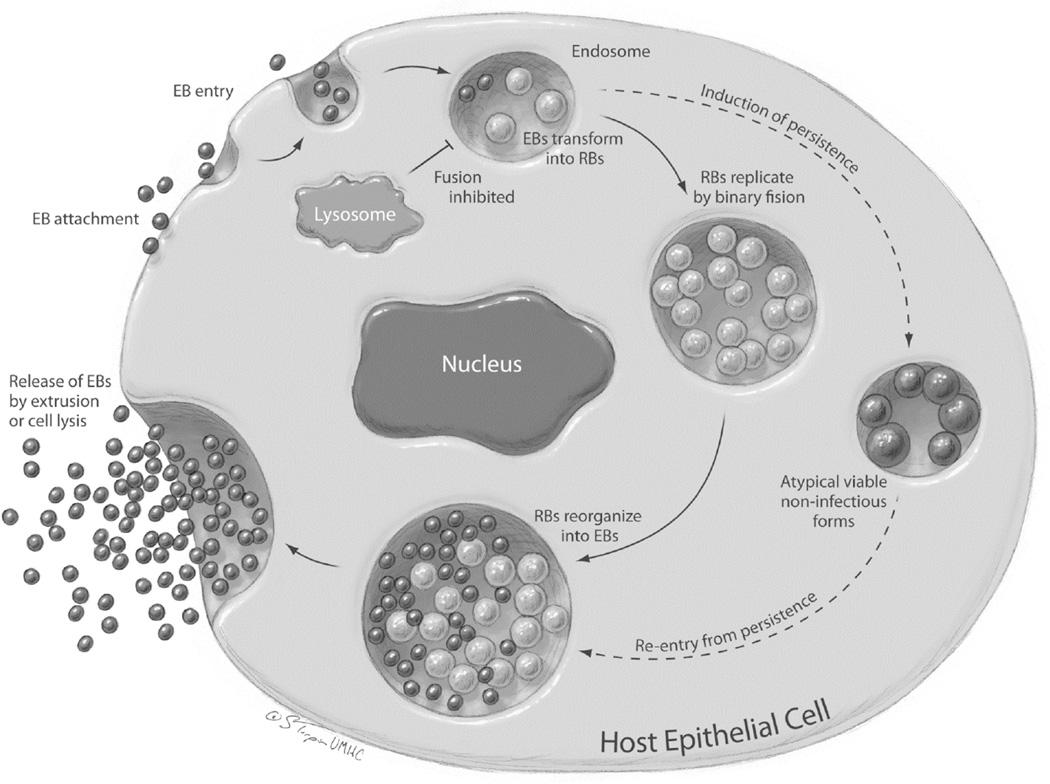
C. trachomatis is an obligate intracellular bacterium with a distinctive biphasic developmental cycle (Figure 1). The bacteria alternate between two forms: the metabolically inactive, infectious elementary body (EB) and the metabolically active, non-infectious, intracellular reticulate body (RB). The ability of C. trachomatis to alternate between resting EBs and replicating, metabolically active RBs enhances the organism’s capacity to survive in the human reproductive tract [5]. Infection of the human female reproductive tract by C. trachomatis serovars D-K most commonly occurs in the endocervix and transitional zone, however bacteria can ascend into the upper reproductive tract [6]. Although selectivity for genital columnar and transitional epithelial cells by C. trachomatis serovars D-K is exquisite in vivo, the pathogen can infect a variety of transformed cell types in vitro [6–8]. Binding of EB appears to occur at or near host cell microvilli and attachment induces receptor mediated endocytosis [7, 9–10] of the EB. The ligand receptor pairs involved in chlamydial attachment to the host cell surface are incompletely described, although there is evidence for involvement of the chlamydial heat shock proteins 70 [11] and 60 [12], for host cell heparin sulfate [13–14] and for the host cellular chaperone, protein disulfide isomerase (PDI), which is a component of the estrogen receptor complex [9, 12]. Elementary bodies entering the infected cell are incorporated into phagosomes; these phagosomes subsequently move to the distal region of the Golgi complex [15]. Chlamydiae avoid immediate destruction by host cellular defenses by actively inhibiting fusion of the EB-containing phagosomes with the cellular lysosome [15–16]. EBs within the phagosome then differentiate into metabolically active RBs, which begin to secrete chlamydial proteins into the phagosome and into the cellular cytoplasm and begin to replicate by binary fusion [16–19]. The phagosome grows to fill an increasingly larger proportion of the host cell cytoplasm and is called a chlamydial inclusion. At the completion of the developmental cycle, RB condense back into EB and the EB are released into the extracellular space from the infected cell by extrusion or cell lysis [17, 20]. In the presence of several cell stressors, including exposure to some antibiotics, metabolite deprivation and sub-inhibitory concentrations of interferon gamma (IFNγ), C. trachomatis may enter an alternate developmental pathway in vitro [21–23]. This pathway, termed chlamydial persistence in vitro, is characterized by the presence of morphologically aberrant RB within the chlamydial inclusion, the long-term presence of viable, but non-cultivable chlamydial organisms and continued chlamydial chromosome division in the absence of binary fission of the organism or re-differentiation into EB [24]. Upon removal of external stressors, C. trachomatis organisms can typically return to the normal developmental cycle fairly rapidly in vitro [22–24]. To date, there is no direct evidence of chlamydial persistence in vivo, although multiple clinical attributes of the disease suggest that it occurs (reviewed in [25]).
Figure 1. The C. trachomatis developmental cycle.
C. trachomatis travels between susceptible cells as small, metabolically-inert forms, called the elementary bodies (EBs). EBs attach to host cells and enter into a phagosomal compartment partially derived from the host cell Golgi apparatus. Within the phagosomal compartment, now called an inclusion body, EBs transform into larger, metabolically active forms called reticulate bodies (RBs). RBs reproduce by binary fission, filling the enlarging inclusion body. Late in the C. trachomatis life cycle (36–48 hours), RBs re-organize into EBs and EBs are released into the extracellular space to infect surrounding susceptible cells. Under certain in vitro conditions, C. trachomatis RBs undergo differentiation into large atypical forms that may persist within the infected cell for prolonged periods of time. Clinical and epidemiologic characteristics of C. trachomatis infections also support persistence. Reproduced from: Ibana JA and Schust DJ. Chapter 86, Chlamydia trachomatis. In Winn HN, Chervenak FC, and Romero R (eds), Clinical Maternal Fetal Medicine Online Second Edition, Informa Healthcare 2012, London, with permission.
Although infection with C. trachomatis has been reported to increase cervical shedding of and co-infection by HIV-1 [26–29], the mechanisms by which this occurs remain unknown. Several mechanisms have been proposed. In the uninfected baseline state, the endocervix and transformation zone contain many immune cells and these numbers are increased during cervicitis [30]. Since infection of immune cells expressing the primary HIV receptor, CD4, and HIV’s chemokine co-receptors, CXCR4 or CCR5, is known to cause the major disease manifestations of HIV infection, a local increase in HIV target cells in the presence of chlamydial infection could be assumed to be a primary factor linking increased HIV transmissibility to C. trachomatis infection. C. trachomatis infection has also been shown to decrease epithelial integrity in vitro, as evidenced by decreased transepithelial resistance and cervical epithelial cell shedding in selected C. trachomatis-infected genital epithelial models [31–33]. This could facilitate movement of HIV-1 between mucosal epithelial cells and allow direct contact with infiltrating immune cells. Finally, direct epithelial cell mechanisms could be involved in the genital tract, a possibility suggested by findings in the oral cavity, another mucosal surface across which HIV can be transmitted. Briefly, oral mucosal epithelial cells infected with Porphyromonas gingivalis exhibit increased cell surface expression of the HIV-1 co-receptor, CCR5, and this has been linked to the increased risk for oral-genital HIV transmission in the presence of periodontal disease [34–35].
Sexual transmission of HIV-1 across the endocervical epithelium
We recently developed an immortalized human endocervical epithelial cell line (A2EN) that retains site-appropriate expression of differentiation proteins, cell surface molecules driving innate immune responses [36–37] and that can express HIV co-receptors (current review). Unlike the vaginal mucosa, which is comprised of multiple layers of keratinized squamous cells, the endocervical epithelial barrier is but a single layer of columnar epithelial cells [30]. Although the epithelial mucosal surface of the endocervix is commonly described as glandular, no true glands are formed and the human endocervical architecture may be more correctly described as forming endocervical crypts (Figure 2). Other than a small area of the transformation zone as it approaches the endocervix, the endocervical mucosal epithelium represents the only single-cell layered barrier to pathogen transmission in the lower genital tract [30]. This vulnerability may combine with the subepithelial immune cell infiltrates seen in cervicitis to make the endocervical epithelial barrier a particularly relevant site for HIV transmission in a C. trachomatis-infected genital tract. Our novel cell line allows mechanistic study of this mode of transmission.
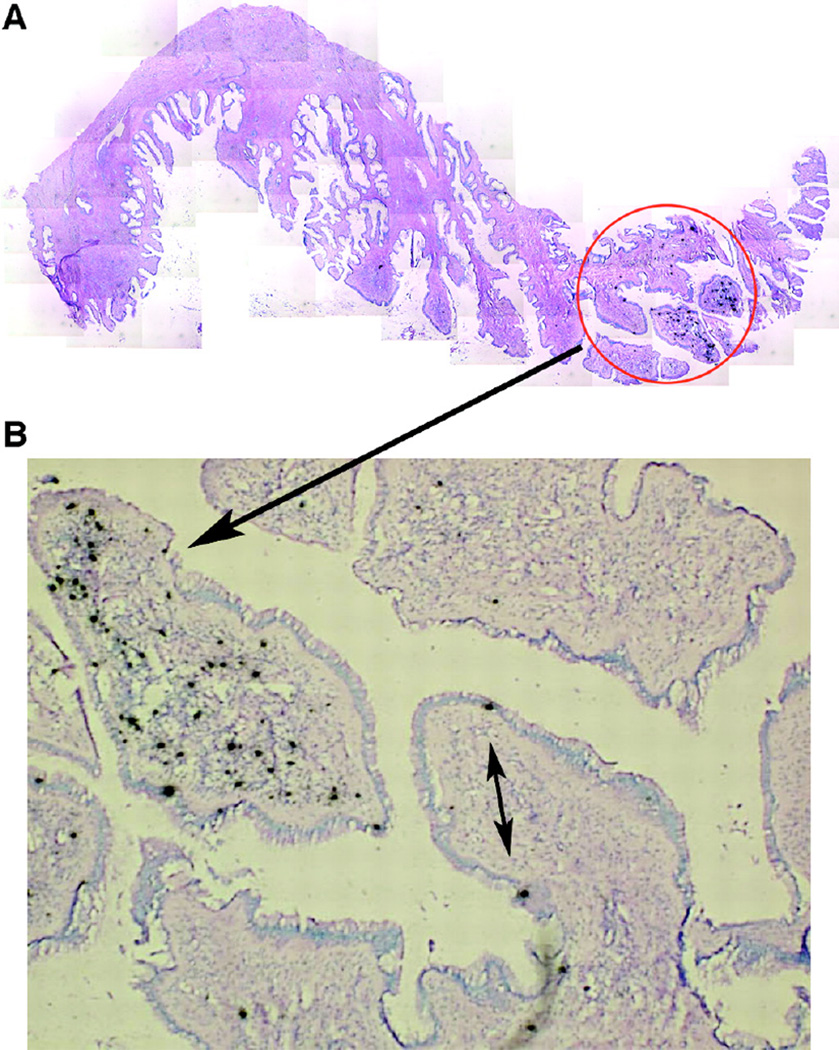
Figure 2. Highly focal productive infection in an endocervix 4 days after intravaginal simian immunodeficiency virus (SIV) inoculation.
A. Montage of images (magnification, ×100) of a single section of endocervix, among 20 sections examined from monkey 31385, in which one focus (encircled) of SIV RNA-positive cells was detectable. B. SIV RNA-positive cells at a higher magnification. The double-headed arrow points to two SIV RNA-positive cells that appear to be intraepithelial lymphocytes. The single-headed arrow points to a focal collection of SIV RNA-positive cells in the endocervical mucosa. SIV RNA was detected by in situ hybridization (ISH) with radiolabeled riboprobes. In the developed radioautographs viewed with transmitted light, the SIV RNA-positive cells appear black because of the large number of silver grains overlying the cell. Original magnification, ×100. Reproduced from: Miller CJ, et. al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol 2005;79(14):9217–9227, with permission.
Lessons from simian immunodeficiency virus transmission models in non-human primates
A series of investigations in non-human primate models has also implicated the endocervix and transformation zone as preferential sites for initial heterosexual transmission of HIV. Studies from the laboratories of Miller and Haase have given us a clear picture of the initial events in transmission of simian immunodeficiency virus (SIV) across the female rhesus macaque genital tract in the absence of genital microtrauma [38–41]. These important studies used high dose [105 50% tissue culture infectious dose (TCID50) and atraumatic SIV vaginal exposures. The investigators have demonstrated that transmission across the mucosa of the reproductive tract is very rapid (within hours) and that both T cells and dendritic cells/Langerhans cells are infected [38, 42]. HIV appears to be able to directly infect CD4+ T cells without the assistance of Langerhans cells in ex vivo vaginal organ culture models [43]. In their non-human primate (NHP) models, Miller et al, have shown that SIV virions are trapped in the cervical mucus and are likely released over time to underlying cells [40]. Interestingly, in the NHP female vaginal tract, infection occurred in small and isolated microfoci [39–40] and the endocervical epithelium was specifically implicated in establishing these foci by producing the pDC/T cell chemokine MIP-3α/CCL20 in response to viral challenge [39]. It is also reported that these small founder populations of infected cells must undergo expansion during the first week post-exposure prior to dissemination to secondary lymphoid organs [40]. This establishment of local founder populations may not be relevant after oral and rectal exposures, as post-exposure dissemination of SIV and simian/human immunodeficiency virus (SHIV), respectively, appears to be much more rapid than that after vaginal inoculation with SIV [44–45].
The high dose vaginal SIV/NHP inoculation model has been criticized for its application to human transmission of HIV since exposure levels in humans are much lower than those used in the NHP model [46]. That said, the model does allow for documentation of early events in mucosal transmission across the female genital tract that may never be able to be ethically studied or technically feasible in light of the typically low in vivo virion exposure levels in humans. An argument for the relevance of the high dose vaginal inoculation NHP model lies in its ability to predict the well-described genetic bottleneck seen in after human sexual transmission of HIV. The small number of isolated founder populations detected after vaginal SIV inoculation may explain the significantly lower genetic diversity of HIV virus detected in newly-infected women when compared to their heterosexual transmitting partner [47]. Studies on human heterosexual transmission pairs have demonstrated that most newly-infected partners carry a single virus and the remainder carry only 2–5 viral subtypes [48]. Others have used rare human clinical data to suggest that HIV-1 transmission does not occur via the cervical transformation zone or endocervix. Heterosexual HIV transmission of HIV has been reported in a woman with Meyer-Rokitansky-Kuster-Hauser syndrome, a defect in the development of the female reproductive tract that results in congenital absence of the upper vagina, cervix uterus and fallopian tubes [49]. While this finding clearly demonstrates that heterosexual HIV transmission can occur across the lower vaginal mucosa, it does not suggest preferential sites of transmission and may only indicate that transmission can occur across several sites in the lower female genital tract.
We were particularly intrigued by the high-dose atraumatic vaginal SIV NHP inoculation model since it has consistently shown that the preferential sites for the development of founder infections were detected in the endocervix and in the cervical transformation zone [[39–40]; Figure 2]. This suggested to us that despite the larger surface area of the vaginal mucosa when compared to that of the human endocervix and human cervical transformation zone, the latter tissues likely represent important sites for HIV transmission in humans, a concept further supported by an immune cell mapping study of the human female reproductive tract that showed the highest concentrations of HIV immune target cells are in the transformation zone and in the endocervix during cervicitis [30].
Early events in HIV transmission across genital mucosal epithelium: receptor-ligand pairs and host cell entry
Mechanisms for host cell infection by HIV-1 are better described than those for C. trachomatis. Two proteins expressed at the surface of the HIV virus, gp120 and gp41 interact with several proteins expressed on the host cell surface, including CD4 and CXCR4 and/or CCR5 [50–53], to initiate infection. HIV-1 viral subtypes that preferentially bind to CXCR4 are termed X4 viruses and, in general, they are T cell tropic and induce syncytia formation in infected cells [54]. Those that preferentially bind to the CCR5 are termed R5 viruses, are tropic for macrophages and do not induce syncytialization. Those that bind to both co-receptors are called X4R5 viruses. The majority of clinical isolates are of the R5 or dual tropic variety and about half of R5 viruses evolve to use CXCR4 or become dual tropic over time [55–56].
Although the majority of investigators pose that the primary cells infected by HIV-1 are immune cells localized within the reproductive tract, several studies have demonstrated that certain epithelia in the human gastrointestinal tract and female reproductive tract can be directly infected with HIV-1 [57–61]. Interestingly, Asin, et al. [58], reported both X4 and R5 viruses could enter uterine epithelial cells. However, only the X4 strains were integrated into the uterine cell genome. The R5 viruses were released from reproductive epithelia in an unmodified form, but could still infect surrounding CXCR5-expressing immune cells.
Genital tract epithelial cells do not typically express the primary HIV receptor CD4. They do, however, express more recently described alternative receptors, such as galactosyl ceramide (GalCer) [62–63]. GalCer is an epithelial cell membrane glycolipid found on the surface of gastrointestinal, rectal and endocervical cells [62–65]. At these sites, GalCer aids in the formation of lipid rafts that, in turn, function as platforms for endocytosis and transcytosis [66–68]. The initial interactions between HIV-1 and GalCer involve direct binding of HIV-1 surface glycoproteins, particularly gp41, to GalCer [62]. It appears that binding of host cell GalCer by HIV-expressed gp41 does not induce virus/host cell fusion and productive infection, but instead promotes transcytosis of HIV virions across the epithelial cell [60, 66] that would allow for productive infection of intra- or sub-epithelial dendritic cells and CD4+ T cells. To this point, Alfsen, et al [69] have demonstrated that antibodies targeting gp41 inhibit HIV-1 transcytosis across endometrial and colonic adenocarcinoma cell line monolayers. Genital tract epithelial cells also co-express the classical HIV co-receptors, CXCR4 and CCR5, although expression varies by cell type and location [70–72]. Productive infection of human female genital tract epithelial cell lines has also been infrequently reported [73–74]. In the absence of CD4 expression on genital epithelial cells, these infections perhaps may involve GalCer or other alternative receptors such as heparin sulfate proteoglycans (HSPGs) [73].
Transmission of HIV-1 across the female genital mucosa can also occur via a paracellular route, enabling infection of intraepithelial dendritic and CD4+ T cells directly. Gorodeski, et. al., have used in vitro models of the human endocervix (CaSki epidermoid carcinoma cells) and primary ectocervical epithelial cells to perform a series of elegant experiments looking at the regulation of cervical permeability [75–77]. They and others [33, 78–80] have demonstrated that the ability of fluid and small particles contained within that fluid to traverse the space between these epithelial cells is influenced by the function of several of the molecular components in intercellular junctional complexes, including the N-cadherin/catenin complex and the nectin-afadin complex. Both complexes have been shown to be disrupted by C. trachomatis infection in vitro [31, 33]. These reports were generated using C. trachomatis serovar E to infect cervical adenocarcinoma cell lines [HeLa cells grown in monolayers [31, 33] or under polarizing conditions [33]] or using primary cervical epithelial cells cultured under non-polarizing conditions [31]. They have never been performed on cells derived from the endocervix.
Finally it should be considered that the levels of cytokines, chemokines or growth factors, when modified by C. trachomatis infection of epithelial cells, may likely increase the numbers of HIV-target cells attracted to the cervix, and/or modify their phenotype. This would further increase the potential for local viral amplification.
Mechanisms for increased HIV-1 transmission across C. trachomatis-infected human endocervical epithelial monolayers
Our interest in C. trachomatis as a co-factor in sexual transmission of HIV-1 in women recently led us to develop an in vitro model for dual infection with C. trachomatis and HIV-1. Since the human endocervix is a site of primary infection by genital serovars of C. trachomatis and it appears to be a preferred site for HIV-1 transmission, this model utilizes a primary-like immortalized human endocervical epithelial cell line [37]. These cells were derived from primary endocervical cells isolated from an HLA-A2-positive female and are, therefore, called A2EN cells; they retain site-appropriate expression of differentiation proteins and molecules driving innate immune responses [36–37]. We hypothesize that C. trachomatis infection could promote increased HIV transmission across the human endocervical epithelium through a combination of entry- and non entry-mediated mechanisms. While we have recently polarized the A2EN endocervical epithelial cell model [37], the results discussed in this manuscript are mainly limited to cells grown as a non-polarized monolayer. That said, preliminary results in A2EN endocervical cells grown under strict polarizing conditions indicate that infection with C. trachomatis serovar D exerts quite minimal and transient effects on endocervical epithelial integrity (Buckner, Schust and Quayle, unpublished observations). This possible discrepancy between our current findings and those of others [31],[33] may be due to the infected cell type, C. trachomatis serovar choice (see above), and/or chlamydial preference for an exocytic versus a lytic exit strategy [17, 81]. These issues are currently under further investigation in our polarized model. Using non-polarized monolayer cultures of A2EN endocervical epithelial cells, we here show that: 1) C. trachomatis infection of A2EN cells increases cell surface expression of HIV-1 primary and co-receptors, and 2) C. trachomatis infection increases HIV-1 binding to A2EN cells and, subsequently, increases levels of virus in co-cultures of HIV exposed A2EN and susceptible CD4+ lymphocytes. We also report that active infection of the human endocervix with C. trachomatis generally results in a local accumulation of CD4+ T cells that express the HIV co-receptors CXCR4 and CCR5.
Infection of endocervical epithelial cells with C. trachomatis increases cell surface expression of CXCR4, CCR5 and GalCer
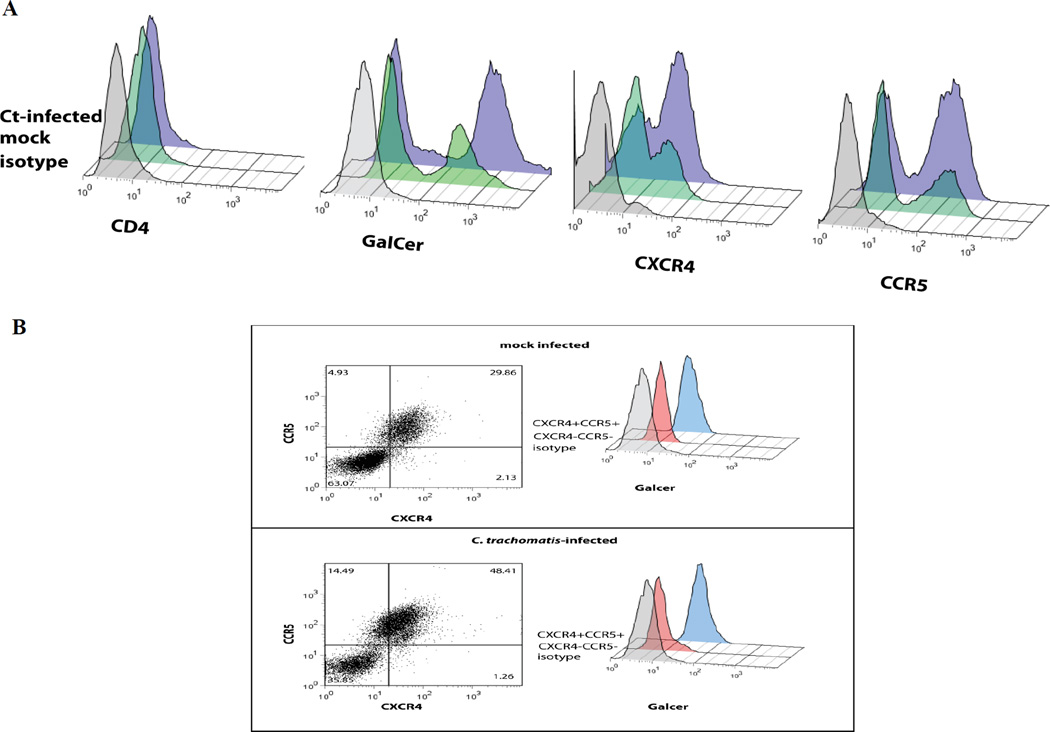
Real-time PCR and western blotting techniques confirmed low-level constitutive expression of CXCR4 and CCR5 and GalCer, but not CD4 in A2EN cells (data not shown). A2EN cells were infected with C. trachomatis serovars L2 and D under conditions allowing greater than 90% infection. Cell surface expression of CCR5, CXCR4 and GalCer was evaluated at 24 hours post-infection (hpi) using flow cytometry. As shown in Figure 3A, the cell surface expression of GalCer, CXCR4 and CCR5 are all upregulated in endocervical cells upon infection with C. trachomatis serovar D (results for serovar L2 were similar and are not shown). In further experiments, these cells were subfractionated by GalCer expression using flow cytometry. GalCer, CXCR4 and CCR5 were all found to be expressed on the same endocervical cell subpopulation (Figure 3B).
Figure 3. C. trachomatis infection of A2EN cells increases surface expression of HIV-1 primary and co-receptors.
A2EN cells were mock-infected or infected for 24 hours with C. trachomatis serovar D at levels promoting 90% infection. Cells were then immunostained for HIV receptors and co-receptors and analyzed by flow cytometry. C. trachomatis infection increased CCR5, CXCR4 and GalCer cell surface expression in a subpopulation of cells. CD4 was not detected on A2EN cells nor was it upregulated by C. trachomatis infection. Experiment is representative of 3 replicates. Legend: dashed line-isotype control; solid line – mock infected; filled histogram- C. trachomatis serovar D-infected.
C. trachomatis serovar D infection of A2EN cells increases HIV-1 transmission
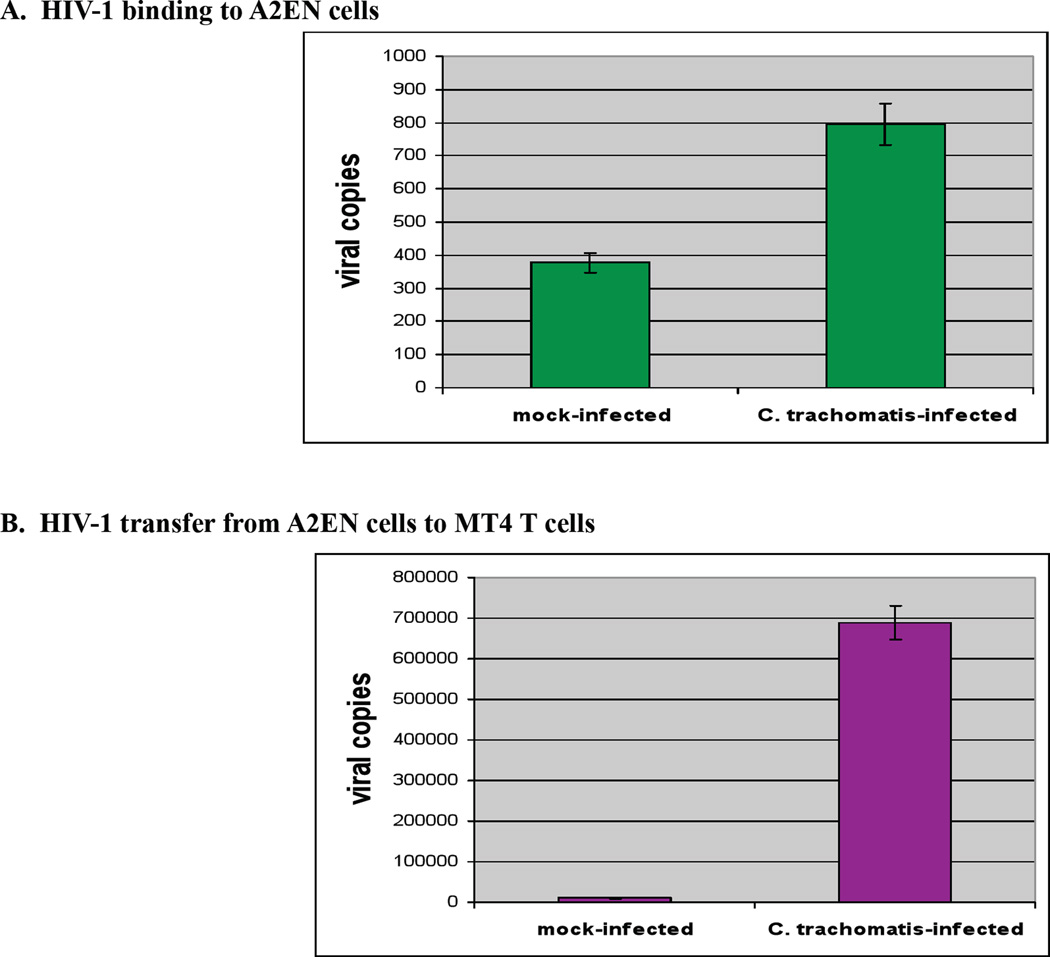
To evaluate how genital infections of C. trachomatis may enhance transmission of HIV, our group has conducted in vitro HIV binding and infection studies. Endocervical epithelial cells (A2EN) were mock-infected or exposed to C. trachomatis serovar D, cultured for 40 hours, then exposed to cell-free HIV-1BAL for 1 hour. Following extensive washes, the monolayers were lysed and the levels of HIV bound to the cells were quantified by RT-PCR. A two-fold increase in the level of bound HIV was observed in Chlamydia-infected A2EN cells as compared to the mock-infected controls (Figure 4A). A second set of experiments evaluated whether virus bound to A2EN could be amplified in co-cultures with CD4+, CCR5+, CXCR4+ T-Cells. Mock-infected and C. trachomatis-infected A2EN cells were exposed to HIV-1BAL as above, washed to remove unbound HIV-1 virions and co-cultured with a susceptible CD4+ T cell line, MT4-R5, for 4 days. The level of HIV RNA in the culture supernatants and T-cells was quantified. A 10-fold increase in HIV-1 viral copies was observed in the samples from pre-infected C. trachomatis A2EN compared to mock-infected controls (Figure 4B). These initial experiments in the A2EN cell line provide a unique model to further dissect the mechanisms by which C. trachomatis infections enhance the attachment of HIV to the genital epithelium, augment the transfer of HIV to lymphoid cells, and potentially enhance replication of HIV in susceptible cells.
Figure 4. A2EN cells bind HIV-1 and C. trachomatis infection of A2EN cells increases HIV-1 binding and transfer.
A2EN cells were mock-infected or infected with C. trachomatis serovar D (at a level to achieve 90% infection) and cultured for 40 hours post-infection. Cells were then exposed to HIV-1BAL for 1 hour at 37°C at a concentration corresponding to 10 ng/ml p24 then washed extensively to remove unbound HIV-1 virions. A. Cells were lysed for RNA purification using Trizol reagent (Invitrogen) and the amount of HIV bound to cells was determined using real-time quantitative RT-PCR specific for HIV-1 RNA [83]. B. C. trachomatis infected HIV-exposed A2EN cells (as above) were placed in culture with 5 × 105 MT4-R5 T -cells for 4 days at 37°C. MT4 cells and culture supernatants were removed on day 4 and free virions in the culture fluid as well as MT4 cells were recovered by high speed centrifugation. RNA was purified from the resulting pellet and HIV RNA levels quantified using real-time quantitative RT-PCR. Experiments were done in triplicate and repeated twice. Error bars represent the SD of the two means.
Immune cells isolated from the endocervix in C. trachomatis-infected women contain increased numbers of CD4+ cells expressing CXCR4 and CCR5
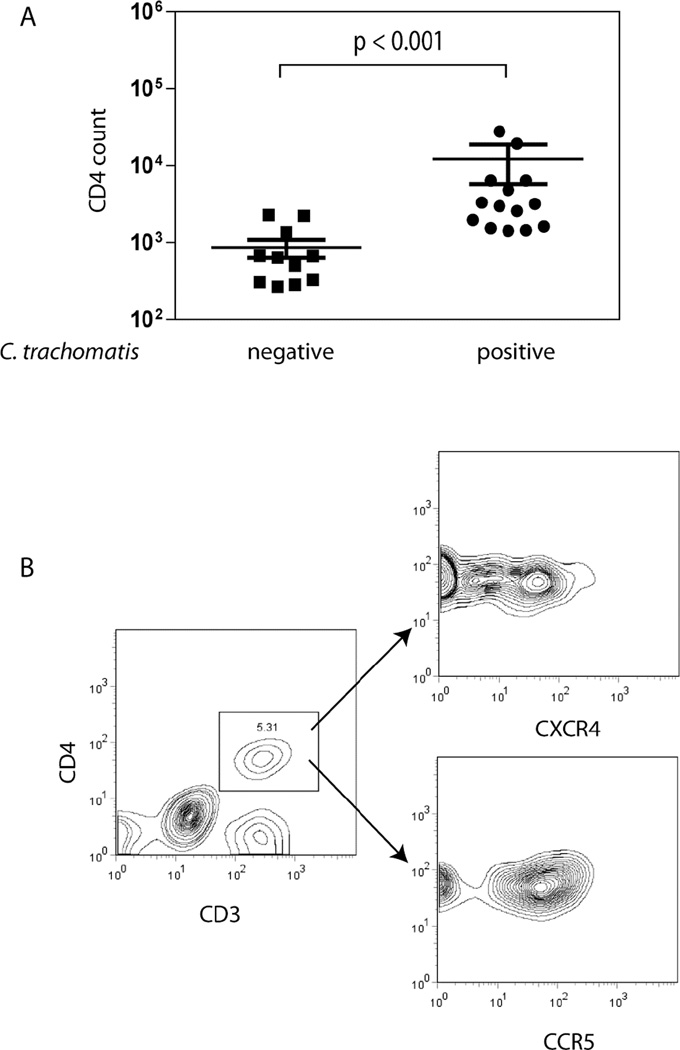
Immune cells were isolated from cytobrush specimens collected from the endocervices of volunteers who were either tested negative for C. trachomatis infection or who were currently infected with C. trachomatis (C. trachomatis-positive by NAAT and culture). Cells were immunostained and analyzed using multicolor flow cytometry. The total number of CD4+ T cells retrieved from cervical cell samples from 11 C trachomatis-negative and 14 C. trachomatis-positive participants is plotted in Figure 5A. Women who were positive for C. trachomatis infection had more than a log-fold increase in the number of CD4+ cells collected from the endocervix. CD3+ CD4+ T cells were further evaluated for their cell surface expression of the HIV-1 co-receptors CXCR4 and CCR5 (Figure 5B). The majority of cells that were CD4+ also expressed CXCR4 and/or CCR5.
Figure 5. CD4 T cell counts are elevated in the endocervices of C. trachomatis-infected women.
Consenting women ages 18–30 years old attending the Delgado Clinic, New Orleans were enrolled in a study approved by Louisiana State University Health Sciences Center. Participants of the study were tested for C. trachomatis infection by nucleic acid amplification test (NAAT) and by culture. Cervical cells were collected from C. trachomatis-negative (n=11) and C-trachomatis-positive (n=14) women using a cytobrush as previously described (Ficarra 2008 AJRI). The cells were stained with CD3-PerCp Cy5.5, CD56-APC, CD4-Alexa 700 and CD8-Pacific Blue antibodies. A. CD4 T cell numbers were enumerated using multiparameter flow cytometric analyses by gating for CD3+CD56-CD8-CD4+ cells. The CD4 T cells count from C. trachomatis-positive women was significantly higher than in C. trachomatis-negative women (p<0.001). Statistical analysis was performed using the Mann-Whitney U test. B. Expression of CXCR4 and CCR5 by CD4 T cells was also determined by flow cytometry. The majority of CD4 T cells from the endocervix express CXCR4 and CCR5 as demonstrated by the CD4 T cell profile of a representative woman enrolled in the study.
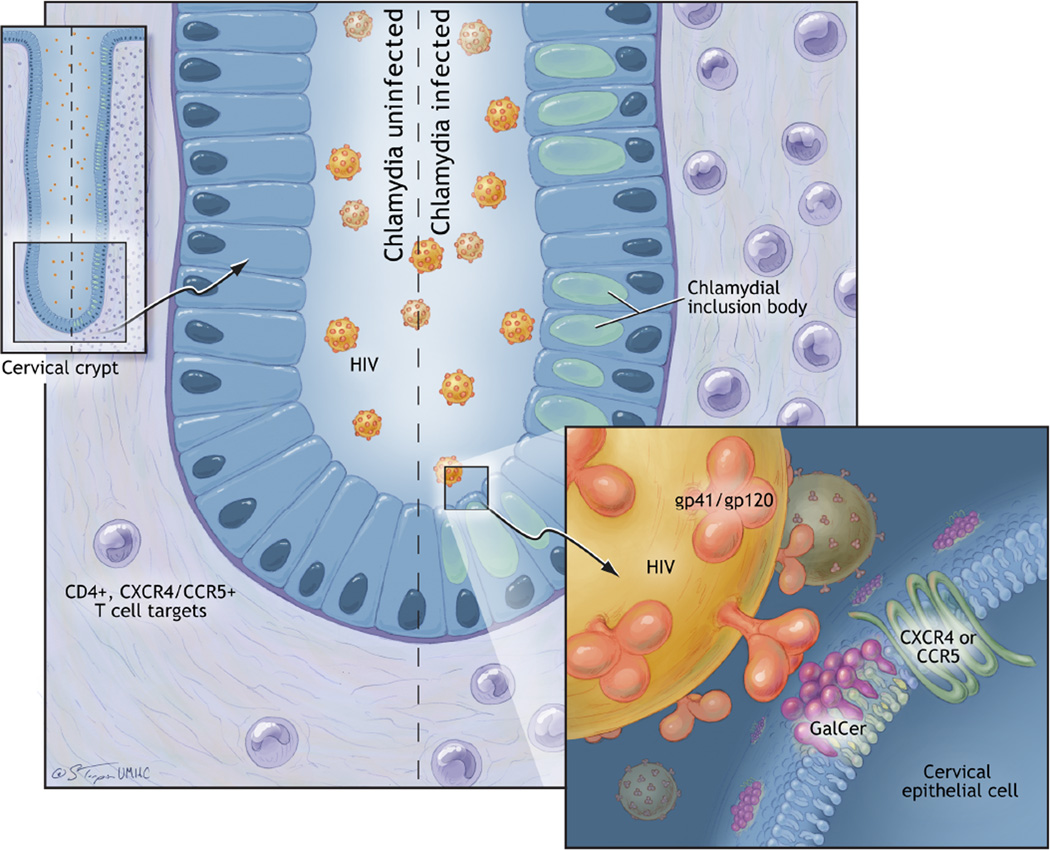
Figure 6. Effects of C. trachomatis infection on HIV-1 transmission across the endocervical epithelium.
The left side of the endocervical crypt depicted is not infected with C. trachomatis; the right side is infected. Both are exposed to HIV-1 at their luminal surfaces. The results of our in vitro modeling experiments in endocervical epithelial cells suggest that endocervical epithelial cells infected with non-disseminating genital C. trachomatis serovars increase their cell surface expression of the HIV-1 primary receptor, GalCer, and the HIV-1 co-receptors, CXCR4 and CCR5 (Figure 3). GalCer is shown at the epithelial cell surface in association with lipid rafts. The CXCR4 and/or CCR5 co-receptors on the endocervical epithelial cell, in conjunction with GalCer, interact with the GP41 stalk/ GP120 HIV-1 surface protein. Increased epithelial receptors and co-receptors in those cells infected with C. trachomatis may be responsible for increased endocervical epithelial cell binding of HIV-1 and release of HIV-1 to susceptible T cells in vitro (Figure 4). Sampling of endocervical immune cells from women actively infected with C. trachomatis reveals a dramatic increase in CD4+ T cells expressing the HIV-1 co-receptors CXCR4 and/or CCR5 when compared to women who are C. trachomatis negative. This increases the availability of HIV-1 immune cells targets in the subepithelial layers of C. trachomatis-infected endocervices..
Summary.
Sexually transmitted infections, including those by C. trachomatis, increase cervical shedding of, and co-infection by, HIV-1 [26–27, 82]. Elegant models of SIV transmission in non-human primates indicate that the virus can establish infection through entry at the endocervix [39, 42]. The epithelium lining this tissue is only a single cell in thickness and acts as the primary reservoir and site of replication for C. trachomatis [6]. We have recently begun to connect these two pathogens in a series of studies that examine the effects of C. trachomatis on possible mechanisms for HIV-1 transmission across the human endocervical epithelium
Although many investigators pose that the primary cells infected by HIV-1 are immune cells localized within the reproductive tract, several have reported that some epithelia in the human gastrointestinal tract and female reproductive tract can be directly infected with HIV-1[57–61]. Genital tract epithelial cells do not typically express the primary HIV receptor CD4. They do, however, express alternative receptors, such as galactosyl ceramide (GalCer) [62–63]. GalCer is an epithelial cell membrane glycolipid found on the surface of gastrointestinal, rectal and endocervical cells [62–65]. At these sites, GalCer aids in the formation of lipid rafts that, in turn, function as platforms for endocytosis and transcytosis [67–68]. The initial interactions between HIV-1 and GalCer involve direct binding of the HIV-1 surface glycoprotein, gp41, to GalCer. Genital tract epithelial cells also co-express the classical HIV co-receptors, CXCR4 and CCR5, although expression varies by cell type and location [70–72]. Cellular entry through GalCer has classically been linked to HIV-1 transcytosis [67, 69, 74] across the epithelial cell, allowing productive infection of intra- or sub-epithelial dendritic cells and CD4+ T cells. Productive infection of endocervical epithelial cells has also been reported [73–74]. In the absence of CD4 expression on genital epithelial cells, these infections may involve GalCer or other alternative receptors such as heparin sulfate proteoglycans (HSPGs) [73]. We demonstrate here (Figure 6) that infection of A2EN cells with genital serovars of C. trachomatis increases their cell surface expression of GalCer, CXCR4 and CCR5, but has no effect on CD4 expression. We further show that, compared to uninfected A2EN cells, A2EN cells infected with C. trachomatis bind increased amounts of HIV-1 and this results in increased levels of virus when cells are co-cultured with susceptible T cells. This log-fold increase in HIV-1 particles may result from several effects. C. trachomatis could increase HIV-1 transcytosis through A2EN cells but the difference in infectious particle production would then rely on paracrine effects of infected A2EN cells on MT4 cells, the latter of which we are currently studying in depth in our laboratories. Alternatively, C. trachomatis infection could increase HIV-1 integration into A2EN cells with amplification of the virions released to infect MT4 cells. We ultimately show in vivo that the chlamydiae-infected endocervix is populated with an increased number of HIV-susceptible CD4+ CXCR4+ CCR5+ T cells when compared to that of uninfected women. Together our results address several ways through which C. trachomatis infection may increase transmission of HIV-1 across the endocervical epithelium. Future studies to dissect the mechanisms by which this occurs will be greatly aided by the polarized A2EN endocervical epithelial model [37], which will allow the study of paracellular and transcellular pathogen entry and innate immune defense mechanisms that are localized to apical or basal cell surfaces.
Ackowledgement
This work is supported by NIH grant AI087899.
References
- 1.CDC. 2009 Sexually transmitted disease surveillance. 2010 Available from: http://www.cdc.gov/std/stats09/chlamydia.htm.
- 2.CDC. Sexually-transmitted diseases: Chlamydia. 2010 Available from: www.cdc.gov/std/chlamydia.
- 3.Millman K, Black CM, Johnson RE, et al. Population-based genetic and evolutionary analysis of Chlamydia trachomatis urogenital strain variation in the United States. J Bacteriol. 2004;186:2457–2465. doi: 10.1128/JB.186.8.2457-2465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis. 2008;35:53–54. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- 5.Paavonen J, Lehtinen M. Chlamydial pelvic inflammatory disease. Hum Reprod Update. 1996;2:519–529. doi: 10.1093/humupd/2.6.519. [DOI] [PubMed] [Google Scholar]
- 6.Swanson J, Eschenbach DA, Alexander ER, Holmes KK. Light and electron microscopic study of Chlamydia trachomatis infection of the uterine cervix. J Infect Dis. 1975;131:678–687. doi: 10.1093/infdis/131.6.678. [DOI] [PubMed] [Google Scholar]
- 7.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braley AE. Inclusion blenorrhea. A study of the pathologic changes in the conjunctiva and cervix. Am J Ophthalmology. 1938;21:1203–1208. [Google Scholar]
- 9.Abromaitis S, Stephens RS. Attachment and entry of Chlamydia have distinct requirements for host protein disulfide isomerase. PLoS Pathog. 2009;5:e1000357. doi: 10.1371/journal.ppat.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyrick PB, Choong J, Davis CH, et al. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun. 1989;57:2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raulston JE, Davis CH, Paul TR, Hobbs JD, Wyrick PB. Surface accessibility of the 70-kilodalton Chlamydia trachomatis heat shock protein following reduction of outer membrane protein disulfide bonds. Infect Immun. 2002;70:535–543. doi: 10.1128/IAI.70.2.535-543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis CH, Raulston JE, Wyrick PB. Protein disulfide isomerase, a component of the estrogen receptor complex, is associated with Chlamydia trachomatis serovar E attached to human endometrial epithelial cells. Infect Immun. 2002;70:3413–3418. doi: 10.1128/IAI.70.7.3413-3418.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taraktchoglou M, Pacey AA, Turnbull JE, Eley A. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infect Immun. 2001;69:968–976. doi: 10.1128/IAI.69.2.968-976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JP, Stephens RS. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]
- 15.Linhares IM, Witkin SS. Immunopathogenic consequences of Chlamydia trachomatis 60 kDa heat shock protein expression in the female reproductive tract. Cell Stress Chaperones. 2010;15:467–473. doi: 10.1007/s12192-010-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hybiske K, Stephens RS. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect Immun. 2007;75:3925–3934. doi: 10.1128/IAI.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci U S A. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–3428. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi M, Gong S, Lei L, Liu Q, Zhong G. A Chlamydia trachomatis OmcB C-terminal fragment is released into the host cell cytoplasm and is immunogenic in humans. Infect Immun. 2011;79:2193–2203. doi: 10.1128/IAI.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd WJ, Caldwell HD. The interaction of Chlamydia trachomatis with host cells: ultrastructural studies of the mechanism of release of a biovar II strain from HeLa 229 cells. J Infect Dis. 1985;151:1037–1044. doi: 10.1093/infdis/151.6.1037. [DOI] [PubMed] [Google Scholar]
- 21.Beatty WL, Byrne GI, Morrison RP. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci U S A. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belland RJ, Nelson DE, Virok D, et al. Transcriptome analysis of chlamydial growth during IFN-gamma-mediated persistence and reactivation. Proc Natl Acad Sci U S A. 2003;100:15971–15976. doi: 10.1073/pnas.2535394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyrick PB. Chlamydia trachomatis persistence in vitro: an overview. J Infect Dis. 2010;201(Suppl 2):S88–S95. doi: 10.1086/652394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beatty WL, Morrison RP, Byrne GI. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect Immun. 1995;63:199–205. doi: 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan RJ, Mathews SA, Mukhopadhyay S, Summersgill JT, Timms P. Chlamydial persistence: beyond the biphasic paradigm. Infect Immun. 2004;72:1843–1855. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 28.Ghys PD, Fransen K, Diallo MO, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d'Ivoire. AIDS. 1997;11:F85–F93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–959. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 30.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 31.Prozialeck WC, Fay MJ, Lamar PC, et al. Chlamydia trachomatis disrupts N-cadherin-dependent cell-cell junctions and sequesters beta-catenin in human cervical epithelial cells. Infect Immun. 2002;70:2605–2613. doi: 10.1128/IAI.70.5.2605-2613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rank RG, Whittimore J, Bowlin AK, Dessus-Babus S, Wyrick PB. Chlamydiae and polymorphonuclear leukocytes: unlikely allies in the spread of chlamydial infection. FEMS Immunol Med Microbiol. 2008;54:104–113. doi: 10.1111/j.1574-695X.2008.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Kintner J, Schoborg RV. The host adherens junction molecule nectin-1 is downregulated in Chlamydia trachomatis-infected genital epithelial cells. Microbiology. 2008;154:1290–1299. doi: 10.1099/mic.0.2007/015164-0. [DOI] [PubMed] [Google Scholar]
- 34.Giacaman RA, Asrani AC, Gebhard KH, et al. Porphyromonas gingivalis induces CCR5-dependent transfer of infectious HIV-1 from oral keratinocytes to permissive cells. Retrovirology. 2008;5:29. doi: 10.1186/1742-4690-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giacaman RA, Nobbs AH, Ross KF, Herzberg MC. Porphyromonas gingivalis selectively up-regulates the HIV-1 coreceptor CCR5 in oral keratinocytes. J Immunol. 2007;179:2542–2550. doi: 10.4049/jimmunol.179.4.2542. [DOI] [PubMed] [Google Scholar]
- 36.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, et al. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 37.Buckner LR, Schust DJ, Ding J, et al. Innate immune mediator profiles and their regulation in a novel polarized immortalized epithelial cell model derived from human endocervix. J Reprod Immunol. 2011;92:8–20. doi: 10.1016/j.jri.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z-Q, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 42.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 43.Hladik F, Sakchalathorn P, Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milush JM, Kosub D, Marthas M, et al. Rapid dissemination of SIV following oral inoculation. Aids. 2004;18:2371–2380. [PubMed] [Google Scholar]
- 45.Miyake A, Ibuki K, Enose Y, et al. Rapid dissemination of a pathogenic simian/human immunodeficiency virus to systemic organs and active replication in lymphoid tissues following intrarectal infection. J Gen Virol. 2006;87:1311–1320. doi: 10.1099/vir.0.81307-0. [DOI] [PubMed] [Google Scholar]
- 46.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 47.Zhu T, Wang N, Carr A, et al. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kell PD, Barton SE, Edmonds DK, Boag FC. HIV infection in a patient with Meyer-Rokitansky-Kuster-Hauser syndrome. J R Soc Med. 1992;85:706–707. doi: 10.1177/014107689208501119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 51.Chan DC, Chutkowski CT, Kim PS. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc Natl Acad Sci U S A. 1998;95:15613–15617. doi: 10.1073/pnas.95.26.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 53.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 54.Berger EA, Doms RW, Fenyo EM, et al. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 55.Connor RI, Ho DD. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richman DD, Bozzette SA. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 57.Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell. 2005;16:4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asin SN, Fanger MW, Wildt-Perinic D, et al. Transmission of HIV-1 by primary human uterine epithelial cells and stromal fibroblasts. J Infect Dis. 2004;190:236–245. doi: 10.1086/421910. [DOI] [PubMed] [Google Scholar]
- 59.Bobardt MD, Chatterji U, Selvarajah S, et al. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 61.Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ Cells: implications for mechanisms of sexual transmission. J Infect Dis. 2003;188:1473–1482. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 62.Alfsen A, Bomsel M. HIV-1 gp41 envelope residues 650–685 exposed on native virus act as a lectin to bind epithelial cell galactosyl ceramide. J Biol Chem. 2002;277:25649–25659. doi: 10.1074/jbc.M200554200. [DOI] [PubMed] [Google Scholar]
- 63.Berlier W, Bourlet T, Lawrence P, et al. Selective sequestration of X4 isolates by human genital epithelial cells: Implication for virus tropism selection process during sexual transmission of HIV. J Med Virol. 2005;77:465–474. doi: 10.1002/jmv.20478. [DOI] [PubMed] [Google Scholar]
- 64.Kumar RB, Maher DM, Herzberg MC, Southern PJ. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithelium: implications for HIV binding and primary oral infection. Virol J. 2006;3:25. doi: 10.1186/1743-422X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yahi N, Baghdiguian S, Moreau H, Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol. 1992;66:4848–4854. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lingwood CA, Branch DR. The role of glycosphingolipids in HIV/AIDS. Discov Med. 2011;11:303–313. [PubMed] [Google Scholar]
- 67.Magerus-Chatinet A, Yu H, Garcia S, et al. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology. 2007;362:67–74. doi: 10.1016/j.virol.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 68.Persaud-Sawin DA, McNamara JO, 2nd, Rylova S, Vandongen A, Boustany RM. A galactosylceramide binding domain is involved in trafficking of CLN3 from Golgi to rafts via recycling endosomes. Pediatr Res. 2004;56:449–463. doi: 10.1203/01.PDR.0000136152.54638.95. [DOI] [PubMed] [Google Scholar]
- 69.Alfsen A, Iniguez P, Bouguyon E, Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J Immunol. 2001;166:6257–6265. doi: 10.4049/jimmunol.166.10.6257. [DOI] [PubMed] [Google Scholar]
- 70.Yeaman GR, Asin S, Weldon S, et al. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology. 2004;113:524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeaman GR, Howell AL, Weldon S, et al. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeaman GR, White HD, Howell A, Prabhala R, Wira CR. The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S57–S62. [PubMed] [Google Scholar]
- 73.Mondor I, Ugolini S, Sattentau QJ. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol. 1998;72:3623–3634. doi: 10.1128/jvi.72.5.3623-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saidi H, Magri G, Nasreddine N, Requena M, Belec L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology. 2007;358:55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 75.Gorodeski GI. The cultured human cervical epithelium: a new model for studying paracellular transport. J Soc Gynecol Investig. 1996;3:267–280. doi: 10.1016/s1071-5576(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 76.Gorodeski GI, De Santis BJ, Goldfarb J, Utian WH, Hopfer U. Osmolar changes regulate the paracellular permeability of cultured human cervical epithelium. Am J Physiol. 1995;269:C870–C877. doi: 10.1152/ajpcell.1995.269.4.C870. [DOI] [PubMed] [Google Scholar]
- 77.Gorodeski GI, Goldfarb J. Seminal fluid factor increases the resistance of the tight junctional complex of cultured human cervical epithelium CaSki cells. Fertil Steril. 1998;69:309–317. doi: 10.1016/s0015-0282(97)00471-8. [DOI] [PubMed] [Google Scholar]
- 78.Gorodeski GI, Peterson DE, De Santis BJ, Hopfer U. Nucleotide receptor-mediated decrease of tight-junctional permeability in cultured human cervical epithelium. Am J Physiol. 1996;270:C1715–C1725. doi: 10.1152/ajpcell.1996.270.6.C1715. [DOI] [PubMed] [Google Scholar]
- 79.Gorodeski GI, Romero MF, Hopfer U, et al. Human uterine cervical epithelial cells grown on permeable support--a new model for the study of differentiation. Differentiation. 1994;56:107–118. doi: 10.1046/j.1432-0436.1994.56120107.x. [DOI] [PubMed] [Google Scholar]
- 80.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 81.Beatty WL. Lysosome repair enables host cell survival and bacterial persistence following Chlamydia trachomatis infection. Cell Microbiol. 2007;9:2141–2152. doi: 10.1111/j.1462-5822.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 82.Haaland RE, Hawkins PA, Salazar-Gonzalez J, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henning TR, Lacour N, Amedee AM. Efficient methodologies for sensitive HIV-1 RNA quantitation from plasma and vaginal secretions. J Clin Virol. 2009;46:309–313. doi: 10.1016/j.jcv.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]