Abstract
Endocannabinoid (eCB) signaling has been identified as a modulator of adaptation to stress, and is integral to basal and stress-induced glucocorticoid regulation. Furthermore, interactions between eCBs and glucocorticoids have been shown to be necessary for the regulation of emotional memories, suggesting that eCB function may relate to the development of post-traumatic stress disorder (PTSD). To examine this, plasma eCBs were measured in a sample (n=46) drawn from a population-based cohort selected for physical proximity to the World Trade Center (WTC) at the time of the 9/11 attacks. Participants received a structured diagnostic interview and were grouped according to whether they met diagnostic criteria for PTSD (no PTSD, n=22; lifetime diagnosis of PTSD = 24). eCB content (2-arachidonoylglycerol (2-AG) and anandamide (AEA)) and cortisol were measured from 8 a.m. plasma samples. Circulating 2-AG content was significantly reduced among individuals meeting diagnostic criteria for PTSD. The effect of reduced 2-AG content in PTSD remained significant after controlling for the stress of exposure to the WTC collapse, gender, depression and alcohol abuse. There were no significant group differences for AEA or cortisol levels; however, across the whole sample AEA levels positively correlated with circulating cortisol, and AEA levels exhibited a negative relationship with the degree of intrusive symptoms within the PTSD sample. This report shows that PTSD is associated with a reduction in circulating levels of the eCB 2-AG. Given the role of 2-AG in the regulation of the stress response, these data support the hypothesis that deficient eCB signaling may be a component of the glucocorticoid dysregulation associated with PTSD. The negative association between AEA levels and intrusive symptoms is consistent with animal data indicating that reductions in AEA promote retention of aversive emotional memories. Future work will aim to replicate these findings and extend their relevance to clinical pathophysiology, as well as to neuroendocrine and molecular markers of PTSD.
Keywords: stress, PTSD, trauma, endocannabinoid, HPA axis, cortisol, N-arachidonylethanolamine, anandamide, 2-arachidonoylglycerol, anxiety
Introduction
The development of post-traumatic stress disorder (PTSD) is related to abnormalities in the regulation of biological stress response systems, specifically the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (Krystal and Neumeister, 2009; Yehuda, 2009). Current theories suggest that increased responsivity of glucocorticoid receptors resulting in reduced cortisol levels at the time of a traumatic exposure, or immediately thereafter, will result in increased noradrenergic transmission associated with a prolonged state of distress (Pervanidou and Chrousos, 2010). This state of arousal will result in the ‘hyperconsolidation’ of emotional memories, and ultimately, could lead to the development of PTSD. However, since not all persons exposed to trauma develop PTSD, it has also been of interest to identify hormones or signaling molecules that could be responsible for either increasing the probability of PTSD or for promoting resistance to PTSD development. By examining a population-based cohort of individuals exposed to the World Trade Center (WTC) collapse, we have recently identified genetic markers related to glucocorticoid signaling in individuals who developed PTSD (Yehuda et al., 2009; Sarapas et al., 2011). Accordingly, further investigation of systems involved in the regulation and actions of glucocorticoid hormones was undertaken to explore the underlying biological mechanisms specific to the development of PTSD.
The endocannabinoid (eCB) system represents an ideal candidate system to investigate with respect to the pathophysiology of PTSD (Hill and Gorzalka, 2009; Neumeister, 2013). The eCB system is primarily composed of a central CB1 receptor and two endogenous ligands (N-arachidonylethanolamine [anandamide; AEA] and 2-arachidonoylglycerol [2-AG]). In addition, there are also CB2 receptors, whose expression is primarily restricted to immune cells of macrophage lineage but may also be expressed in the CNS, as well as a family of fatty acid ethanolamides, such as palmitoylethanolamide and oleoylethanolamide, which share biosynthetic and catabolic pathways with AEA, but are not ligands for the CB receptors. The eCB system is known to constrain activation of the stress response through distributed actions in limbic and hypothalamic circuits in the brain (Riebe and Wotjak, 2011; Hill and Tasker, 2012). More so, eCB signaling is responsive to glucocorticoid hormones (Di et al., 2003; Hill et al., 2010a), and the recruitment of eCB signaling by glucocorticoids has been found to mediate many of the physiological actions of these hormones, including negative feedback termination of HPA axis activity (Evanson et al., 2010; Hill et al., 2011) and modulation of emotionally salient cognitive processes (Campolongo et al., 2009; Atsak et al., 2012). In addition to the role of eCBs in mediating the actions of glucocorticoids, eCB signaling is involved in many processes which are dysregulated in PTSD, such as the extinction of emotionally aversive memories (Marsicano et al., 2002; Plendl and Wotjak, 2010; Gunduz-Cinar et al., 2013), habituation and adaptation to stress (Patel et al., 2005b; Hill et al., 2010b) and release of catecholamines from sympathetic nerve terminals (Ishac et al., 1996; Bellocchio et al., 2013).
Based on these converging lines of evidence and the neurobiology of PTSD, we hypothesize that reductions in circulating concentrations of eCB represent a biomarker of stress vulnerability, and that deficient eCB signaling is involved in the biological processes related to PTSD. To examine these hypotheses, we evaluated circulating concentrations of the eCBs AEA and 2-AG in a sample derived from a population-based cohort selected for physical proximity to the WTC at the time of the 9/11 attacks. A subsample of this cohort was previously selected for a genome-wide association study of PTSD (Yehuda et al., 2009). This is an ideal cohort in which to search for biological identifiers of PTSD, as risk for exposure was based on proximity to the WTC, and was uncomplicated by the confound that exposure often introduces to genetic association studies (e.g., familial risk for exposure to interpersonal violence). Furthermore, circulating eCB concentrations are elevated in response to acute stress (Hill et al., 2009b; Dlugos et al., 2012), whereas deficient recruitment of eCB signaling in response to acute stress, or low basal eCB contents in the circulation, have been related to excessive stress-induced activation of the HPA axis (Chouker et al., 2010; Dlugos et al., 2012) and negative long-term outcomes following exposure to stressful events, such as cardiac surgery (Hauer et al., 2012). Our data indicate that PTSD is associated with reduced concentrations of 2-AG in the circulation and that both 2-AG and AEA concentrations associate with specific symptom clusters within PTSD.
Methods
Participants
Subjects were 46 participants who comprised a subset of a population based sample (n=109) evaluated at the Mount Sinai School of Medicine (MSSM) four to six years following the 9/11 attacks (Yehuda et al., 2009). The subjects studied at the MSSM were those who responded positively to a mailing asking participants to have an in-person diagnostic evaluation, complete self-report questionnaires and permit an 8 am blood draw. The study was approved by the Institutional Review Board (IRB) at the Mount Sinai School of Medicine; all subjects provided written informed consent and were subsequently screened to establish eligibility. Subjects were excluded if they met criteria for primary psychotic disorder, bipolar illness, alcohol or substance dependence, or major endocrine, neurological, or other medical illness, including diabetes. Although subjects with PTSD showed greater number of lifetime psychiatric diagnoses than those without PTSD (Table 1), as has been previously described (Breslau et al. 2000), none were receiving psychiatric treatment or taking psychotropic medications at the time of participation. The 46 subjects included in this report were those with remaining frozen samples available for endocannabinoid assay who had previously provided consent for analyses of compounds unrelated to the goals of the initial investigation, i.e., associations with genotype. Selection was based purely on availability of biological sample in conjunction with appropriate consent, and not on any other inclusion or exclusion criteria, clinical or otherwise. Subjects with endocannabinoid determinations (n=46) were similar in age, gender distribution, and lifetime trauma exposure histories to the remaining members of the original cohort (n=63). Of 46 subjects, 22 were deemed to have suffered direct, high magnitude exposure to the events of 9/11 (direct exposure to the events of 9/11), whereas 24 reported indirect exposure. ‘Direct exposure’ was assigned to participants who were in the vicinity of the World Trade Towers at the time of the attacks with immediate threat to their safety or survival, or had suffered the loss of family members or intimate friends on 9/11. ‘Indirect exposure’ was attributed to those who witnessed collapse of the Towers from a safe distance, were informed of the attacks while out of town, or observed the events on TV, without enduring direct threat to self or family members.
Table 1.
Demographic Characteristics of Sample Population (n=46)
| Demographics and Clinical Characteristics |
No PTSD | Lifetime PTSD | Group Comparisons |
|---|---|---|---|
| (n=22) | (n=24) | ||
| Mean ± SD or n (%) | Mean ± SD or n (%) | F 1,44, p - or - χ2, df=1, p | |
| Age (yrs) | 57.09 ± 13.44 | 51.92 ± 15.54 | F=1.44, ns |
| Gender (M / F) | 10 M / 12 F | 12 M / 12 F | Χ2=.11, ns |
| Race: White | 21 (45.7%) | 21 (45.7%) | Χ2=3.92, df=3, ns |
| African-American | 3 (0%) | 2 (4.3%) | |
| Latino | 1 (2.2%) | 0 (0%) | |
| Asian / Pacific Islander | 0 (0%) | 1 (2.2%) | |
| Education (yrs) | 15.3 ± 3.3 | 15.6 ± 3.7 | F=0.58, ns |
| Childhood Trauma Questionnaire (Total) | 7.33 ± 2.00 | 8.71 ± 3.53 | F=2.38, ns |
| Directly affected by WTC | 8 (36.4%) | 14 (58.3%) | χ=2.22, ns |
| PTSD severity (CAPS1 total score) | 14.67 ± 11.85 | 59.95 ± 27.57 | F=41.28, p<.0005 |
| CAPS intrusive subscale | 7.22 ± 6.59 | 19.68 ± 9.11 | F=22.50, p<.0005 |
| CAPS avoidance subscale | 2.22 ± 3.77 | 20.58 ± 13.28 | F=31.93, p=0.001 |
| CAPS hyperarousal subscale | 5.22 ± 4.63 | 19.74 ± 10.55 | F=28.77, p=0.001 |
| Current MDD | 2 (11.1%) | 3 (20.0%) | Χ2=.503, ns |
| Past MDD | 4 (22.2%) | 8 (53.3%) | Χ2=3.42, (p=.064) |
| Past Alcohol Abuse | 0 (0%) | 4 (25.0%) | Χ2=5.10, .024 |
| Past Drug Abuse | 2 (11.1%) | 1 (6.2%) | Χ2=.244, ns |
Clinician Administered PTSD Scale
Clinical Evaluation
Psychologists with established interrater reliability administered the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995) and the Structured Clinical Interview for the DSM-IV (Spitzer et al., 1995) to determine the presence of PTSD and other psychiatric disorders. 24 of the 46 subjects interviewed reached threshold criterion for PTSD in their lifetime, 22 cases did not meet criteria for lifetime presence of this disorder. Participants also completed the self-report Posttraumatic Stress Diagnostic Scale (Foa et al., 1993).
Blood Drawing and Processing
Fasting blood samples were obtained between 08:00 and 08:30 hours, into EDTA containing tubes (BD Vacutainer, K2 EDTA (K2E) Plus Blood Collection Tubes), placed on ice, spun within 40 min of collection − C until analysis. Plasma cortisol levels were determined using commercially-available RIA kits (DiaSorin Inc., Stillwater, MN). The intra- and inter-assay coefficients of variation were 2.3% and 6.1% for cortisol, respectively.
Determination of the plasma concentrations of the eCBs 2-AG and AEA, as well as two other fatty acid ethanolamides, palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) was performed using a previously published method (Hill et al., 2008). PEA and OEA are non-cannabinoid fatty acid ethanolamides which share biosynthetic and metabolic pathways with AEA, but do not activate cannabinoid receptors. In brief, all extractions were performed using Bond Elut C18 solid-phase extraction columns (1 ml; Varian Inc, Lake Forest, CA). Plasma samples (0.5 ml each) were thawed and made up to 15% ethanol, to which the internal standards [2H8]-AEA (16.9 pmol) and [2H8]-2-AG (46.5 pmol) (Cayman Chemicals, Ann Arbor, MI) were added. Samples were vortexed and centrifuged at 1000 × g for 4 min. The supernatant was loaded on C18 columns, which have been conditioned with 1 ml redistilled ethanol and 3 ml of double distilled water (ddH2O). The remaining pellet was washed with 100 µl of 15% ethanol and centrifuged again for 3 min. The resulting supernatant was also loaded onto the C18 column. Columns were washed with 5 ml ddH2O and eluted with 1 ml of ethyl acetate. The ethyl acetate layer in the resulting elute was removed and dried under N2. Lipids in the residual ddH2O phase were extracted by mixing with an additional 1 ml of ethyl acetate, which was added to the original ethyl acetate solution. Once dried, samples were resuspended in 20 µl of methanol and stored at −80°C. AEA and 2-AG were quantified using isotope-dilution, atmospheric pressure, chemical ionization liquid chromatography/mass spectrometry (LC-APCI-MS) as described previously (Patel et al., 2005a). For analysis, the m/z transitions for each molecule were as follows: AEA 348.3, AEA-d8 356.3, 2-AG 379.3, 2-AG-d8 387.3, OEA 326.3 and PEA 300.3. For analysis of 2-AG, given that it isomerizes to 1,3-AG during the extraction process (and since there is virtually no naturally occurring 1,3-AG), both 2-AG and 1,3-AG peaks were integrated and added together to determine levels of 2-AG.
Statistical Analysis
Eight am plasma 2-AG, AEA, OEA and PEA, and cortisol concentrations were compared between subjects with and without PTSD, using univariate analysis of variance (ANOVA), and co-variance (ANCOVA), with PTSD diagnosis as the fixed factor, and gender as the covariate. Severity of exposure (‘direct’ versus ‘indirect’ exposure) to WTC related events was compared for both PTSD and non-PTSD groups using chi-square tests, and used as a covariate in comparisons of endocannabinoids between subjects with and without PTSD. There were no significant differences between the PTSD and no-PTSD groups in age, and none of the outcome variables were themselves related to age. Despite this, comparisons were performed with and without covariation for age. Analyses of covariance were additionally conducted for lifetime alcohol abuse and depression diagnoses to evaluate the extent to which results were influenced by these variables. Bivariate correlations were performed to examine relationships of plasma eCBs with cortisol, as well as with CAPS and PDS ratings for overall PTSD symptom severity, and with PTSD symptom clusters (avoidance, hyperarousal, intrusive). Finally intercorrelations among the eCBs and ethanolamides were evaluated for the entire sample, and within the PTSD and no-PTSD groups separately.
Results
Table 1 shows the clinical characteristics of the sample. There were no differences in any variables except for CAPS scores of PTSD severity, lifetime diagnosis of major depression and past alcohol abuse, all of which were higher in the PTSD compared to the no-PTSD sample. Interestingly, despite differences in CAPS scores, there were no differences between the PTSD and no-PTSD groups in actual exposures to traumatic events, including childhood adversity or sexual abuse. Single time point analysis of 8am cortisol demonstrated no significant differences in basal cortisol between individuals who did or did not have a diagnosis of PTSD [F(1,43)=.001, ns. PTSD, 14.95±4.64 ug/dL; no PTSD, 14.91±5.41 ug/dL (M±SD); cortisol by PTSD, covaried for gender [F(1,42)=.004, ns. PTSD, 14.87±1.04 ug/dL; no PTSD, 15.01±1.11 ug/dL (M±SE).
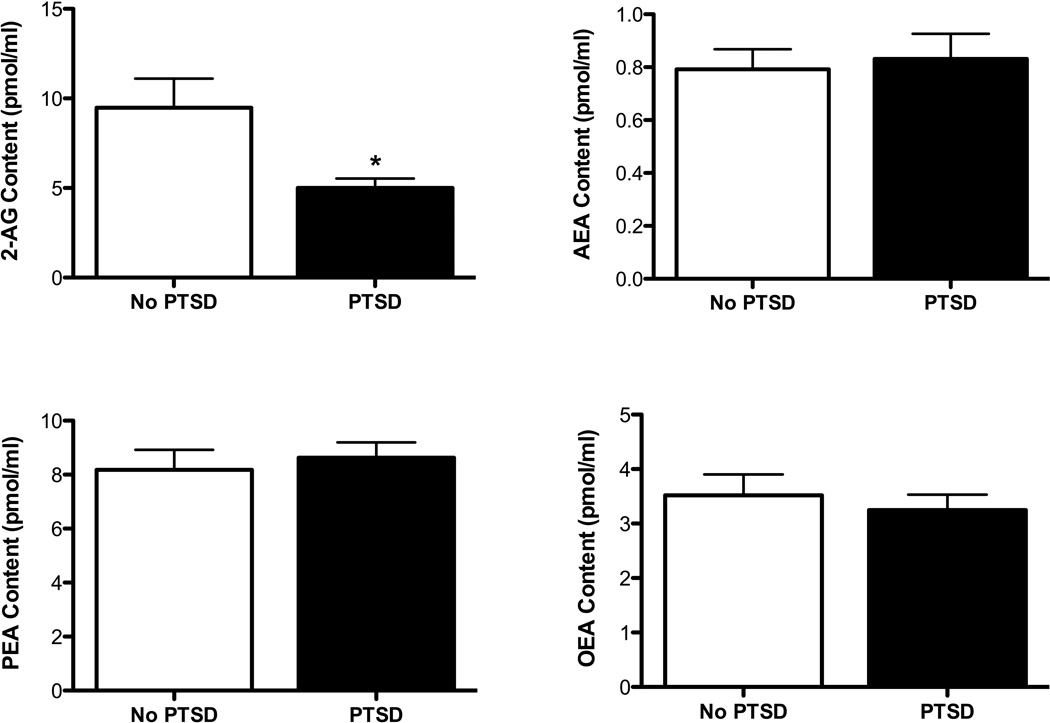
Examination of 2-AG concentrations in the circulation, comparing individuals with and without lifetime PTSD, revealed a significant main effect for PTSD diagnosis [F(1,43)= 6.94, p=0.012; 2-AG by PTSD, covaried for gender F(1,43)= 7.42, p=.009; Fig. 1], such that PTSD was associated with significantly lower circulating concentrations of 2-AG. There was no significant difference in AEA [F(1,43)=0.16, ns; Fig. 1], OEA [F(1,43)=0.44, ns; Fig. 1] or PEA [F(1,43)=0.27, ns; Fig. 1] between subjects with and without PTSD. Since individuals with PTSD reported significantly greater lifetime alcohol abuse, we covaried for this diagnosis, which did not affect the significance of the difference in 2-AG levels between subjects with and without PTSD [F (1, 33) = 4.72, p = 0.037]. Similarly, as a greater proportion of subjects with PTSD also reported lifetime depressive diagnoses (major depression or dysthymia), we covaried for depression [F (1, 34) = 7.95, p = 0.008] which likewise did not influence the significant reduction in 2-AG seen for those with PTSD.
Figure 1.
Plasma 2-arachidonoylglycerol (2-AG), anandamide (AEA), palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) concentrations in individuals who were diagnosed with post-traumatic stress disorder (PTSD) or healthy age and gender matched controls (No PTSD). Plasma levels of 2-AG, but not AEA, PEA or OEA, are found to be reduced in PTSD. Data are presented as mean +/− SEM. Significant diferences (p < 0.05) are denoted by *.
To further explore the apparent relationship of low concentrations of circulating 2-AG to lifetime PTSD diagnosis, we examined whether severity of exposure to the WTC collapse alone was sufficient to influence circulating 2-AG. A significant effect of intensity of exposure was noted for 2-AG [indirect exposure (9.81±1.01), direct exposure, (4.85±1.08); F(1,43)=11.20, p=.005]. Similar to the result for PTSD, there was no effect of exposure to the WTC collapse on plasma levels of AEA [indirect exposure (0.82±0.09), direct exposure (0.87±0.10); F(1,43)=0.14, ns], PEA [indirect exposure (8.41±0.67), direct exposure (8.66±0.72); F(1,43) = 0.07, ns] or OEA [indirect exposure (3.03±0.32), direct exposure (3.73±0.34); F(1,43)=2.23, ns]. Given that the extent of exposure to the WTC collapse was related to reduced levels of 2-AG, an analysis of covariance was performed to determine if the relationship between PTSD and 2-AG was mediated by the exposure. Controlling for intensity of WTC exposure revealed significant effects for both exposure [F(1,43)=6.25, p=.016] and PTSD [F(1,43)=4.71, p=.036]. Thus, even after controlling for exposure to the WTC collapse, the reduction in circulating 2-AG levels in individuals with lifetime PTSD remained significant. These data do suggest, however, that exposure to a traumatic stressor alone may have residual effects to diminish circulating 2-AG levels.
A series of correlation analyses were undertaken to assess associations among the eCBs and ethanolamides, as well as the relationships of these measures to circulating cortisol, and to symptom profiles of the participants. For the entire sample, there was no significant relationship of 2-AG with AEA, PEA or OEA (r’s between .05 and .18). However, there were significant intercorrelations among AEA, PEA and OEA (r’s between .59 and .80, all p’s <.0005), suggesting that 2-AG is regulated independently from AEA, PEA and OEA, which are regulated in concert. These intercorrelation results were similar for the no-PTSD and PTSD subgroups.
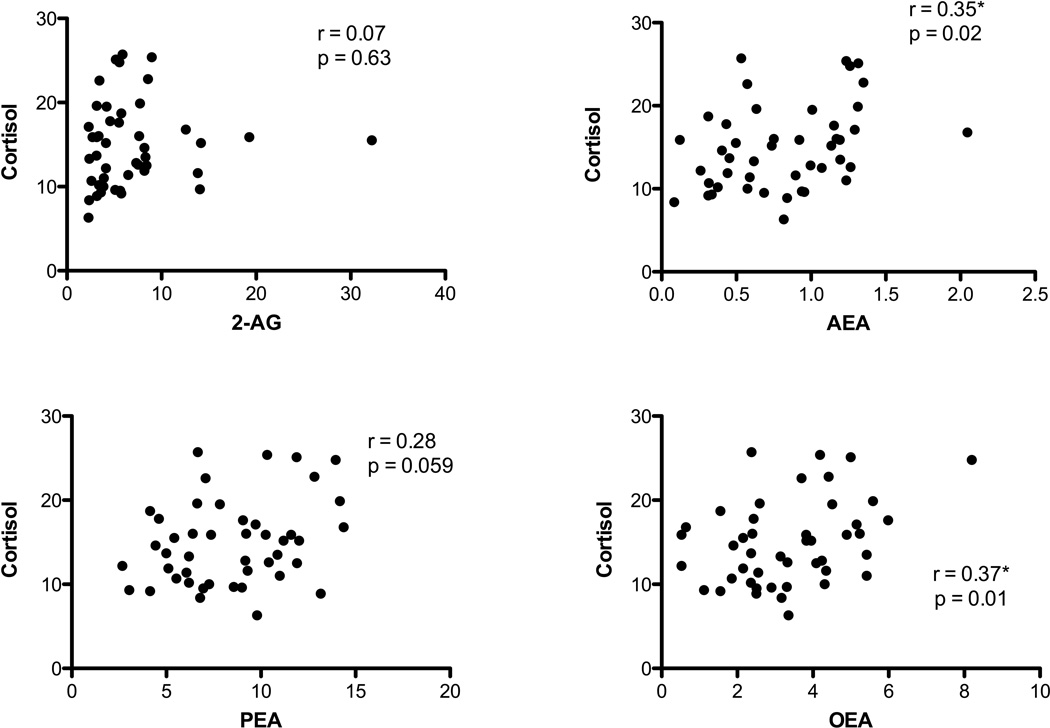
We also examined correlations between cortisol and eCB, OEA and PEA concentrations (see Fig. 2). For the entire sample, there was a significant positive relationship between cortisol and OEA (r=0.373, n=45, p=0.012) and AEA (r=0.345, n = 45, p = 0.02) and positive relationships of cortisol at trend levels of significance with PEA (r = 0.284, n=45, p = 0.059), indicating that all of the fatty acid ethanolamides exhibit some degree of positive association with circulating cortisol concentrations. In the entire sample, there was no relationship between cortisol and 2-AG concentrations (r = 0.07, n = 45, ns). Interestingly, when data from the PTSD group alone are analyzed, the correlation between AEA and cortisol concentrations is lost, while a positive and significant correlation between 2-AG and cortisol emerges (r=0.434, n=24, p=0.034). This suggests that relationships between eCB concentrations and glucocorticoids could differ depending on the presence or history of PTSD.
Figure 2.
Correlations between plasma cortisol and 2-arachidonoylglycerol (2-AG), anandamide (AEA), palmitoylethanolamide (PEA) or oleoylethanolamide (OEA). All of AEA, PEA and OEA, but not 2-AG, were found to correlate with cortisol in the entire sample (both healthy control and those with post-traumatic stress disorder). Significant correlations (p < 0.05) are denoted by *.
For analysis of how the eCBs and ethanolamides correlated to PTSD symptom clusters, correlations were performed only within the PTSD group. 2-AG concentration was significantly associated with current CAPS subscale scores for avoidance (r=.553, n=19, p=.019) and with number of CAPS avoidance symptoms (r=.618, n=19, p=.005). 2-AG was also related to the current PTSD symptom total score, but only at a trend level of significance (r=.424, n=19, p=.070), and with total number of current PTSD symptoms (r=.530, n=19, p=.020). AEA concentrations were negatively associated with current CAPS intrusive subscale scores (r=−.532, n=19, p=.019), and with number of intrusive symptoms (r=−.527, n=19, p=.020), but was not significantly associated with the current PTSD symptom total score or number. Lifetime CAPS scores were associated with AEA concentration showing a marginally significant association with lifetime intrusive subscale scores (r=−.455, n=19, p=.050), and with lifetime number of intrusive symptoms (r=−.472, n=19, p=.041). Thus, among subjects with PTSD, clinician rated CAPS subscale scores for avoidance and intrusions were differentially associated with 2-AG and AEA, respectively.
Discussion
These data demonstrate that individuals recruited following the WTC collapse, who met criteria for PTSD in their lifetime, exhibit lower concentrations of the endocannabinoid 2-AG, but not AEA, in the circulation. The relationship between 2-AG and PTSD was also related to the trauma of being exposed to the WTC collapse as even individuals who did not develop PTSD, but had a high degree of exposure to the WTC collapse also exhibited reductions in plasma concentrations of 2-AG. Importantly, despite the fact that individuals with PTSD exhibited higher rates of lifetime depression and alcohol abuse, covariance analyses for each of these comorbidities demonstrated that they did not mediate the significant relationship between PTSD and 2-AG levels. Cigarette smoking was not accounted for in the current study, and might have influenced the results, since smoking rates tend to be elevated among individuals with PTSD (Fu et al, 2007). The recent observation, however, of no significant difference in circulating 2-AG between smokers and non-smokers argues against this possibility (Hauer et al., 2013). Collectively, these data indicate that a complex relationship between stress exposure, PTSD and circulating levels of 2-AG exists.
The reduction in plasma 2-AG concentrations seen in individuals with PTSD is similar in magnitude to that previously reported for individuals with major depression (Hill et al., 2008; Hill et al., 2009b). That both PTSD and depression are stress-related mental illnesses suggests that reduced concentrations of 2-AG could represent a peripheral biomarker of vulnerability to stress-related mood and anxiety disorders. Preclinical research has found that 2-AG signaling is recruited within the brain during acute stress, which contributes to termination of the stress response (Evanson et al., 2010; Hill et al., 2011). Under conditions of repeated stress, an up-regulation of 2-AG signaling is required to facilitate adaptation or habituation to stress exposure (Patel et al., 2005b; Hill et al., 2010b). Similarly, in humans, exposure to an acute social stress has been found to increase circulating levels of 2-AG (Hill et al., 2009b; however, it should be noted that a subsequent study found circulating concentrations of AEA, but not 2-AG, were elevated in response to acute stress; Dlugos et al., 2012), suggesting the possibility of an evolutionarily conserved role for 2-AG signaling in buffering the effects of stress. For instance, exaggerated stress responses were described in humans who did not show elevations in 2-AG following exposure to parabolic flight stress (Chouker et al., 2010). These data suggest that stress-induced increases in 2-AG are an adaptive response that functions to buffer the potential deleterious effects of stress (Hill and Tasker, 2012). Accordingly, one possibility is that a trait deficit in 2-AG signaling might represent a vulnerability factor predisposing an individual to an adverse response following stressful or traumatic exposure. As such, an integral role for 2-AG in stress regulation may explain the finding of reduced 2-AG levels in subjects with PTSD. However, detailed prospective studies will be required to determine if reduced 2-AG is predisposing, or a consequence of PTSD.
It is interesting that despite significant differences in 2-AG levels between those with and without PTSD, analyses of the entire sample did not detect significant relationships between 2-AG and any clinical variable. However, within the PTSD group, 2-AG correlates positively with avoidance and total CAPS scores, as well as with cortisol levels. These associations would suggest that for individuals with PTSD, both higher basal cortisol and greater degree of symptom expression are related to higher levels of circulating 2-AG. As peripheral 2-AG is known to be responsive to stress exposure, this would suggest that elevation in 2-AG within individuals most afflicted by PTSD symptoms may reflect augmented levels of stress. Thus, despite the observation of diminished mean levels of 2-AG in a cohort characterized by lifetime PTSD, 2-AG may be relatively elevated among substantially stressed individuals in a direction to constrain the stress response. Consistent with this formulation for the behavior of the eCB system in PTSD, individuals with major depression exhibit reductions in basal levels of circulating 2-AG but still possess the capacity to mount a 2-AG response following exposure to stress (Hill et al., 2009b). This model is analogous to the results of a recent study that demonstrated sustained elevations of 2-AG (and AEA) levels in a trauma exposed population with PTSD, in which it was suggested that the magnitude of stress was associated with parallel activation of the eCB system (Hauer et al., 2013). While Hauer and colleagues (2013) found a main effect of elevated, rather than reduced, 2-AG in PTSD, their suggestion that eCBs are engaged to counter the effects of stress is consistent with the current formulation. Future research will be required to understand which factors may be most relevant in determining whether mean 2-AG is elevated or reduced for PTSD subjects; these variables could include duration of stress exposure, acuity of stress exposure relative to measurement of eCB levels and nature of the traumatic stressor. It should be noted though, that while the findings in the study of Hauer and colleagues (2013) are inconsistent with the current data, our findings of reduced eCB content in PTSD are relatively consistent with another recent report by Neumeister and colleagues (2013), which found reduced circulating levels of AEA (but not 2-AG) in PTSD.
There were no differences in AEA concentrations between the PTSD and no- PTSD groups, but a robust negative relationship between AEA levels and expression of intrusive symptoms was demonstrated; that is, individuals with lower levels of plasma AEA exhibited the highest rates of intrusive symptoms. Both clinical and preclinical studies suggest an integral role for AEA signaling in the regulation of amygdala reactivity and the suppression of emotionally aversive memories (Hariri et al., 2009; Hill et al., 2009a; Gunduz-Cinar et al., 2013), which may explain this relationship. Rodent studies indicate that during extinction of aversive memories, there is a recruitment of AEA signaling within the amygdala and that facilitation of this response augments extinction, while blockade of AEA/CB1 receptor signaling may result in the perseveration of the aversive memory (Marsicano et al., 2002; Chhatwal et al., 2005; Gunduz-Cinar et al., 2013). Further, increased AEA signaling is known to dampen stress responses in rodents (Patel et al., 2004; Hill et al., 2009a), and, in humans, to result in lower levels of trait anxiety and accelerated habituation of the amygdala in response to threat cues (Gunduz-Cinar et al., 2013). More so, it has been demonstrated in both healthy individuals (Dlugos et al., 2012), and those with major depression (Hill et al., 2008), that circulating AEA concentrations negatively correlate with anxiety levels. Since impairment in fear extinction may result in the emergence of intrusive symptoms, the negative association between AEA signaling and aversive memories suggests that reduced AEA signaling may likewise be associated with impaired extinction of aversive memories in the current sample, with an associated increase in intrusive symptoms. Given these findings, we propose that while AEA concentrations are not significantly reduced in association with PTSD in the current cohort, individuals who possess lower concentrations of AEA could be more susceptible to intrusive symptoms.
The relationship between cortisol and concentrations of the NAEs is of interest given the context of an ever-growing body of research demonstrating a functional relationship between glucocorticoids and the eCB system (Hill and McEwen, 2010). Interestingly, we have previously demonstrated in rodents that the administration of glucocorticoids causes a rapid elevation in AEA throughout the limbic system (Hill et al., 2010a). Given the overlap between the biosynthetic and metabolic pathways of AEA, PEA and OEA (Ahn et al., 2008), it is not surprising that circulating concentrations of these molecules correlate with each other. Accordingly, the possibility exists that cortisol positively correlates with concentrations of these molecules through an ability of cortisol either to increase their shared biosynthesis or reduce their metabolism. An earlier study found that PEA concentrations in the circulation positively correlate with cortisol in healthy subjects following exposure to the Trier social stress test (Dlugos et al., 2012). More importantly, a recent study found that circulating AEA concentrations correlated with cortisol levels, and that individuals with PTSD exhibited reductions in both AEA and cortisol concentrations in blood, relative to healthy and trauma exposed controls (Neumeister et al., 2013). As the current PTSD sample did not exhibit reduced levels of cortisol on the basis of a single 8am blood draw, it is perhaps not surprising that we did not detect reduced levels of AEA. Taken together, these data suggest that reduced eCB signaling could be a feature of PTSD, and that relative reductions in AEA or 2-AG may be related to alterations in glucocorticoid signaling. Future studies based on larger samples will be required to examine relationships between eCB levels and cortisol to determine which biological factors predict alterations in AEA vs. 2-AG.
One major limitation of this study is that it is based a single time point analysis, both with regard to time of day for biological sampling and the time course of PTSD. However, four studies based on data derived from a single time point have revealed reduced circulating levels of eCB molecules in individuals diagnosed with depression or PTSD (Hill et al., 2008; Hill et al., 2009b; Neumeister et al, 2013; current data; however, see Hauer et al., 2013 discussed above for opposite findings). Second, the current study is based exclusively on peripheral measures, which have been used to support hypotheses that relate to central nervous system function. While it is unlikely that circulating concentrations of eCBs are from the same signaling pool as eCBs in the CNS, there is an interesting functional correlation between stimulae that modulate central and peripheral eCB signaling (Hillard et al., 2012). Studies examining the effects of stress on circulating eCBs in humans have generally reached the same conclusions as those performed in animals (Hill et al., 2009b; Chouker et al., 2010; Evanson et al., 2010; Hill et al., 2011). Further, multiple studies have revealed significant relationships between circulating eCB levels and distinct neurobehavioral domains and/or symptom clusters, particularly those related to anxiety (McPartland et al., 2005; Hill et al., 2008; Mangieri et al., 2009; Gunduz-Cinar et al., 2013). While it is possible that a third variable could contribute to these relationships, the fact that similar associations have held across studies and species suggests that peripheral eCB content is related to eCB signaling in the CNS. The recent finding that low concentrations of circulating AEA in PTSD are significantly correlated with an up-regulation of CB1 receptor binding sites within forebrain regions associated with stress regulation and emotional processing supports this relationship (Neumeister et al., 2013), suggesting that a deficit in eCB signaling detected at the peripheral level may be associated with a compensatory up-regulation of central CB1 receptors.
In conclusion, the finding of reduced concentrations of circulating 2-AG in PTSD is consistent with current biological formulations of PTSD. For example, in addition to the relationship between eCBs (AEA and 2-AG), cortisol and the stress response (Hill and Tasker, 2012), there are substantial interactions between eCBs and other systems found to be dysfunctional in PTSD. CB1 receptors on sympathetic terminals, for instance, regulate noradrenaline release (Ishac et al., 1996), and decrements in eCB signaling at noradrenergic terminals have been shown to result in elevated sympathetic outflow (Srivastava and Lutz, 2012), as is seen in PTSD (Pervanidou and Chrousos, 2010). Furthermore, eCBs (as well as PEA and OEA) possess anti-inflammatory actions (Hansen, 2010), such that reduced eCB signaling could contribute to a basal pro-inflammatory state (Beyer et al., 2010), as has also been documented for PTSD (Spivak et al., 1997; Baker et al., 2001; Plantinga et al., 2013). Finally, eCB signaling promotes the release of NPY (Gamber et al., 2005), which has been demonstrated in association with stress resiliency (Morgan et al., 2000; Yehuda et al., 2006; Sajdyk et al., 2008; Cohen et al., 2012). Reductions in NPY have been found in PTSD (Rasmusson et al., 2000; Sah et al., 2009), which have increased in association with PTSD recovery (Yehuda et al., 2006). Thus, diminished eCB function may additionally result in a deficit in the recruitment of NPY. These biological findings, in the context of known involvement of the eCB system in emotional memory extinction and recall, stress buffering and adaptation, as well as HPA function, suggest the possibility of a pivotal role for the eCB system in PTSD. A deficit in eCB function is consistent with all of the major symptom dimensions of PTSD, and represents a novel candidate system for further investigation in the pathophysiology and treatment of the disorder. Trials examining the effect of agents that potentiate eCB signaling in PTSD, either alone or in conjunction with treatments that modulate glucocorticoid signaling, will provide proof of principal for the contribution of eCB signaling to PTSD, and will help to elucidate more precisely the respective functional roles of 2-AG and AEA in PTSD development and expression.
Acknowledgments
The authors would like to thank Kara Stuhr for her technical assistance with the running of these experiments. This research was supported by National Institute of Mental Health (NIMH) Innovation Award 1R56MH077321-01 (RY) and NIH grants DA026996 (CJH) and MH41256 (BSM), operating grants from the Lightfighter Foundation (RY and BSM) and the Canadian Institutes of Health Research (MNH). MNH is the receipient of a Tier II Canada Research Chair in the Neurobiolgy of Stress. Partial funding for this study came from the Research and Education Initiative Fund, a component of the Advancing a Healtheir Wisconsin endowment at the Medical College of Wisconsin. Funding was also supported, in part, by 5MO1 RR00071 for the Mount Sinai General Clinical Research Center from the National Center for Research Resources. Support for Dr. Yehuda and Dr. Golier is provided by the Department of Veterans Affairs. Dr. Yehuda reported research funding from Eli Lilly and Company and Dr. McEwen reported research funding from Johnson and Johnson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Drs. Hill, Bierer, Makotkine, Golier and Hillard declare no conflicts of interest.
Contributors
MN Hill performed the endocannabinoid analysis and wrote the first draft of the manuscript. LM Bierer assisted in collection of the samples, contributed to the clinical assessments and contributed to the writing and editing of the manuscript. I Makotkine peformed the cortisol analysis. JA Golier contributed to subject recruitment and the clinical assessment. BS McEwen helped to design the study and edit the manuscript. CJ Hillard assisted with the endocannabinoid analysis and edited the manuscript. R Yehuda designed the study, contributed to the clinical assessments and helped to finalize the manuscript.
References
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsak P, Hauer D, Campolongo P, Schelling G, McGaugh JL, Roozendaal B. Glucocorticoids interact with the hippocampal endocannabinoid system in impairing retrieval of contextual fear memory. Proc Natl Acad Sci U S A. 2012;109:3504–3509. doi: 10.1073/pnas.1200742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD., Jr Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Soria-Gomez E, Quarta C, Metna-Laurent M, Cardinal P, Binder E, Cannich A, Delamarre A, et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB(1) receptor blockade. Proc Natl Acad Sci U S A. 2013;110:4786–4791. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, Chan K, Manners MT, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–155. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: The post-traumatic stress disorder-major depression connection. Biol Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chouker A, Kaufmann I, Kreth S, Hauer D, Feuerecker M, Thieme D, Vogeser M, Thiel M, Schelling G. Motion sickness, stress and the endocannabinoid system. PLoS One. 2010;5:e10752. doi: 10.1371/journal.pone.0010752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast Feedback Inhibition of the HPA Axis by Glucocorticoids Is Mediated by Endocannabinoid Signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, Joseph AM. Post-traumatic stress disorder and smoking: A systematic review. Nicotine Tob Res. 2007;9:1071–1084. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- Gamber KM, MacArthur KM, Westfall TC. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005;49:646–652. doi: 10.1016/j.neuropharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Macpherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Godlweski G, Ramikie TS, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp Neurol. 2010;224:48–55. doi: 10.1016/j.expneurol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer D, Weis F, Campolongo P, Schopp M, Beiras-Fernandez A, Strewe C, Giehl M, Toth R, et al. Glucocorticoid-endocannabinoid interaction in cardiac surgical patients: relationship to early cognitive dysfunction and late depression. Rev Neurosci. 2012;23:681–690. doi: 10.1515/revneuro-2012-0058. [DOI] [PubMed] [Google Scholar]
- Hauer D, Schelling G, Gola H, Campolongo P, Morath J, Roozendaal B, Hamuni G, Karabatsiakis A, et al. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS One. 2013;8:e62741. doi: 10.1371/journal.pone.0062741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010a;35:1333–1338. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 2010b;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009a;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, Karatsoreos IN, Mackie K, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009b;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–229. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Hong KI, Piomelli D, Sinha R. An endocannabinoid signal associated with desire for alcohol is suppressed in recently abstinent alcoholics. Psychopharmacology (Berl) 2009;205:63–72. doi: 10.1007/s00213-009-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Giuffrida A, King J, Skinner E, Scotter J, Musty RE. Cannabimimetic effects of osteopathic manipulative treatment. J Am Osteopath Assoc. 2005;105:283–291. [PubMed] [Google Scholar]
- Morgan CA, 3rd, Wang S, Southwick SM, Rasmusson A, Hazlett G, Hauger RL, Charney DS. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry. 2000;47:902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- Neumeister A. The endocannabinoid system provides an avenue for evidence-based treatment development for PTSD. Depress Anxiety. 2013;30:93–96. doi: 10.1002/da.22031. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, Potenza MN, Bailey CR, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.61. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005a;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005b;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Neuroendocrinology of post-traumatic stress disorder. Prog Brain Res. 2010;182:149–160. doi: 10.1016/S0079-6123(10)82005-9. [DOI] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: A twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30:4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress. 2011;14:384–397. doi: 10.3109/10253890.2011.586753. [DOI] [PubMed] [Google Scholar]
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, Geracioti TD., Jr Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M, Rein T, Schmeidler J, et al. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Dis Markers. 2011;30:101–110. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute Biometrics Research. 1995 [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- Srivastava RJ, Lutz B. Dysregulation of hypothalamic-pituitary-adrenal axis in mice lacking CB1 receptors in adrenergic and noradrenergic neurons; P1–28, 22nd Annual Symposium of the International Cannabinoid Research Society; 2012. [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]